ABSTRACT
Circular RNAs (circRNAs) are an evolutionarily conserved form of noncoding RNA with covalently closed loop structures. Initial studies established a functional role for circRNAs as potent microRNA sponges and many other studies have focussed solely on this. However, the biological functions of most circRNAs are still undetermined and other functional roles are gaining traction. These include protein sponges and regulators, and coding for proteins with an alternative mechanism of translation, potentially opening up a whole new transcriptome. The first step to gaining insight into circRNA function is accurate identification and various software platforms have been developed. Specialized detection software has now evolved into whole bioinformatics pipelines that can be used for detection, de novo identification, functional prediction, and validation of circRNAs. However, few cardiovascular circRNA studies have utilized these tools. This review summarizes current knowledge of circRNA biogenesis, bioinformatic detection tools and the emerging role of circRNAs in cardiovascular disease.
Introduction
The first endogenous circRNAs in humans were reported in the early 1990s as non-polyadenylated transcripts with a ‘scrambled’ exon structure (i.e. exons joined at consensus splice sites but in a different order to the primary pre-mRNA transcript) [Citation1,Citation2]. As most early transcriptomic studies used isolation methods that enriched for poly-A transcripts, circular RNAs, with their lack of poly-A tails, went undetected. However, with the evolution of next-generation sequencing kits that interrogated ribosomal-depleted total RNA (rather than poly-adenylated RNA), studies identifying and functionally characterizing circRNAs quickly proliferated. Fast forward 30 years and we now know that circRNAs can contain exonic and/or intronic sequences [Citation3,Citation4], are conserved across species and are associated with many different disease states, including cardiovascular disease [Citation5–8]. Despite these advances, our understanding of their functional roles is still in its infancy and for many circRNAs, the functional mechanism has not been assigned.
CircRNA transcripts may be located in either the nucleus or the cytoplasm. Whereas exon-intron and intronic circRNAs remain in the nucleus and often appear to regulate the gene encoding them (parental gene) [Citation9,Citation10], exonic circRNAs are more commonly exported to the cytoplasm where they exert multiple functions [Citation4]. CircRNAs are highly stable due to a lack of free ends that are vulnerable to exonuclease activity and can accumulate in non-proliferating tissues [Citation11] and biofluids including plasma, saliva and urine [Citation12–14].
This review is divided into four sections. The first section provides a brief summary of the current knowledge of circRNA biogenesis, focussing on the most recent developments in the field. The second section provides a comprehensive list of current bioinformatic detection and downstream in silico functional annotation tools. The third section describes circRNA functions, including roles as miRNA sponges, protein sponges, protein scaffolds and protein-coding transcripts. The final section discusses the emerging roles of circRNAs in cardiovascular disease, including as biomarkers and regulatory molecules in atherosclerosis, myocardial infarction/ischaemia-reperfusion injury, heart failure, cardiac hypertrophy, and cardiac fibrosis.
Biogenesis
Most human genes produce multiple circularized transcripts, a process termed alternative circularization [Citation3,Citation4,Citation8]. In contrast to splicing of pre-mRNA to linear mRNA, exon-containing circRNAs are formed from non-canonical splicing of linear pre-mRNA, whereby a downstream 5ʹ splice site is joined to an upstream 3ʹ splice site in what is known as ‘back-splicing’. This results in a 3ʹ, 5ʹ phosphodiester bond at the back-splicing junction forming a covalently closed, circular transcript and a linear transcript with a skipped exon [Citation15] ()). Mutation of the canonical splice sites or inhibition of splicing by isoginkgetin (a general inhibitor of both the major and minor spliceosomes) diminishes circRNA production [Citation16,Citation17], suggesting that back-splicing of circRNAs utilizes the canonical spliceosome and the same splice sites as linear splicing.
