ABSTRACT
The bacterial σ factor plays the central role in promoter recognition by RNA polymerase (RNAP). The primary σ factor, involved in transcription of housekeeping genes, was also shown to participate in the initiation of RNA synthesis and promoter escape by RNAP. In the open promoter complex, the σ finger formed by σ region 3.2 directly interacts with the template DNA strand upstream of the transcription start site. Here, we analysed the role of the σ finger in transcription initiation by four alternative σ factors in Escherichia coli, σ38, σ32, σ28 and σ24. We found that deletions of the σ finger to various extent compromise the activity of RNAP holoenzymes containing alternative σ factors, especially at low NTP concentrations. All four σs are able to utilize NADH as a noncanonical priming substrate but it has only mild effects on the efficiency of transcription initiation. The mediators of the stringent response, transcription factor DksA and the alarmone ppGpp decrease RNAP activity and promoter complex stability for all four σ factors on tested promoters. For all σs except σ38, deletions of the σ finger conversely increase the stability of promoter complexes and decrease their sensitivity to DksA and ppGpp. The result suggests that the σ finger plays a universal role in transcription initiation by alternative σ factors and sensitizes promoter complexes to the action of global transcription regulators DksA and ppGpp by modulating promoter complex stability.
Introduction
The initiation of DNA transcription is one of the main regulatory steps in the control of gene expression. Bacterial promoters are recognized by the RNA polymerase (RNAP) holoenzyme consisting of the catalytic core enzyme and a variable σ factor (reviewed in [Citation1,Citation2]). This provides an elegant way for switching the RNAP specificity depending on the bound initiation factor. All known bacteria have at list one primary σ factor responsible for transcription of housekeeping genes (σ70 in Escherichia coli, σA in others). Alternative σ factors control transcription of genes during adaptation to various growth conditions, stress response or specific developmental programmes [Citation1–4].
The six alternative σ factors encoded in the genome of E. coli belong to two unrelated families, σ70 and σ54. The members of the σ70 family include σ38 (σS), σ32 (σH), σ28 (σF or FliA), σ24 (σE) and σ19 (FecI) factors. RNAP containing σ38 transcribes genes involved in stress response, survival at the stationary phase, regulation of secondary metabolism and biofilm formation [Citation5–8]. σ32 is responsible for transcription of genes during heat shock response [Citation9–11]. σ28 controls genes involved in flagella synthesis and cell mobility [Citation12–14]. σ24 and σ19 belong to the most diverse ECF (extra cytoplasmic functions) family of σ factors that regulate transcription in response to signals in the periplasm or in the cell membrane [Citation3,Citation15,Citation16]. σ24 is involved in the membranous/periplasmic heat shock response [Citation17–19], while σ19, the shortest σ in E. coli, transcribes only one operon necessary for transport of iron(III) dicitrate in iron starvation conditions [Citation20,Citation21]. Finally, the σ54 factor is a regulator of nitrogen metabolism and a variety of other cellular processes. It shares no sequence homology with the σ70 family and uses a distinct mechanism of transcription initiation (reviewed in ref. [Citation22]).
Members of the σ70 family contain up to four conserved regions further divided into subregions [Citation2,Citation4,Citation23]. Promoters recognized by σ factors of the σ70 family usually contain two conserved motifs, the −10 and −35 elements, interacting with σ regions 2 and 4, respectively [Citation1,Citation2,Citation24]. In addition, promoters of the primary σ70 factor can contain the extended −10 motif (TG upstream of the −10 hexamer) and a specific discriminator motif downstream of the −10 element, recognized by region 3.0 (aka region 2.5) and region 1.2, respectively [Citation23–28]. σ38 recognizes promoter elements similar to promoters of σ70 [Citation29,Citation30], while promoters of other σs considerably differ from σ70 [Citation14,Citation31–33]. However, recent analyses of the open promoter complexes (RPo) formed by RNAP holoenzymes containing σ70, σ38, σ28 and σ24 factors revealed that they all have a common architecture (). The presence of two conserved DNA binding domains, formed by σ regions 2 and 4, and the similar organization of promoter complexes suggests a universal mechanism of promoter recognition for all alternative σs from the σ70 family [Citation1,Citation2,Citation34–38].
Figure 1. Structure of the σ finger in the principal and alternative σ factors in E. coli. (A-E) Structures of promoter complexes formed by RNAP holoenzymes containing the principal and alternative σ factors: σ70 (A, PDB 4YLO [Citation25]), σ70 with DksA and ppGpp (G4P) (B, PDB:5VSW [Citation55]), σ38 (C, PDB:5IPL [Citation34]), σ28 (D, PDB 6PMI [Citation38], σ24 (E, PDB 6JBQ [Citation35]). The σ factor is shown in ochre, the σ finger (region 3.2) is orange, deletions in the σ finger analysed in this study are brown. The template DNA strand (T) is light blue, the nontemplate DNA strand (NT) is dark blue, the RNA primer is black, the bridge helix (BH) is green, magnesium ions (Mg) bound in the active site are magenta. (F) Scheme of transcription initiation. Changes in the σ finger during RNA priming are indicated with arrows. (G) Sequences of the σ finger (shown in the orange box, corresponding to the region highlighted in orange in panels A-E) in the principal and alternative σs in E. coli. Deleted amino acid residues are shown in bold. Position of the σ finger in σ24 is based on the published structure of the RNAP σ24 holoenzyme [Citation35]
![Figure 1. Structure of the σ finger in the principal and alternative σ factors in E. coli. (A-E) Structures of promoter complexes formed by RNAP holoenzymes containing the principal and alternative σ factors: σ70 (A, PDB 4YLO [Citation25]), σ70 with DksA and ppGpp (G4P) (B, PDB:5VSW [Citation55]), σ38 (C, PDB:5IPL [Citation34]), σ28 (D, PDB 6PMI [Citation38], σ24 (E, PDB 6JBQ [Citation35]). The σ factor is shown in ochre, the σ finger (region 3.2) is orange, deletions in the σ finger analysed in this study are brown. The template DNA strand (T) is light blue, the nontemplate DNA strand (NT) is dark blue, the RNA primer is black, the bridge helix (BH) is green, magnesium ions (Mg) bound in the active site are magenta. (F) Scheme of transcription initiation. Changes in the σ finger during RNA priming are indicated with arrows. (G) Sequences of the σ finger (shown in the orange box, corresponding to the region highlighted in orange in panels A-E) in the principal and alternative σs in E. coli. Deleted amino acid residues are shown in bold. Position of the σ finger in σ24 is based on the published structure of the RNAP σ24 holoenzyme [Citation35]](/cms/asset/8dde47c4-9746-40a9-b9b8-bc6833d0558d/krnb_a_1889254_f0001_oc.jpg)
The primary σ factor contains a flexible linker between regions 2 and 4, formed by region 3.2 and called the σ finger. The σ finger deeply penetrates into the active site cleft and directly interacts with the template DNA strand upstream of the active site in the open promoter complex (). These contacts are important for the positioning of the template strand in the active site and for binding of initiating NTPs and priming of RNA synthesis [Citation24,Citation39–44]. At later steps of initiation, the σ finger clashes with the growing RNA transcript, leading to abortive initiation and transcriptional pausing (). Further displacement of the σ finger from the RNA exit channel facilitates disassembly of the promoter complex and dissociation of the σ factor during promoter escape [Citation39–43,Citation45-48].
Surprisingly, despite its significant role in transcription initiation by the primary σ factor, the sequence of the σ finger is not conserved in σ38, σ32 and σ28 except for the presence of a few negatively charged amino acid residues. Moreover, σ24 and σ19 completely lack region 3 which is replaced by a short linker of unrelated sequence () [Citation4]. Nevertheless, recent structural analysis demonstrated that in promoter complexes formed by σ38 and σ28, the σ finger occupies a similar position to σ70 in the active site cleft () [Citation34,Citation38]. Even in the case of σ24, the linker between domains 2 and 4 inserts into the active site cleft in a way similar to the holoenzymes of other σs containing region 3 () [Citation35]. At the same time, beyond this structural homology the role of the σ finger during transcription initiation by alternative σs remains largely unknown.
Transcription factor DksA and the alarmone ppGpp are key regulators of transcription during the stringent response [Citation49–51]. The major result of the synergetic action of DksA and ppGpp on the σ70 RNAP holoenzyme is a reduction of ribosome synthesis, mainly due to the decreased activity of promoters of ribosomal RNA and proteins [Citation50,Citation52,Citation53]. DksA binds RNAP within the secondary channel and destabilizes promoter complexes formed by the σ70 holoenzyme () [Citation50,Citation53–55]. The alarmone ppGpp binds to two sites on RNAP, site 1 at the β’-ω interface outside of the DNA binding cleft and site 2 at the interface between RNAP and DksA in the secondary channel, thus potentiating the action of DksA () [Citation49,Citation52,Citation55,Citation56]. DksA and ppGpp together strongly suppress the activity of promoters that form unstable complexes with RNAP, including ribosomal promoters [Citation49,Citation50,Citation53,Citation56,Citation57]. Previously, we demonstrated that deletions and substitutions in the σ finger in σ70 increase the stability of promoter complexes formed by RNAP and thus make them resistant to the action of DksA and ppGpp, suggesting that the σ finger plays an important role in transcription regulation during stress response [Citation58]. DksA and ppGpp also have major effects on the expression of genes in the regulons controlled by alternative σ factors, many of which are activated possibly due to re-distribution of RNAP from the inhibited σ70 promoters [Citation49,Citation59]. At the same time, the ability of DksA and ppGpp to directly regulate transcription by RNAP holoenzymes containing alternative σ factors remains poorly investigated. Furthermore, a possible role of the σ finger in alternative σ factors in the control of promoter complex stability and its sensitivity to DksA and ppGpp is unknown.
In this study, we have investigated the effects of deletions in the σ finger in σ38, σ32, σ28 and σ24 on transcription initiation by E. coli RNAP. We have shown that the σ finger is important for efficient transcription initiation and that it modulates promoter complex stability for all σ factors. DksA and ppGpp destabilize promoter complexes of RNAP holoenzymes containing alternative σ factors. For σ32, σ28 and σ24, deletions in the σ finger increase the stability of promoter complexes and make them less sensitive to the action of DksA and ppGpp. Therefore, the σ finger plays a conserved function in transcription initiation by various σ factors, by promoting transcription initiation but at the same time making promoter complexes susceptible to transcription regulation by the stringent response factors.
Results
Deletions in the σ finger compromise the transcriptional activity of RNAP holoenzymes containing alternative σ factors
For analysis of the functional role of the σ finger in transcription initiation, we obtained deletions in region 3.2 of the alternative σ factors in positions roughly corresponding to the position of the previously studied deletion Δ513-519 in σ70 () [Citation42,Citation43]. For σ32, the size of the deletion was larger because of an insertion of several residues in region 3.2 in this σ in comparison with σ70 and σ38. In the case of σ24, the position of the deletion was selected based on the positioning of the σ2-σ4 linker in the published promoter complex structure () [Citation35]. For analysis of the activity of RNAPs variants containing alternative σ factors, we selected several previously characterized promoters that are recognized by corresponding RNAP holoenzymes [Citation60]: adhEp1 [Citation61,Citation62], talAp2 [Citation63] and ecnBp [Citation64,Citation65] for σ38, dnaKp1 [Citation66] for σ32, flgMp [Citation13] and vesP [Citation67] for σ28, and yieEp [Citation68] for σ24 (Fig. S1).
Since mutations in the σ finger in σ70 were previously shown to affect the binding of initiating NTPs and thus impair RNA priming [Citation42,Citation43], we measured RNAP activity in the presence of different NTP concentrations (‘high’ and ‘low’, 200 μM and 30 μM, respectively). It should be noted that these concentrations are lower than measured NTP pools in exponentially growing E. coli cells, but are comparable to the cellular NTP concentrations at the stationary phase of growth (e.g [Citation69].). Most experiments were performed with the adhEp1, dnaKp1, flgMp and yieEp promoters, which were highly active with corresponding RNAP holoenzymes in vitro (). It was shown that deletions in the σ finger markedly decreased the efficiency of RNA synthesis (quantified in percent of full-length RNA relative to the wild-type σ) for each RNAP holoenzyme, with the exception of σ28 for which the deletion had only a minor effect on the total RNAP activity (). At the same time, the deletion decreased the activity of the σ28 Δfinger RNAP holoenzyme on another tested promoter, vesP (Fig. S2). Furthermore, for the σ38 adhEp1 and σ32 dnaKp1 promoters, the defects of mutant RNAPs were much more pronounced at low NTP concentrations (, compare lanes 1,4 with lanes 7,10). This suggested that deletions of the σ finger may impair RNA priming during transcription initiation by alternative σ factors.