Figure 1. Components involved with linear mRNA or circular RNA biogenesis. (a) The top panel shows a pre-mRNA transcript with four exons. Long flanking introns containing inverted repeat elements such as Alu elements or trans-acting RBP proteins bring the downstream splice site into close proximity with the upstream splice site to favour back splicing and thus circRNA production. Conversely, introns bound by ADAR1 ‘melt’ RNA secondary structures by disrupting intronic base pairing to disrupt circularization. (b) Intronic circRNA biogenesis: Linear splicing produces lariat structures that are usually debranched and hydrolysed. However, GU rich and C rich intronic motifs escape debranching to form ciRNAs (intronic circRNAs). ADAR adenosine deaminases acting on RNA, BP: Branch points. RBP: RNA binding proteins. ALU: Alu repeat elements, mRNA messenger RNA
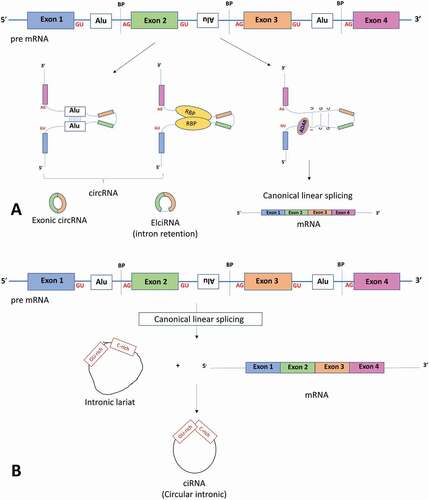
Certain genomic features appear to favour circRNA biogenesis. First, single exon circRNAs tend to have longer exons on average (~3-fold longer [Citation18]), whereas multi-exon circRNAs are of more regular length. Second, exceptionally long introns that contain inverted repeat elements are significantly enriched in circRNA loci compared to linear controls, with nearly 90% of circRNAs in humans having ALU repeats in their flanking introns [Citation19]. Third, conserved binding motifs for trans-acting RNA-binding proteins (RBPs) in flanking introns promote circularization by allowing RBP-associated intronic base pairing to facilitate the interaction of a downstream intron with an upstream intron ()). Conversely, the RNA-editing enzyme, adenosine deaminase acting on RNA (ADAR) appears to inhibit circularization by ‘melting’ the secondary structures within the introns that facilitate circRNA biogenesis, thereby promoting the formation of linear transcripts ()) [Citation19]. Fourth, complementary binding of inverted repeat sequences or binding of RBP proteins promotes circularization by bringing the back-splice junctions into close proximity. This allows circularization of either the exon(s) only or, to a lesser extent, the exon with a retained intron (termed circRNAs containing exonic and intronic sequences, EIciRNAs, )). Along with EIciRNAs, ciRNAs (circRNAs containing exclusively intronic sequences) are thought to reside in the nucleus and regulate their parental gene [Citation9,Citation10].
In contrast to exon-containing circRNAs, ciRNAs are generated during linear splicing when the 5ʹ splice site of the excised intron joins to an adenosine at the branch point, forming a lariat structure ()). Lariat structures are usually removed by a debranching enzyme that hydrolyses the 2ʹ-5ʹ phosphodiester bond at the branch point to produce linear molecules. ciRNAs appear to be protected from the debranching process by consensus RNA motifs – a GU-rich sequence near the 5ʹ splice site and a C-rich sequence near the branch point ()) – although the precise mechanism is unknown [Citation10].
For export of exonic circRNAs from the nucleus to the cytoplasm the cell employs one of two pathways depending on circRNA length [Citation20]. Initial work in Drosophila found that depletion of DExH/D box RNA helicase Hel25E resulted in accumulation of long circRNAs in the nucleus. Two human orthologues of this gene were found which shared 90% identity with Hel25E – UAP56 which regulates export of long (>800 nt) circRNAs and URH49 which regulates short circRNAs [Citation20].
In summary, the current model of circRNA biogenesis is that back splicing is promoted by a looping of the intron(s) bringing the (back) splice sites into close proximity. This ‘looping’ appears to be facilitated by longer introns, inverted repeat sequences and RNA binding proteins [Citation3,Citation4,Citation21]. Given that the same spliceosome and splice sites are used and that many circRNAs consist of exons, it may be tempting to speculate that circRNA biogenesis could be a regulator of mRNA production. However, accumulating evidence shows that circRNAs have their own function independent to that of the linear transcript.
CircRNA detection
CircRNA detection became possible as sequencing libraries evolved from poly-A enriched to ribosomal rRNA-depleted total RNA. Furthermore, circRNAs could be enriched by the addition of 3′-5′ exonuclease Ribonuclease R (RNAse R) which degrades linear RNA (although certain circRNAs are sensitive to RNAse R treatment [Citation22,Citation23]). However, RNAse R does not remove RNA with highly structured 3ʹ ends such as small nuclear RNAs (snRNAs) and histone mRNAs, or RNAs with G-rich G-quadruplex (G4) secondary structures, and circRNA library preparation methods were further refined, using RNAse R treatment followed by polyadenylation of transcripts and then poly(A)+ RNA depletion [Citation24–26].
Following RNA sequencing, bioinformatic detection of circRNAs remains challenging. For a list of circRNA detection and functional prediction software see . While numerous programmes are freely available, there appears to be limited overlap in the circRNAs identified, suggesting a high rate of false positives and that combining outputs from several algorithms is recommended. The reader is directed to the following reviews for a detailed comparison between circRNA software programmes [Citation27,Citation28]. However, the field is rapidly evolving, and several programmes have been updated and new programmes developed since these articles were published.