Figure 2. Transcription initiation by RNAP holoenzymes containing alternative σ factors with deletions in the σ finger. (A) σ38 on the adhEp1 promoter. (B) σ38 on the dnaKp1 promoter. (C) σ38 on the flgMp promoter. (D) σ24 on the yieEp promoter. The reactions contained high (200 μM ATP, GTP, CTP and 10 μM UTP) or low (30 μM ATP, GTP, CTP and 10 μM UTP) nucleotide concentrations. Primers added to the reactions are indicated as X (dinucleotide primers corresponding to each promoter, Fig. S1) and N (NADH). For each RNAP, RNA products were separated in one or two parallel polyacrylamide gel of different densities (15 and 23%) for analysis of abortive products (AP) and full-length (RO, run-off) RNA products. Positions of full length products initiated in the absence (A) or in the presence of NADH are indicated. For most promoters, additional RNA products observed below the RO product likely correspond to RNAP stalling before the end of the linear DNA fragment (see Fig. S3). The marker (M) lanes contain abortive RNA products of known lengths synthesized during transcription by the σ70 RNAP holoenzyme on the T7A1cons promoter [Citation43]. RNAP activities calculated in percent of full-length RNA products relative to the wild-type RNAP are indicated below the gels (means from 2 independent measurements). The scanning profiles of full-length and short RNA products synthesized in reactions performed at high nucleotide concentrations (lanes 1,2 and 7,8 in each panel) are shown on the right in each panel. The profiles were drawn for reactions separated on the same gel and were normalized to the level of RO synthesis. The ratio of RO RNA to the sum of short RNA products (indicated in the profiles) in the same reactions is shown at the bottom
![Figure 2. Transcription initiation by RNAP holoenzymes containing alternative σ factors with deletions in the σ finger. (A) σ38 on the adhEp1 promoter. (B) σ38 on the dnaKp1 promoter. (C) σ38 on the flgMp promoter. (D) σ24 on the yieEp promoter. The reactions contained high (200 μM ATP, GTP, CTP and 10 μM UTP) or low (30 μM ATP, GTP, CTP and 10 μM UTP) nucleotide concentrations. Primers added to the reactions are indicated as X (dinucleotide primers corresponding to each promoter, Fig. S1) and N (NADH). For each RNAP, RNA products were separated in one or two parallel polyacrylamide gel of different densities (15 and 23%) for analysis of abortive products (AP) and full-length (RO, run-off) RNA products. Positions of full length products initiated in the absence (A) or in the presence of NADH are indicated. For most promoters, additional RNA products observed below the RO product likely correspond to RNAP stalling before the end of the linear DNA fragment (see Fig. S3). The marker (M) lanes contain abortive RNA products of known lengths synthesized during transcription by the σ70 RNAP holoenzyme on the T7A1cons promoter [Citation43]. RNAP activities calculated in percent of full-length RNA products relative to the wild-type RNAP are indicated below the gels (means from 2 independent measurements). The scanning profiles of full-length and short RNA products synthesized in reactions performed at high nucleotide concentrations (lanes 1,2 and 7,8 in each panel) are shown on the right in each panel. The profiles were drawn for reactions separated on the same gel and were normalized to the level of RO synthesis. The ratio of RO RNA to the sum of short RNA products (indicated in the profiles) in the same reactions is shown at the bottom](/cms/asset/a926786b-d571-4e6f-abf6-ac172497c9c1/krnb_a_1889254_f0002_oc.jpg)
We also tested the effects of short dinucleotide primers, corresponding to the transcription start site in each promoter (Fig. S1), on transcription initiation. The primers had only minor effects on the pattern of RNA products synthesized on most promoters, except for dnaKp1, in the case of which the activity of RNAP was significantly stimulated at low NTP concentrations (, compare lanes 4 and 5, 10 and 11).
The decrease in the full-length RNA synthesis caused by the σ finger deletions was accompanied by changes in the quantity and pattern of abortive RNA products synthesized during transcription. In particular, the amounts of short RNAs were significantly decreased for the σ38 Δfinger mutant, as a result of overall defects in transcription initiation (, lanes 7–12). At the same time, the relative amounts of short RNA products (8–12 nt) synthesized by RNAP containing the mutant σ38 subunit were comparable or even higher than in the case of wild-type σ38, when the profiles of synthesized RNAs were normalized to the amounts of full-length RNA. Similar effects were observed for reactions performed with and without the dinucleotide primer (, compare scanning profiles for lanes 1, 2 and 7, 8). The ratio of the amounts of the full-length transcript to 8–12 nt products (RO/AP) was also somewhat higher for wild-type RNAP than for the mutant (). Deletions of the σ finger also increased the efficiency of short RNA synthesis relative to full-length RNA in the case of σ32 and σ24 RNAPs ( and ), as is evident from both comparison of the scanning profiles of RNA products and the RO/AP ratio (lanes 1, 2 and 7, 8). In contrast, deletion of the σ finger in σ28 did not significantly affect the efficiency of promoter escape but it changed the pattern of short RNA products synthesized by σ28 RNAP on both the flgM and ves promoters. In paritcular the amounts of RNAs shorter than 8–9 nts were significantly decreased for this RNAP variant ( and Fig. S2). Overall, these results indicated that the mutations in the σ finger impair promoter escape by the mutant variants of the RNAP holoenzyme.
Incorporation of noncanonical priming nucleotides with alternative σ factors
Bacterial RNAP containing the principal σ factor was previously shown to incorporate noncanonical priming substrates during transcription initiation, including NAD+/NADH, FAD, desphospho-CoA and others [Citation70–74]. However, the ability of RNAP holoenzymes containing alternative σ factors to initiate transcription with noncanonical priming nucleotides has never been studied, except for σ38 [Citation71]. Since changes in the σ finger affect the binding of initiating nucleotides, it is also possible that deletions of the σ finger in alternative σ factors might affect utilization of noncanonical priming substrates such as NADH, one of the most common capping nucleotides. We therefore compared the activities of the wild-type and mutant RNAP variants containing alternative σ factors in the absence and in the presence of NADH.
It was found that all four alternative σs could incorporate NADH during transcription initiation, as revealed by the appearance of a lower mobility RNA transcript in comparison with control reactions (Fig. S3 and ). When NADH was present in excess over ATP, the canonical priming substrate for all four promoters, most transcripts were initiated from NADH (Fig. S3). Notably, secondary channel factors GreB or DksA had no effect on the relative efficiency of transcription initiation from ATP or NADH under these conditions (Fig. S3).
We then compared the effects of NADH on transcription performed with wild-type or mutant σ variants at different NTP concentrations. Similarly to wild-type RNAPs, all mutant RNAPs incorporated NADH during initiation, resulting in shifts in the position of abortive and full-length RNAs in the gel (, compare lanes 3,6 and 9,12 in each panel). NADH had a mild stimulatory effect on the activity of mutant RNAP variants, which was more pronounced at low NTP concentrations (~1.5–2 fold increase in RNA synthesis of σ38, σ32 and σ24 Δfinger mutants; ). The effect of NADH on abortive transcription was most obvious for σ38, for which the whole range of short RNA transcripts was shifted by one nucleotide, apparently as a result of incorporation of NADH instead of initiating ATP (, lanes 3,6,9,12).
Sensitivity of RNAP holoenzymes containing alternative σ factors to DksA and ppGpp
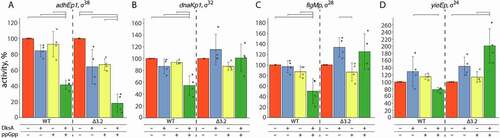
DksA and ppGpp were previously shown to have a crucial role in the regulation of RNAP activity during stringent response (see Introduction). To test whether promoters analysed in this study can be directly regulated by DksA and ppGpp, we measured the activity of RNAP holoenzymes containing alternative σs in the presence of either one of these factors or both. DksA or ppGpp alone had only minor effects on the overall RNAP activity for all four σs under the conditions of our experiments (). However, when both factors were present together, the activity of alternative RNAP holoenzymes was decreased ~2-fold in comparison with reactions containing no factors (with the exception of σ24, for which the effect was smaller than for other σs) (, wild-type σs).
Figure 3. Effects of DksA and ppGpp on the activity of RNAP holoenzymes containing wild-type and mutant σ factors. For each σ factor, wild-type or mutant, transcription was performed in the absence or in the presence of DksA and ppGpp and normalized to the activity in the absence of regulators (see Materials and Methods for details). Means and standard deviations from four independent measurements are shown. Significant differences in activities are shown with brackets above the plots (p-values < 0.05)
To measure the effects of DksA and ppGpp on the stability of RNAP-promoter complexes, we incubated preformed promoter complexes with the competitive inhibitor of DNA binding heparin and measured the residual activity of RNAP after increasing time intervals, by monitoring full-length RNA synthesis (). For promoter complexes analysed in this study, the half-life times for wild-type RNAPs varied between >10 min and <5 s (810 s for σ38 adhEp1, 480 s for σ24 yieEp, 78 s for σ28 flgMp and <5 s for σ32 dnaKp1) (, ).
Table 1. Promoter complex half-life times for RNAP holoenzymes containing wild-type and mutant σ factors, in the absence and in the presence of DksA and ppGpp
Figure 4. Stabilities of promoter complexes formed by wild-type and mutant σ factors in the absence and in the presence of DksA/ppGpp. Preformed promoter complexes were incubated for increasing time intervals with heparin, followed by the addition of NTPs (in the control samples heparin was added together with NTPs). Positions of the run-off RNA transcripts are indicated. The plots show the kinetics of the decay of RNAP activity (averages from 3–4 independent experiments). The data were fitted to a single-exponential equation. The half-life times for each promoter complex are shown in
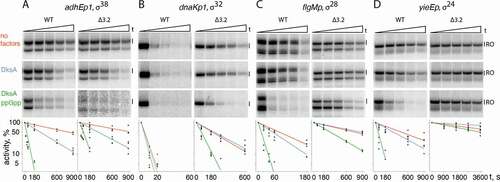
DksA and ppGpp decreased promoter complex stabilities for all four σ factors (the effect for σ32 was barely detectable because the dnaKp1 promoter complex was already unstable even in the absence of these factors) (). In all cases, DksA by itself moderately decreased the promoter complex half-life, from 1.5 to 2.8 fold for various σ factors (). However, the effects were much larger when both DksA and ppGpp were present in the reaction (from 4 to 25 fold) (, ), indicating that ppGpp potentiates the action of DksA for various alternative σ factors. Together, these results indicate that DksA and ppGpp can decrease promoter complex stability and suppress the activity of RNAP holoenzymes containing alternative σ factors.
Deletions in the σ finger stabilize promoter complexes and decrease their sensitivity to DksA and ppGpp
In the last set of experiments, we analysed the effects of deletions in the σ finger on the stability of promoter complexes formed by RNAP holoenzymes containing alternative σs and on their sensitivity to DksA and ppGpp. Deletions of the σ finger significantly increased promoter complex stabilities for all σ factors except σ38 (10-fold for σ28 and σ24, ~40-fold for σ32) (, ). Since no effect was observed for σ38 on the adhEp1 promoter, we analysed two additional promoters for this σ, talAp2 and ecnBp (Fig. S1). The stabilities of complexes formed with the Δfinger variant of σ38 on these promoters were also similar to the wild-type σ factor (Fig. S4).
We then tested the effects of DksA and ppGpp on the stability of promoter complexes formed by mutant RNAPs. RNAP holoenzyme containing the Δfinger variant of σ38 was similarly sensitive to DksA and ppGpp to wild-type RNAP (t1/2 in the presence of both factors was decreased ~25-fold for both WT and Δfinger RNAPs, ). At the same time, the other three σ factors revealed markedly decreased sensitivity to the regulatory factors. DksA by itself had essentially no effect on t1/2 for promoter complexes formed by the σ32, σ28 and σ24 Δfinger RNAPs (, ). DksA together with ppGpp decreased half-lives of promoter complexes formed by the mutant RNAPs, but the extent of this effect was smaller than in the case of corresponding wild-type RNAP variants, and the resulting t1/2 values measured for the mutants were much larger than for wild-type RNAPs (). For example, for σ28 the stability of promoter complexes was increased as much as 36-fold in comparison with wild-type RNAP (t1/2 of 7.8 and 285 s for wild-type and Δfinger RNAP variants). Similarly, for σ32 t1/2 was increased from <5 to 42 s, and for σ24 from 30 to 3600 s ().
In agreement with the observed effects of the σ finger deletions on promoter complex stabilities, DksA and ppGpp did not inhibit the activity of RNAPs containing the mutant variants of σ32, σ28 and σ24 during full-length RNA synthesis (). Moreover, the activity of the σ24 Δfinger RNAP was even stimulated in the presence of these factors, indicating that they may facilitate a rate-limiting step in promoter complex formation by the mutant RNAP holoenzyme (). At the same time, the activity of the σ38 Δfinger RNAP was still inhibited by DksA and ppGpp, consistent with the absence of the effects of this deletion on the promoter complex stability (). Overall, we conclude that deletions of the σ finger in all σs except σ38 can greatly stabilize promoter complexes formed by RNAP holoenzymes containing alternative σ factors from various groups and make them resistant to the action of DksA and ppGpp.
Discussion
Recent structural analysis of promoter complexes formed by RNAP holoenzymes containing alternative σ factors of the σ70 family showed that they have a common general structure, with the σ finger occupying similar positions inside the DNA binding cleft and contacting the template DNA strand upstream of the active site () [Citation34–38,Citation41]. The sequences of the σ finger are relatively well conserved in primary σ factors but are very diverse in alternative σs () [Citation4,Citation23]. Nevertheless, we have demonstrated that changes in the σ finger have similar effects on the transcriptional properties of alternative σ factors. Deletions of the σ finger impair the activity of RNAP holoenzymes containing σ38, σ32, σ24, and σ28 factors, at least on a subset of promoters tested in this study. Deletions of the σ finger also change the pattern of abortive RNA products synthesized during transcription initiation, suggesting that the σ finger is involved in first steps of RNA synthesis, similarly to the primary σ70 factor [Citation42,Citation43]. In particular, the relative amounts of short RNAs synthesized by the mutant RNAPs were increased in comparison with full-length RNA, indicative of problems in promoter escape for these RNAP holoenzymes. In all analysed σ factors, the σ finger contains negatively charged residues () that may directly interfere with the 5ʹ-triphosphate group during RNA extension and thus promote release of abortive RNAs or displacement of σ during promoter escape by RNAP [Citation39–43]. Deletion of the σ finger also changes the pattern of abortive RNA transcripts in the case of σ70 [Citation42,Citation43], and increases the retention of σ within the transcription elongation complex for both σ70 and σ38 [Citation43,Citation65], indicating that the σ finger facilitates dissociation of the σ factor during promoter escape by RNAP.
The σ70 and σ38 RNAP holoenzymes were previously shown to incorporate noncanonical initiating substrates into the 5ʹ-end of RNA, including NAD+/NADH, 3′-desphospho-CoA, FAD, UDP-glucose and UDP-N-acetylglucosamine [Citation70–72,Citation74]. Here, we have demonstrated that NADH can be incorporated into the nascent RNA by all four analysed alternative σ factors. Deletions of the σ finger to various extent impair transcription initiation from NADH for three out of four alternative σs, except for σ28. This contrasts the previous report showing that incorporation of noncanonical substrates by the σ70 RNAP holoenzyme on an RNA I promoter was not strongly affected by the σ finger deletion in σ70 [Citation71]. Therefore, the role of the σ finger in incorporation of various initiating substrates may vary for different σ factors and for different tested promoters.