Table 1. Software for circular RNA detection and downstream applications
CircRNA detection software distinguishes circRNAs from their linear counterparts by exploiting the fact that circRNAs contain back-spliced junctions. Detection strategies can be split into three groups – those that use a multi-stage mapping approach, those that directly detect the back-spliced junction reads using split or chimeric reads and those that forgo the alignment step and assemble kmers (short sequences of nucleotides). In the multi-stage mapping approach, there is an initial mapping step where reads that align continuously to the reference genome are filtered out and only unmapped reads are further analysed. These ‘unmapped’ reads are then aligned to pseudo-sequences which are built around putative back-spliced junctions (BSJs) [Citation29]. The newer aligners, including STAR2, HISAT or TopHat2 [Citation30–32], are ‘splice aware’ (reads are split when aligning back to the reference to account for intronic sequences), which allows chimeric reads to be directly aligned to the human reference genome (). Care must be taken here as chimeric reads that are in the opposite order can also be generated from other trans splicing instances in the genome such as intergenic (gene fusion) or intragenic (exon/gene duplications) [Citation33]. These false positives can be reduced by selecting only those reads that occur in the same gene (to eradicate intergenic trans splicing and using biological replicates (to minimize intragenic trans splicing). Lastly, newer software packages are opting to forgo the mapping stage and instead use the reference and annotation to assemble and store all kmers that are located near exon boundaries. Each sequencing read is then examined for kmers which are matched to the stored kmers (for example, Circ DBG builds a De Bruijn graph from the kmers [Citation34]). When two kmers from a single read are out of order compared to the reference, this suggests the presence of a circRNA. As this strategy assembles all possible kmers from the reference and annotation files, only circRNAs from annotated exons will be predicted, and no de novo circRNAs will be detected.
Figure 2. Three different strategies of identifying back-splice junction (BSJ) reads employed by circRNA detection software. (a) Pseudo reference strategy: Reads are first aligned to the reference (grey boxes) any unmapped reads (red boxes) are taken forward and aligned against pseudo references, which are all possible combinations of exon junctions. In this way, any reads that align identify the exons involved in the back-spliced junctions. (b) Split alignment strategy: Splice-aware aligners split the sequencing reads which allows direct alignment to the reference. Reads can be selected to choose the BSJ reads. (c) Kmer strategy: The actual step of read alignment is forgone. Instead, all possible kmers (short stretches of sequence) are created from the exon boundaries. Kmers are then matched to the reads. Matched kmers that are in sequence with the reference are considered linear spliced reads (top exons). Matched kmers that are out of sequence to the reference are considered circRNA, back-spliced junction reads (bottom exons)
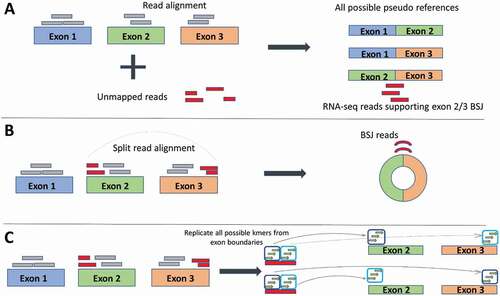
Although circRNAs may align with multiple reads in an RNA Sequencing run, only the reads aligning to the back splice can be unambiguously assigned to the circRNA, as the other reads may originate from its linear mRNA counterparts. For this reason, the read counts for circRNAs are relatively low. Accordingly, caution must be taken when inferring the internal sequence of circular RNAs for in silico functional predictions (such as miRNA and RBP binding sites), and this is only possible for single exon circular RNAs. Unfortunately, this does not appear to be the case for several circRNA databases, which include many multi-exonic transcripts that have not been experimentally validated (currently 20 databases: 14 noncurated and six curated, containing 1953–1,223,114 noncurated or 249–3181 curated circRNAs, respectively) [Citation35]. For an excellent review of the current state of circRNA databases see Vromman et al [Citation35].
CircRNA functions
CircRNAs as microRNA sponges
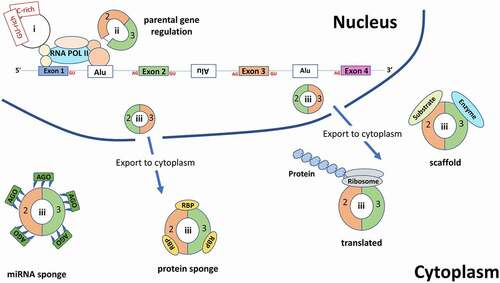
The first papers to demonstrate functional roles for circRNAs found that the circRNAs miR-7 (ciRS-7) and sex-determining region Y (circSRY) bound and sequestered microRNAs miR-7 and miR-138 via 70 and 16 conserved binding sites, respectively [Citation58,Citation59]. Both circRNAs strongly suppressed the ability of the miRNA to bind to its target mRNA, leading to increased expression of the mRNA and suggesting a competing endogenous RNA regulation of the mRNA. This sponge-like mechanism caused great excitement and anticipation of a general functional role for circRNAs. However, bioinformatic analyses suggest that miRNA-binding sites are no more enriched on circRNA than would be expected by chance [Citation60], and sequestering miRNAs appears to be just one of several emerging functional roles for circRNAs ().