Similarly to σ70, deletions of the σ finger in three out of four analysed alternative σs, except for σ38, stabilize promoter complexes formed by the RNAP holoenzyme. This indicates that the collision of the σ finger with the template DNA strand may have a general destabilizing effect on RNAP-promoter interactions. This effect may likely be stronger for promoters that form intrinsically unstable complexes with RNAP, such as rrnB P1 for σ70 that has a suboptimal spacer between the −10 element and the transcription start site [Citation58,Citation75] or dnaKp1 for σ32 (). Interestingly, the deletion of the σ finger in σ38 has no significant effect on the promoter complex stability on three tested promoters. While comparison of available structures of the open promoter complexes of the σ38 and σ70 RNAP holoenzymes does not reveal major differences in the positioning of the σ finger () [Citation25,Citation34], the result suggests that it does not destabilize promoter complexes during transcription initiation by the σ38 holoenzyme, in contrast to σ70 and other alternative σ factors.
Importantly, we have demonstrated that the σ finger in the ECF σ24 factor likely plays the same functions in transcription initiation as in the case of other σ factors, consistent with its similar positioning in the open promoter complex [Citation35,Citation37,Citation41]. Previously, deletion or replacement of the whole σ finger in E. coli σ24 with an unrelated sequence was shown to dramatically impair promoter complex formation and full-length RNA synthesis by RNAP [Citation35]. In our study, deletion of the central part of the σ finger in σ24 has a more specific effect on transcription initiation, by decreasing the overall RNAP activity, increasing promoter complex stability and changing RNAP sensitivity to the stringent response factors.
We have shown that DksA and ppGpp strongly destabilize promoter complexes formed by all four alternative σs. They also decrease the efficiency of transcription initiation from the analysed promoters, although the effects are smaller than in the case of highly regulated σ70 promoters. The concentration of DksA is relatively constant during cell growth, and its functional activity is dependent on the varying concentration of ppGpp [Citation50]. Consistently, we have observed that the effects of DksA on the promoter complex stability and activity of RNAP holoenzymes containing alternative σ factors are strongly potentiated by ppGpp. Deletions in the σ finger oppose the effects of DksA and ppGpp, likely by increasing the stability of promoter complexes. Similarly, mutations in the σ finger in σ70 were shown to change the regulation of the rrnB P1 promoter by the stringent response factors, by increasing the stability of promoter complexes, decreasing their apparent affinity to DksA, and relieving the inhibitory effects of DksA in vitro and in vivo [Citation58]. From the structural point of view, stabilization of promoter complexes by the σ finger deletion may counteract destabilization of downstream RNAP-DNA contacts caused by DksA/ppGpp [Citation55,Citation57,Citation58].
During stringent response, DksA and ppGpp have complex effects on the expression of regulons controlled by alternative σ factors, first of all, σ38. In most cases, they were proposed to control transcription indirectly, through re-distribution of RNAP freed from the σ70 promoters [Citation51,Citation53,Citation76]. DksA and ppGpp were also shown to activate the σ38 regulon by direct stimulation of σ70 promoters responsible for transcription of key regulators of translation and proteolysis of σ38 [Citation77]. Such direct activation of σ70 transcription by DksA and ppGpp may result from their effects on isomerization of intermediate complexes formed during promoter recognition [Citation56,Citation78]. These effects likely overwhelm the possible inhibitory action of DksA and ppGpp on σ38 promoters that we have observed in vitro.
In contrast, DksA and ppGpp were shown to inhibit flagellar transcription, by directly inhibiting σ70-dependent expression of σ28 itself and of its master regulator FlhDC [Citation79]. Consistently, deletion of the dksA gene results in activation of the σ28 regulon [Citation76]. Previous studies did not observe direct effects of DksA on transcription by the σ28 RNAP holoenzyme on the fliA and fliC promoters [Citation79]. We have demonstrated that DksA and ppGpp can inhibit the activity of the σ28 RNAP holoenzyme and decrease the stability of promoter complexes on the flgM promoter suggesting that DksA/ppGpp may also directly regulate the activity of σ28 promoters in vivo.
Finally, the activity of the σ24 regulon was shown to be increased by ppGpp and DksA upon entry to the stationary phase [Citation80]. Some promoters recognized by the σ24 RNAP holoenzyme (rpoHp3 and rybBp) could be activated in vitro by the combined action of DksA and ppGpp, despite their negative effect on the promoter complex stability [Citation81]. Similarly, the DksA homolog TraR encoded in the F factor was shown to activate σ24 promoters independently of ppGpp [Citation82]. In contrast, we show that DksA/ppGpp do not activate the yieEp promoter recognized by the σ24 holoenzyme and strongly decrease the stability of its complexes with RNAP. A possible role for the observed inhibition of the σ24 transcription by DksA/ppGpp for gene regulation in vivo remains to be investigated.
It was previously proposed that DksA/ppGpp and TraR may target particular kinetic intermediates during promoter complex formation by the σ70 holoenzyme resulting in different outcomes depending on the promoter sequence [Citation56,Citation78,Citation83]. Furthermore, we demonstrated that mutations in the σ finger in σ70 have different effects on the RNAP activity and its sensitivity to DksA/ppGpp depending on the stability of promoter complexes [Citation58]. Thus, the reported differences in the effects of DksA/ppGpp on various promoters of σ24 and other σ factors may likely result from different kinetic pathways of open complex formation on these promoters.
In conclusion, our data suggest that the σ finger play important functions during transcription initiation by bacterial RNAP holoenzymes containing various alternative σ factors. It likely participates in RNA priming and promoter escape but at the same time destabilizes promoter complexes thus making them amenable for transcription regulation by the stringent response factors. These functions of the σ finger are apparently conserved even in the most diverse ECF σ factors of the σ70 family that completely lack conserved region 3 [Citation4,Citation84]. Moreover, an unrelated region RII.3 in σ54 was proposed to play a similar role in RNA priming during transcription initiation [Citation22,Citation85]. Notably, the B-reader motif in the general transcription initiation factor TFIIB of eukaryotic RNAP II [Citation86] and its counterparts in RNAP I [Citation87] and RNAP III [Citation88,Citation89] are also positioned in the open promoter complex in a similar fashion to the σ finger in bacterial RNAP, and may play similar functions in transcription initiation. The requirement for the contacts of an initiation factor with the template DNA strand and growing RNA in the promoter complex may therefore be a fundamental feature of transcription initiation in various systems, likely connected to the ability of RNAPs to initiate RNA synthesis de novo.
The involvement of the σ finger in several steps of transcription initiation opens additional opportunities for the regulation of gene expression. In various σ factors, the σ finger may likely modulate the action of other transcription regulators in addition to DksA and ppGpp. Indeed, recent structural analysis of promoter complexes of M. tuberculosis RNAP demonstrated that actinobacteria-specific factor RbpA directly contacts the σ finger and may rely on these contacts to affect initial steps of RNA synthesis [Citation90]. The contacts of rifamycin antibiotics with the σ finger observed in the E. coli σ70 RNAP holoenzyme [Citation91,Citation92] may explain the effects of rifampicin on the binding of initiating NTPs, prior to its steric occlusion of the RNA exit [Citation43,Citation73,Citation91]. Antibiotic fidaxomycin binds RNAP near the σ finger and these contacts possibly contribute to its effects on transcription [Citation90,Citation93,Citation94]. The σ finger may therefore serve as a target for novel antibacterial compounds and antibiotics.
Materials and methods
Proteins
The E. coli genes encoding the σ38, σ32, σ28 and σ24 factors, respectively, were amplified from genomic by PCR and cloned into the pET29 (rpoS, rpoH, rpoF) or pLATE52 (rpoE) vectors as described previously [Citation65,Citation95]. Mutant genes with deletions of the σ finger (Δ228-234, Δ188-197, Δ149-156, Δ101-111 for σ38, σ32, σ28 and σ28, respectively) were obtained by PCR and cloned in the same way. The wild-type and mutant σ38, σ32, σ28 variants were expressed and purified from E. coli strain C3013 (New England Biolabs) by MonoQ anion exchange chromatography as described previously [Citation65,Citation95]. σ24 containing an N-terminal His6 tag was purified using Co2+-affinity chromatography as described [Citation95]. The DksA protein was purified as in [Citation45]. Wild-type E. coli core RNAP was purified from E. coli BL21(DE3) (Novagen) containing the expression plasmid pVS10 as described previously [Citation96], including polyethylenimine precipitation, heparin affinity chromatography, Ni-affinity chromatography and MonoQ anion exchange chromatography steps.
In vitro transcription
Wild-type promoter fragments were obtained by PCR from genomic DNA (see Supplementary Fig. S1 and Table S1 for promoter and primer sequences). Promoter fragments with substitutions around the transcription start site were obtained by PCR from oligonucleotide templates, cloned into the pJET1.2 vector using CloneJET PCR Cloning Kit, and amplified by PCR using the same primers (Table S1).
Promoter complexes were assembled by mixing 200 nM core enzyme, 500 nM σ factors and 30 nM DNA fragments in 10 μl of transcription buffer containing 40 mM Tris-HCl pH 7.9, 40 mM KCl, 10 mM MgCl2, and incubating the mix for 10 min at 37°C. The reaction was started by adding NTP substrates at high (200 μM ATP, GTP, CTP, 10 μM UTP, 2.5 μCi of α-[32P]-UTP; most experiments) or low (30 μM ATP, GTP, CTP, 10 μM UTP, 2.5 μCi of α-[32P]-UTP; experiments in and Fig. S2) concentrations, together with 10 μg/ml heparin. To detect the ability of RNAP holoenzymes with alternative σs to initiate transcription starting from non-canonical substrates, dinucleotide primers corresponding to positions −1/+1 (20 μM) or NADH (2 mM) were added together with the NTP mix. The primers used for each promoter fragment are shown in Fig. S1. When indicated, DksA (2 μM) and/or ppGpp (100 μM, TriLink BioTechnologies, Inc.) were added and the samples were incubated for additional 5 min. To measure half-life times of promoter complexes, heparin (10 μg/ml) was added for different time intervals prior to NTPs. The reactions were stopped after 3 min (for half-life time measurements) or after 7 min (for all other experiments) by adding 10 μl of stop-buffer containing 8 M urea, 20 mM EDTA, 2×TBE. RNA products were analysed by denaturing 15–23% PAGE and quantified by phosphorimaging using a Typhoon 9500 scanner (GE Healthcare). The activity of RNAP in the absence of any factors was set as 100%, all other measurements were normalized to this value. The difference across experimental conditions was evaluated with the ANOVA test followed by the post-hoc Tukey test for multiple comparison of means with the standard significance level of 0.05. The half-life times (t1/2) of promoter complexes were calculated by nonlinear least-squares estimation of the parameters of a single-exponential decay model, y = 100×exp(-kobs×t), where t1/2 equals ln2/kobs.
Supplemental Material
Download PDF (1,023.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Chen J, Boyaci H, Campbell EA. Diverse and unified mechanisms of transcription initiation in bacteria. Nat Rev Microbiol. 2021;19:95–109.
- Feklistov A, Sharon BD, Darst SA, et al. Bacterial sigma factors: a historical, structural, and genomic perspective. Annu Rev Microbiol. 2014;68:357–376.
- Paget MS. Bacterial sigma factors and anti-sigma factors: structure, function and distribution. Biomolecules. 2015;5:1245–1265.
- Gruber TM, Gross CA. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol. 2003;57:441–466.
- Chen AI, Goulian M. A network of regulators promotes dehydration tolerance in Escherichia coli. Environ Microbiol. 2018;20:1283–1295.
- Battesti A, Majdalani N, Gottesman S. The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol. 2011;65:189–213.
- Schellhorn HE. Function, evolution, and composition of the RpoS regulon in Escherichia coli. Front Microbiol. 2020;11:560099.
- Hengge R. Stationary-phase gene regulation in escherichia coli section sign. EcoSal Plus. 2011;4. DOI:10.1128/ecosalplus.5.6.3
- Grossman AD, Erickson JW, Gross CA. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984;38:383–390.
- Yura T. Regulation of the heat shock response in Escherichia coli: history and perspectives. Genes Genet Syst. 2019;94:103–108.
- Zhao K, Liu M, Burgess RR. The global transcriptional response of Escherichia coli to induced sigma 32 protein involves sigma 32 regulon activation followed by inactivation and degradation of sigma 32 in vivo. J Biol Chem. 2005;280:17758–17768.
- Arnosti DN, Chamberlin MJ. Secondary sigma factor controls transcription of flagellar and chemotaxis genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989;86:830–834.
- Chilcott GS, Hughes KT. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar typhimurium and Escherichia coli. Microbiol Mol Biol Rev. 2000;64:694–708.
- Koo BM, Rhodius VA, Campbell EA, et al. Mutational analysis of Escherichia coli sigma28 and its target promoters reveals recognition of a composite −10 region, comprised of an ‘extended −10ʹ motif and a core −10 element. Mol Microbiol. 2009;72:830–843.
- Maeda H, Jishage M, Nomura T, et al. Two extracytoplasmic function sigma subunits, sigma(E) and sigma(FecI), of Escherichia coli: promoter selectivity and intracellular levels. J Bacteriol. 2000;182:1181–1184.
- Helmann JD. Where to begin? Sigma factors and the selectivity of transcription initiation in bacteria. Mol Microbiol. 2019;112:335–347.
- Raivio TL, Silhavy TJ. Periplasmic stress and ECF sigma factors. Annu Rev Microbiol. 2001;55:591–624.
- Mecsas J, Rouviere PE, Erickson JW, et al. The activity of sigma E, an Escherichia coli heat-inducible sigma-factor, is modulated by expression of outer membrane proteins. Genes Dev. 1993;7:2618–2628.
- Ades SE. Regulation by destruction: design of the sigmaE envelope stress response. Curr Opin Microbiol. 2008;11:535–540.
- Angerer A, Enz S, Ochs M, et al. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. Fecl belongs to a new subfamily of sigma 70-type factors that respond to extracytoplasmic stimuli. Mol Microbiol. 1995;18:163–174.
- Braun V, Mahren S. Transmembrane transcriptional control (surface signalling) of the Escherichia coli Fec type. FEMS Microbiol Rev. 2005;29:673–684.
- Danson AE, Jovanovic M, Buck M, et al. Mechanisms of sigma(54)-dependent transcription initiation and regulation. J Mol Biol. 2019;431:3960–3974.
- Campbell EA, Muzzin O, Chlenov M, et al. Structure of the bacterial RNA polymerase promoter specificity sigma subunit. Mol Cell. 2002;9:527–539.