Figure 3. An overview of the various functions for circRNAs. Within the nucleus, intronic circRNAs (ciRNAs (I)) and exon and intron containing circRNAs (EIciRNAs (ii)) are involved in regulation of their parental gene. Exonic circRNAs(iii) are exported to the cytoplasm where they can either act as miRNA sponges (which also bind the Argonaute protein – an essential component of the RNA-induced silencing complex (RISC) which acts upon the targeted mRNA), RBP sponges, protein scaffolds or are translated. RBP: RNA binding protein, Ago: Argonaute protein, Alu: Alu elements
Three in vitro methods are typically used to demonstrate circRNA/miRNA interaction. First, luciferase reporter assays, which involve co-transfection of the circRNA sequence along with the miRNA of interest downstream of the luciferase gene [Citation61], demonstrate miRNA binding to the circRNA sequence in vitro but not the actual circRNA in vivo. Second, biotin labelled probes can be used to co-precipitate or ‘pull down’ the circRNA/miRNA complex in vivo [Citation62]. Third, RNA immunoprecipitation (RIP) or crosslinking-immunoprecipitation (CLIP) of the circRNA by an argonaute 2 protein (AGO2) antibody (part of the miRNA silencing machinery) demonstrates both circRNA/miRNA interaction and the RNA silencing mechanism. Each method should be validated as non-specific binding between circRNAs, and miRNAs can occur. For example, Lim et al [Citation63] showed that biotin pull down of circSlc8a1-bound miRNAs identified 14 miRNAs which, after prioritization, led to five candidate miRNAs. Three of these showed significant and consistent inhibition of luciferase activity, with two having several in silico predicted binding sites. It was not until they performed an inverse pull-down assay to test for endogenous circSlc8a1 binding by using biotinylated miRNA mimics that they demonstrated only one of these miRNAs had a robust endogenous interaction to the circRNA.
CircRNAs as protein sponges, regulators, and scaffolds
CircRNAs can act as (i) protein sponges to block protein activity [Citation64,Citation65], (ii) positive regulators of polymerase II to regulate the transcription of their parental genes in the nucleus (examples include circEIF3J, circPAIP2 and ci-ankrd52 [Citation9,Citation10]), and (iii) protein scaffolds to colocalise enzymes and their substrates in the cytoplasm, thereby facilitating recruitment [Citation66] and enzyme activity [Citation67,Citation68]. For a comprehensive review see Du et al [Citation69].
CircRNAs as protein-coding transcripts
The notion of an alternative circRNA transcriptome is appealing given that circRNAs are predominantly located in the cytoplasm, contain exonic sequences and could potentially have an infinite open reading frame (ORF) because of their circular structure [Citation70,Citation71]. Using ribosome profiling, initial studies examined whether circRNA back spliced reads overlapped ribosome protected fragments (suggesting translation) but found no evidence [Citation4,Citation60]. Demonstrating circRNA translation via ribosomal footprinting is restricted by only being able to count the reads spanning the back spliced junction which is a fraction of total reads in the experiment which may account for the lack of success in early attempts.
An alternative to the canonical mechanism of translation initiation would be needed as circRNA lack the 5ʹ cap which is used for linear mRNA translation – circRNAs would need a cap-independent mechanism. Work on viruses and cellular physiological stress responses identified an alternative, 5ʹ cap-independent translation mechanism involving RNA structures known as internal ribosome entry sites (IRESs), which recruit ribosomes to an internal start codon to initiate translation [Citation72]. It has been shown that circRNAs synthesized in vitro carrying engineered IRESs can be translated in this manner [Citation73] but endogenous IRESs are rare. Recent bioinformatic analysis identified IRES-like AU-rich hexamers that were found to bind trans-acting factors and drive circRNA cap-independent translation in a green fluorescent protein (GFP) cell-based reporter system [Citation74]. The authors predicted that sequences longer than 50 nucleotides would contain an IRES-like hexamer by chance. As >99% of circRNAs are >100 nucleotides in length, most circRNAs could potentially be translated by this mechanism [Citation74].
In addition to IRESs, methylated adenosine residues (N6-methyladenosines (m6A), the most abundant base modification of RNA) in the 5ʹ UTR region, can directly recruit the eukaryotic initiation factor 3 (eIF3) and initiate translation in the absence of the cap-binding factor eIF4E [Citation75]. N6-methyladenosine (m6A) consensus motifs are enriched in circRNAs and a single m6A site was found to be sufficient to drive translation in human cells [Citation76]. For example, Legnini et al used a CRISPR/Cas9 system in mouse embryonic stem cells to produce constructs expressing tagged circ-ZNF609 transcripts which were translated into protein [Citation77].