- Murakami KS, Masuda S, Campbell EA, et al. Structural basis of transcription initiation: an RNA polymerase holoenzyme-DNA complex. Science. 2002;296:1285–1290.
- Zuo Y, Steitz TA. Crystal structures of the E. coli transcription initiation complexes with a complete bubble. Mol Cell. 2015;58:534–540.
- Feklistov A, Barinova N, Sevostyanova A, et al. A basal promoter element recognized by free RNA polymerase sigma subunit determines promoter recognition by RNA polymerase holoenzyme. Mol Cell. 2006;23:97–107.
- Haugen SP, Berkmen MB, Ross W, et al. rRNA promoter regulation by nonoptimal binding of sigma region 1.2: an additional recognition element for RNA polymerase. Cell. 2006;125:1069–1082.
- Barne KA, Bown JA, Busby SJ, et al. Region 2.5 of the Escherichia coli RNA polymerase sigma70 subunit is responsible for the recognition of the ‘extended-10ʹ motif at promoters. Embo J. 1997;16:4034–4040.
- Gaal T, Ross W, Estrem ST, et al. Promoter recognition and discrimination by EsigmaS RNA polymerase. Mol Microbiol. 2001;42:939–954.
- Checroun C, Bordes P, Leroy O, et al. Interactions between the 2.4 and 4.2 regions of sigmaS, the stress-specific sigma factor of Escherichia coli, and the −10 and −35 promoter elements. Nucleic Acids Res. 2004;32:45–53.
- Koo BM, Rhodius VA, Campbell EA, et al. Dissection of recognition determinants of Escherichia coli sigma32 suggests a composite −10 region with an ‘extended −10ʹ motif and a core −10 element. Mol Microbiol. 2009;72:815–829.
- Nonaka G, Blankschien M, Herman C, et al. Regulon and promoter analysis of the E. coli heat-shock factor, sigma32, reveals a multifaceted cellular response to heat stress. Genes Dev. 2006;20:1776–1789.
- Zhao K, Liu M, Burgess RR. Adaptation in bacterial flagellar and motility systems: from regulon members to ‘foraging’-like behavior in E. coli. Nucleic Acids Res. 2007;35:4441–4452.
- Liu B, Zuo Y, Steitz TA. Structures of E. coli sigmaS-transcription initiation complexes provide new insights into polymerase mechanism. Proc Natl Acad Sci U S A. 2016;113:4051–4056.
- Fang C, Li L, Shen L, et al. Structures and mechanism of transcription initiation by bacterial ECF factors. Nucleic Acids Res. 2019;47:7094–7104.
- Lin W, Mandal S, Degen D, et al. Structural basis of ECF-sigma-factor-dependent transcription initiation. Nat Commun. 2019;10:710.
- Li L, Fang C, Zhuang N, et al. Structural basis for transcription initiation by bacterial ECF sigma factors. Nat Commun. 2019;10:1153.
- Shi W, Zhou W, Zhang B, et al. Structural basis of bacterial sigma(28) -mediated transcription reveals roles of the RNA polymerase zinc-binding domain. Embo J. 2020;39:e104389.
- Murakami KS, Masuda S, Darst SA. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution. Science. 2002;296:1280–1284.
- Basu RS, Warner BA, Molodtsov V, et al. Structural basis of transcription initiation by bacterial RNA polymerase holoenzyme. J Biol Chem. 2014;289:24549–24559.
- Li L, Molodtsov V, Lin W, et al. RNA extension drives a stepwise displacement of an initiation-factor structural module in initial transcription. Proc Natl Acad Sci U S A. 2020;117:5801–5809.
- Kulbachinskiy A, Region MA. 3.2 of the sigma subunit contributes to the binding of the 3ʹ-initiating nucleotide in the RNA polymerase active center and facilitates promoter clearance during initiation. J Biol Chem. 2006;281:18273–18276.
- Pupov D, Kuzin I, Bass I, et al. Distinct functions of the RNA polymerase sigma subunit region 3.2 in RNA priming and promoter escape. Nucleic Acids Res. 2014;42:4494–4504.
- Zhang Y, Feng Y, Chatterjee S, et al. Structural basis of transcription initiation. Science. 2012;338:1076–1080.
- Petushkov I, Esyunina D, Mekler V, et al. Interplay between sigma region 3.2 and secondary channel factors during promoter escape by bacterial RNA polymerase. Biochem J. 2017;474:4053–4064.
- Duchi D, Bauer DL, Fernandez L, et al. RNA Polymerase Pausing during Initial Transcription. Mol Cell. 2016;63:939–950.
- Dulin D, Bauer DLV, Malinen AM, et al. Pausing controls branching between productive and non-productive pathways during initial transcription in bacteria. Nat Commun. 2018;9:1478.
- Nickels BE, Garrity SJ, Mekler V, et al. The interaction between sigma70 and the beta-flap of Escherichia coli RNA polymerase inhibits extension of nascent RNA during early elongation. Proc Natl Acad Sci U S A. 2005;102:4488–4493.
- Gourse RL, Chen AY, Gopalkrishnan S, et al. Transcriptional responses to ppGpp and DksA. Annu Rev Microbiol. 2018;72:163–184.
- Paul BJ, Barker MM, Ross W, et al. DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell. 2004;118:311–322.
- Sanchez-Vazquez P, Dewey CN, Kitten N, et al. Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc Natl Acad Sci U S A. 2019;116:8310–8319.
- Ross W, Vrentas CE, Sanchez-Vazquez P, et al. The magic spot: a ppGpp binding site on E. coli RNA polymerase responsible for regulation of transcription initiation. Mol Cell. 2013;50:420–429.
- Lemke JJ, Sanchez-Vazquez P, Burgos HL, et al. Direct regulation of Escherichia coli ribosomal protein promoters by the transcription factors ppGpp and DksA. Proc Natl Acad Sci U S A. 2011;108:5712–5717.
- Perederina A, Svetlov V, Vassylyeva MN, et al. Regulation through the secondary channel–structural framework for ppGpp-DksA synergism during transcription. Cell. 2004;118:297–309.
- Molodtsov V, Sineva E, Zhang L, et al. Allosteric Effector ppGpp Potentiates the Inhibition of Transcript Initiation by DksA. Mol Cell. 2018;69:828–839.
- Ross W, Sanchez-Vazquez P, Chen AY, et al. ppGpp binding to a site at the RNAP-DksA interface accounts for its dramatic effects on transcription initiation during the stringent response. Mol Cell. 2016;62:811–823.
- Shin Y, Qayyum MZ, Pupov D, et al. Structural basis of ribosomal RNA transcription regulation. Nat Commun. 2021;12:528.
- Pupov D, Petushkov I, Esyunina D, et al. 3.2 of the sigma factor controls the stability of rRNA promoter complexes and potentiates their repression by DksA. Nucleic Acids Res. 2018;46:11477–11487.
- Osterberg S, Del Peso-Santos T, Shingler V. Regulation of alternative sigma factor use. Annu Rev Microbiol. 2011;65:37–55.
- Keseler IM, Mackie A, Santos-Zavaleta A, et al. The EcoCyc database: reflecting new knowledge about Escherichia coli K-12. Nucleic Acids Res. 2017;45:D543–D50.