Importantly, additional to ribosomal footprinting, recent studies based on the use of mass spectrometry have allowed detection of several endogenous circRNA translated proteins. The first study by Pamudurti et al [Citation78] detected protein encoded by circMbl which is generated from the muscleblind locus in Drosophila. Other studies to detect actual circRNA translated proteins via mass spectrometry are by Zhang et al [Citation79] who showed that the circular form of the SNF2 histone linker PHD RING helicase (SHPRH) gene contained an open reading frame driven by IRES to encode a novel 146 amino acid which had tumour suppressor activity in human glioblastoma. Yang et al [Citation80] demonstrated CircFBXW7 encodes a 185 amino acid protein with tumour suppressor activity also in human glioblastomas, while Liang et al [Citation81] detected a 370 amino acids novel circβ-catenin isoform which promoted liver cancer cell growth, both in vitro and in vivo. Zheng et al [Citation82] demonstrated circRNA hsa_circ_0000423 (termed as circPPP1R12A) encoded a 73 amino acid protein which promoted the tumour pathogenesis and metastasis in colon cancer tissues. And lastly, Xia et al [Citation83] showed that Circ-AKT3 encodes a 174 amino acid (aa) novel protein, overexpression of which decreased the cell proliferation, radiation resistance and in vivo tumorigenicity of glioblastoma multiforme (GBM) cells.
In summary, from the literature, it would appear that the predominant functional mechanism of circRNAs is to sequester cytoplasmic miRNAs and participate in regulation of their downstream mRNA targets. However, this focus on miRNAs may reflect the fact that the first circRNAs to be discovered were potent miRNA sponges [Citation58,Citation59] and the relative technical ease of demonstrating this in vitro. By comparison, demonstrating circRNA protein translation is technically demanding. Early studies may have suffered from a lack of read depth to detect back spliced junctions for ribosomal footprinting and an inability to stimulate and detect circRNA protein expression owing to inadequate molecular tools. A recent paper by Mo et al [Citation70] demonstrated an expression vector in mammalian cells that increased circRNA formation up to fivefold compared to previous methods, which revealed previously undetected translation of circRtn4. Furthermore, it is important to validate these circRNA encoded proteins in vivo and the use of highly sensitive and specific methods of protein detection, such as mass spectrometry are helping to overcome this technical challenge.
Circular RNAs in cardiovascular disease
Cardiovascular diseases (CVDs) are a leading cause of mortality and morbidity globally accounting for an estimated 17.9 million lives lost each year [Citation84]. There is growing evidence that circRNAs have potential functional roles in cardiac pathologies including atherosclerosis, myocardial infarction, cardiac fibrosis, cardiac hypertrophy, and heart failure (as demonstrated in ). As we unravel the role of circRNAs in the development and progression of CVD, their potential as therapeutic targets to reverse pathophysiological remodelling or enhance protective mechanisms will become apparent [Citation85].
Table 2. circRNAs as potential biomarkers
Table 3. circRNAs associated with atherosclerosis
Circular RNAs as novel biomarkers
Since the discovery of miRNAs in plasma in the mid 1990s, extracellular RNA in biofluids has emerged as an exciting source of biomarkers. Biofluids enable non-invasive, swift sampling, making them an attractive alternative to tissue biopsies.
CircRNAs have been reported in plasma, serum, saliva and urine [Citation13,Citation86–90] and are enriched in these compared to tissues [Citation90,Citation91]. In addition, circRNAs can be packaged into exosomes a subset of extracellular vesicles thought to function in neighbouring and distant cellular communication by transporting proteins, lipids and genetic material between cells [Citation89]. Li et al [Citation90] were the first to show that circRNAs are enriched in exosomes compared to the producer cells. They identified over 1000 circRNAs in human serum exosomes and were able to distinguish colon cancer patients from healthy controls thus demonstrating their value for biomarker application. After exosomes are released into the extracellular fluid they can deliver their internal cargo to initiate a variety of functional responses [Citation92–94], strongly suggesting that there is molecular regulation on the packaging of circRNAs into the exosomes. Bioinformatic analysis showed that circRNAs containing the purine-rich motif 5ʹGMWGVWGRAG-3ʹ were enriched in exosomes compared to other cellular compartments and speculated that RNA binding proteins recognize and package circRNAs containing this motif into exosomes [Citation95]. Due to their closed structure circRNAs are considerably more stable than linear mRNAs – in cells the half-life of circRNAs exceed 48 hours compared to <20 hours for linear RNA [Citation4] – making them excellent candidate biomarkers and potential therapeutic targets for a range of cardiovascular diseases. Presently, only a few studies have demonstrated the use of circRNAs as biomarkers for coronary artery disease (CAD) and myocardial infarction (MI) (summarized in ).
The first study to demonstrate circulating circRNAs as potential biomarkers for CVD was Vausort et al [Citation96] who showed that levels of the circRNA myocardial infarction-associated circular RNA (MICRA) in peripheral blood were a strong predictor of left ventricular dysfunction in 642 MI patients discovery cohort 409 patients, validation cohort 233 patients compared to 86 healthy controls with an odds ratio of 0.53 (95% CI: 0.29 to 0.97). A second study by Salgado-Somoza et al [Citation97] in 472 MI patients confirmed the association between MICRA in peripheral blood and the extent of left ventricular dysfunction and showed that MICRA levels could predict the development of heart failure and help risk stratify patients after MI. MICRA is notable as it is one of the few potential circRNA biomarkers for CVD to have been validated independently. MICRA is an 874-nucleotide long circRNA isoform of the gene ZNF609 (formed mainly from exon 1) [Citation96]. To date, the function of MICRA is not known and further research is needed to determine whether a reduction in MICRA in peripheral blood cells (or other tissues) is directly involved in the development of left ventricular dysfunction and heart failure or forms part of the downstream inflammatory response.
Subsequently, Zhao et al identified circ_0124644 could predict CAD with a receiver operating characteristic area under the curve (ROC AUC) of 0.769 (95% confidence interval, CI, 0.710–0.827, p < 0.001) and a sensitivity and specificity of 0.861 and 0.626, respectively. Two further studies identified three other circRNAs from peripheral blood as potential biomarkers for CAD: Wang et al [Citation98] established that hsa_circ_0001879 and hsa_circ_0004104 were significantly higher in CAD patients compared with controls (hsa_circ_0001879: ROC AUC 0.703 95% CI 0.656–0.750; p < 0.001; hsa_circ_0004104: ROC AUC 0.700 95% CI: 0.646–0.755; p < 0.001), whereas Vilades et al demonstrated hsa_circ_0001445 levels were proportional to coronary atherosclerotic burden in 200 patients with suspected stable CAD [Citation12]. These associations have yet to be validated in independent studies.
Three studies have investigated exosomal circRNAs in cardiovascular disease [Citation99–101]. In the first study to use RNA sequencing for circulating circRNA analysis, Wu et al [Citation99] performed an initial screen of exosomal circRNA expression levels in 3 CAD patients and 3 controls and later validated their findings in two independent cohorts (58 CAD patients versus 35 controls and 47 CAD patients versus 51 controls). They found circ_0005540 was consistently associated with CAD (ROC AUC 0.853; 95% confidence interval = 0:799 − 0:906, P < 0:001 after adjustment for risk factors). In contrast, using cultured cells and a mouse MI model, Wang et al [Citation100] demonstrated that hypoxic cardiomyocytes released circHIPK3 rich exosomes which reduced the infarct area by promoting angiogenesis at the infarct border. CircHIPK3 promoted cardiac endothelial cell proliferation, migration, and tube formation in vitro via miR-29a-mediated regulation of Vascular Endothelial Growth Factor A (VEGFA).
Although these studies highlight the potential of circRNAs as biomarkers, we should proceed with caution. Almost all studies suffer from small cohort sizes, a lack of consideration for confounding medications commonly used for the treatment of CAD and, except for MICRA, a lack of independent replication. Circulating circRNAs levels may be rapidly changing and so the timing of collection during the course of the disease needs to be considered. As yet, there is little consensus between studies as to which circRNAs are the lead candidates, and more work is needed to identify these. Moreover, except for the study by Wu et al [Citation99], these studies have been carried out using targeted technologies of microarray or RT-qPCR, highlighting the potential for more discoveries to come with the use of more sensitive RNA sequencing coupled with specialized library preparation protocols for circRNA and exosome enrichment.
CircRNAs in atherosclerosis (AS)
Atherosclerosis is a silent, chronic inflammatory condition characterized by a narrowing of the arteries from the build-up of fats, cholesterol, and other substances, which can reduce blood flow to the heart muscle. Over the decades, our understanding of atherosclerosis has evolved from a model of passive cholesterol deposition to a dynamic, complex interplay of different cell types and cytokines (cell signalling molecules) resulting in a chronic inflammatory disease.
summarizes current knowledge of the involvement of circRNAs in atherosclerosis. Of the 10 papers published to date, 8 studies have identified the mechanism underlying the association between the circRNA and atherosclerosis, with seven identifying in vitro circRNA/miRNA associations and one showing a circRNA/protein interaction. Notably, for three circRNAs (circANRIL, circCHFR and circ_0003204), associations with atherosclerosis have been reported by two independent studies. However, except for circ_0003204, the independent studies propose different functions or mechanisms of action for the circRNA.