- Aristarkhov A, Mikulskis A, Belasco JG, et al. Translation of the adhE transcript to produce ethanol dehydrogenase requires RNase III cleavage in Escherichia coli. J Bacteriol. 1996;178:4327–4332.
- Membrillo-Hernandez J, Lin EC. Regulation of expression of the adhE gene, encoding ethanol oxidoreductase in Escherichia coli: transcription from a downstream promoter and regulation by fnr and RpoS. J Bacteriol. 1999;181:7571–7579.
- Lacour S, Landini P. SigmaS-dependent gene expression at the onset of stationary phase in Escherichia coli: function of sigmaS-dependent genes and identification of their promoter sequences. J Bacteriol. 2004;186:7186–7195.
- Bishop RE, Leskiw BK, Hodges RS, et al. The entericidin locus of Escherichia coli and its implications for programmed bacterial cell death. J Mol Biol. 1998;280:583–596.
- Petushkov I, Esyunina D, Kulbachinskiy A. sigma38-dependent promoter-proximal pausing by bacterial RNA polymerase. Nucleic Acids Res. 2017;45:3006–3016.
- Cowing DW, Bardwell JC, Craig EA; Cowing DW, Bardwell JC, Craig EA, Woolford C, Hendrix RW, Gross CA. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985;82:2679–2683.
- Yamada M, Nagamitsu H, Izu H, et al. Characterization of the ves gene, which is expressed at a low temperature in Escherichia coli. J Mol Microbiol Biotechnol. 2002;4:163–169.
- Rhodius VA, Suh WC, Nonaka G, et al. Conserved and variable functions of the sigmaE stress response in related genomes. PLoS Biol. 2006;4:e2.
- Buckstein MH, He J, Rubin H. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J Bacteriol. 2008;190:718–726.
- Bird JG, Zhang Y, Tian Y, et al. The mechanism of RNA 5ʹ capping with NAD+, NADH and desphospho-CoA. Nature. 2016;535:444–447.
- Julius C, Bacterial YY. RNA polymerase caps RNA with various cofactors and cell wall precursors. Nucleic Acids Res. 2017;45:8282–8290.
- Malygin AG, Shemyakin MF. Adenosine, NAD and FAD can initiate template-dependent RNA synthesis catalyzed by Escherichia coli RNA polymerase. FEBS Lett. 1979;102:51–54.
- McClure WR, Cech CL. On the mechanism of rifampicin inhibition of RNA synthesis. J Biol Chem. 1978;253:8949–8956.
- Julius C, Noncanonical YY. RNA-capping: discovery, mechanism, and physiological role debate. Wiley Interdiscip Rev RNA. 2019;10:e1512.
- Winkelman JT, Chandrangsu P, Ross W, et al. Open complex scrunching before nucleotide addition accounts for the unusual transcription start site of E. coli ribosomal RNA promoters. Proc Natl Acad Sci U S A. 2016;113:E1787–95.
- Aberg A, Fernandez-Vazquez J, Cabrer-Panes JD, et al. Similar and divergent effects of ppGpp and DksA deficiencies on transcription in Escherichia coli. J Bacteriol. 2009;191:3226–3236.
- Girard ME, Gopalkrishnan S, Grace ED, et al. DksA and ppGpp regulate the sigma(S) stress response by activating promoters for the small RNA DsrA and the anti-adapter protein IraP. J Bacteriol. 2018;200. DOI:10.1128/JB.00463-17
- Paul BJ, Berkmen MB, Gourse RL. DksA potentiates direct activation of amino acid promoters by ppGpp. Proc Natl Acad Sci U S A. 2005;102:7823–7828.
- Lemke JJ, Durfee T, Gourse RL. DksA and ppGpp directly regulate transcription of the Escherichia coli flagellar cascade. Mol Microbiol. 2009;74:1368–1379.
- Costanzo A, Ades SE. Growth phase-dependent regulation of the extracytoplasmic stress factor, sigmaE, by guanosine 3ʹ,5ʹ-bispyrophosphate (ppGpp). J Bacteriol. 2006;188:4627–4634.
- Costanzo A, Nicoloff H, Barchinger SE, et al. ppGpp and DksA likely regulate the activity of the extracytoplasmic stress factor sigmaE in Escherichia coli by both direct and indirect mechanisms. Mol Microbiol. 2008;67:619–632.
- Grace ED, Gopalkrishnan S, Girard ME, et al. Activation of the sigmaE-dependent stress pathway by conjugative TraR may anticipate conjugational stress. J Bacteriol. 2015;197:924–931.
- Chen J, Gopalkrishnan S, Chiu C, et al. E. coli TraR allosterically regulates transcription initiation by altering RNA polymerase conformation. eLife. 2019;8. DOI:10.7554/eLife.49375
- Staron A, Sofia HJ, Dietrich S, et al. The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Mol Microbiol. 2009;74:557–581.
- Glyde R, Ye F, Jovanovic M, et al. Structures of bacterial RNA polymerase complexes reveal the mechanism of DNA loading and transcription initiation. Mol Cell. 2018;70:1111–20 e3.
- Sainsbury S, Niesser J, Cramer P. Structure and function of the initially transcribing RNA polymerase II-TFIIB complex. Nature. 2013;493:437–440.
- Engel C, Gubbey T, Neyer S, et al. Structural basis of RNA polymerase I transcription initiation. Cell. 2017;169:120–31 e22.
- Abascal-Palacios G, Ramsay EP, Beuron F, et al. Structural basis of RNA polymerase III transcription initiation. Nature. 2018;553:301–306.
- Vorlander MK, Khatter H, Wetzel R, et al. Molecular mechanism of promoter opening by RNA polymerase III. Nature. 2018;553:295–300.
- Boyaci H, Chen J, Lilic M, et al. Fidaxomicin jams Mycobacterium tuberculosis RNA polymerase motions needed for initiation via RbpA contacts. eLife. 2018;7. DOI:10.7554/eLife.34823
- Artsimovitch I, Vassylyeva MN, Svetlov D, et al. Allosteric modulation of the RNA polymerase catalytic reaction is an essential component of transcription control by rifamycins. Cell. 2005;122:351–363.
- Molodtsov V, Nawarathne IN, Scharf NT, et al. X-ray crystal structures of the escherichia coli RNA polymerase in complex with benzoxazinorifamycins. J Med Chem. 2013;56:4758–4763.
- Lin W, Das K, Degen D, et al. Structural Basis of Transcription Inhibition by Fidaxomicin (Lipiarmycin A3). Mol Cell. 2018;70:60–71 e15.
- Morichaud Z, Chaloin L, Regions BK. 1.2 and 3.2 of the RNA polymerase sigma subunit promote DNA melting and attenuate action of the antibiotic lipiarmycin. J Mol Biol. 2016;428:463–476.
- Shikalov AB, Esyunina DM, Pupov DV, et al. The sigma(24) subunit of escherichia coli RNA polymerase can induce transcriptional pausing in vitro. Biochemistry (Mosc). 2019;84:426–434.
- Svetlov V, Artsimovitch I. Purification of bacterial RNA polymerase: tools and protocols. Methods Mol Biol. 2015;1276:13–29.