CircANRIL [Citation65,Citation102] originates from chromosome 9p21, a region in the genome that has been identified by genome‐wide association studies (GWAS) as being strongly associated with cardiovascular disease, including coronary artery disease [Citation103]. This locus is devoid of protein-coding genes but houses a long noncoding RNA called antisense noncoding RNA in the INK4 locus (ANRIL). Increased linear ANRIL is associated with increased atherosclerosis [Citation104] and multiple linear isoforms as well as circular isoforms have been identified [Citation65,Citation105]. CircANRIL is expressed in human vascular tissue, smooth muscle cells and monocyte/macrophages, all of which play an important role in atherogenesis. A notable study by Holdt et al (add ref) using a large CAD cohort suggests circANRIL confers atheroprotection. However, a later study looking at the effect of circANRIL on the inflammatory response in a rat model of atherosclerosis by Song et al [Citation102] suggests that expression of circANRIL was correlated with the expression of inflammatory factors in vascular endothelial cells (ECs). Over-expression of circANRIL exacerbated inflammation and promoted atherosclerosis. Further studies are needed to confirm whether circANRIL has a protective or antagonistic role in atherosclerosis [Citation102].
The two studies involving Circ_CHFR suggested two different miRNA/protein interactions as its target. Yang et al [Citation106] suggest circ_CHFR inhibits the proliferation and migration of vascular smooth muscle cells via acting as a sponge to miR-370 which targets the 3ʹUTR of FOXO1. Conversely, Zhuang et al [Citation107] advocate that circ_CHFR promotes cell growth, migration and inflammation in oxidized low-density lipoprotein treated human vascular smooth muscle cells. In contrast, both studies by Zhang et al [Citation108] and Liu et al [Citation109] identifying circ_0003204 as a marker for atherosclerosis propose that it inhibits proliferation and angiogenesis in vascular endothelial cells.
Of the seven circRNA/miRNA studies, only two used the more compelling RNA pulldown method with the remaining studies using the less compelling luciferase method [Citation110,Citation111]). Shen et al [Citation110] identified circ_0044073 as a potential modulator of JAK/STAT levels by binding miR-107 whereas Kong et al [Citation111] identified that circSirt1 may alter nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) by binding miR-132/212 . Among the remaining five studies, Mao et al [Citation112] proposed circSATB2 acts on Stromal Interaction Molecule 1 protein via miR-939, Sun et al [Citation113] proposed circRUSC2 acts on Tyrosine-protein kinase via miR-661 and Zhang et al [Citation108] who propose circ_0003204 inhibits proliferation, migration and tube formation of endothelial cells in atherosclerosis via a miR-370-3p/TGFβR2/phosph-SMAD3 axis. The remaining two studies to use the luciferase method suggest two different miRNAs involved with circCHFR [Citation106,Citation107]: Yang et al [Citation108] who suggests circCHFR facilitates the proliferation and migration of vascular smooth muscle cells via miR-370/FOXO1/cyclin D1 pathway and Zhuang et al suggest circCHFR drives cell growth, migration and inflammation via the miR-214-3p/Wnt3/β-catenin pathway. Both studies used a cell model of atherosclerosis where human vascular smooth muscle cells were stimulated with oxidized low-density lipoprotein.
Together, these studies provide evidence to suggest circRNAs play a regulatory role in the development and progression of atherosclerosis. To date four studies [Citation12,Citation65,Citation107,Citation110] have reported altered levels of circRNAs in biofluids in patients with atherosclerosis, highlighting a potential role for these circRNAs (circRNA_0001445, circRNA-0044073, circCHFR, circANRIL) as potential biomarkers.
Myocardial infarction/ischaemia reperfusion injury
The coronary arteries are the major blood vessels supplying the heart muscle. Following the accumulation of atherosclerotic plaque in these arteries, the blood supply to the heart can become restricted and no longer meet the demands of the myocardium. Over time, this can lead to myocardial ischaemia, associated with a cascade of cellular, inflammatory and biochemical events [Citation114], subclinical myocardial dysfunction and ultimately MI [Citation115]. Cardiomyocytes have limited regenerative capacity and restoration of blood supply or reperfusion is the current clinical procedure to limit death of cardiomyocytes, but reperfusion itself can exacerbate injury to the site [Citation116]. There is growing evidence that circRNAs are involved in cellular responses to MI and ischaemia reperfusion (IR), either through miRNA regulation or interaction with proteins in the cell cytoplasm.
In animal models or human cells in vitro, 18 circRNAs have been associated with MI/IR to date. Of these, 13 circRNAs have been suggested to regulate MI/IR-induced apoptosis in cardiomyocytes via interaction with miRNAs () and represent potential therapeutic targets in this setting. Geng et al [Citation61] were the first to suggest this mechanism, suggesting CDR1as acted as a sponge of miR-7 and led to an increase in apoptosis in cultured mouse cardiomyocytes. However, this study demonstrated no direct evidence for this interaction. Instead, overexpression of CDR1as through administration of an expression plasmid via intracardiac injection in a mouse model of MI led to an increase in infarct size which could be reversed by overexpression of miR-7a. A further three studies by Jin et al, Chen et al and Ji et al [Citation117–119] provide no direct evidence for circRNA/miRNA interaction but instead investigated how overexpression of one affected expression of the other. These studies suggested that circ_0010729 modulates mir-145-5p/mTOR and MEK/ERK pathways [Citation117], circDLPAG4 modulates the miR-143/HECTD1 axis [Citation118] and circPAN3 modulates the miR-31-5p/Quaking axis [Citation119]. Two recent studies have further demonstrated a role of circ_0010729 in hypoxic cardiomyocytes using dual-luciferase reporter assays, RNA immunoprecipitation assays and RNA pull-down assay [Citation120,Citation121]. Interestingly, they both suggest the target proteins are tumour necrosis factor receptor-associated factor (TRAF) but suggest these associate with different miRNAs. Lei et al suggest circ_0010729 acts on miR-27a-3p to regulate TRAF5 and Zhang et al suggest circ_0010729 acts on miR-370-3pto regulate TRAF6. Conversely, the study by Wang et al [Citation121] was the first to use the more compelling method of AGO2 pulldown to not only demonstrate direct evidence of circMFACR (mitochondrial fission and apoptosis-related circRNA) interaction with miR-652-3p but also the AGO2 protein in vivo. A further three studies also provide evidence of circRNA/miRNA interaction with the AGO2 pulldown: Li et al [Citation123] for the circ_ncx1/miR-133a-3p/CDIP1 axis; Gan et al [Citation124] for the circ_101237/let-7a-5p/IGF2BP3 axis and Sun et al [Citation125] for the circ_LAS1L/miR-125b/SFRP5 axis, which all demonstrated circRNA regulation of cardiomyocyte apoptosis. Beyond these, four recent studies have demonstrated cardioprotective roles of the circRNA via a circ/miRNA/protein axis. These are, Cai et al [Citation126] who found circ-Ttc3 was markedly upregulated in the ischaemic myocardium and proposed circ-Ttc3 may bind miR-15b to increase ADP ribosylation factor like 2 (Arl2) expression; Zhao et al [Citation127] who suggested circMACF1 may modulate miR-500b-5p which in turn inhibits epithelial membrane protein 1 (EMP1); Zhang et al [Citation128] who demonstrated circ_0007623 promoted cardiac repair after acute myocardial ischaemia and suggest a miR-297/VEGFA axis and lastly Shao et al [Citation129] who suggest circDENND2a binds to miR-34a which may modulate the β-catenin and Ras/Raf/MEK/ERK pathway.
Table 4. circRNAs associated with myocardial infarction or ischaemia reperfusion injury
In addition to circRNA/miRNA interaction, four studies have demonstrated circRNA/protein binding mechanisms involved in MI/IR injury. The first was Du et al [Citation130] who used antibody pulldown for circFoxo3/p21-CDK2 association. This study was followed by three studies in the same year. Zhou et al [Citation131] demonstrating Autophagy-related circRNA (ACR) was able to inhibit autophagy and cell death in cardiomyocytes to protect the heart from reducing I/R induced infarct sizes. The authors demonstrated circ_ACR blocks DNA methyltransferase 3 beta (Dnmt3B) protein methylation of PTEN-induced kinase 1 (Pink1) to activate Pink1 expression – an autophagy associated gene. Huang et al [Citation132] showed interaction between circNfix and the transcription factor Y-Box Binding Protein 1 (Ybx1) which is involved in cardiomyocyte differentiation. Inhibition of circNfix induced cardiac regeneration and angiogenesis and inhibited cardiomyocyte apoptosis after MI, which significantly restored cardiac function and improved the prognosis. Finally, Garikipati et al [Citation133] found that circFndc3b was significantly downregulated in post-MI mouse hearts and in human cardiac tissues of ischaemic cardiomyopathy patients. They used RNA Immunoprecipitation to show circFndc3b interaction with Fused in Sarcoma (FUS) protein to regulate vascular endothelial growth factor-A expression and signalling. Interestingly and perhaps surprisingly, it was not until the study by Huang et al [Citation132] in 2019 that RNA Sequencing was used to identify CircNfix.
As well as circHIPK3 rich exosomes being shown to regulate oxidative stress damage by Wang et al () circHIPK3 was first shown to aggravate myocardial ischaemia-reperfusion injury by binding to miRNA-124-3p. Bai et al [Citation134] determined that circHIPK3 induced apoptosis in cardiomyocytes after MI by upregulating the oxidative stress regulators Bax and Bcl-2. Another study by the same author [Citation135] showed inhibition of circ_010567 with siRNA improved cardiac function, alleviated myocardial fibrosis, and had a significantly lower apoptosis rate of myocardial cells. They suggest the regulatory mechanism may be related to the inhibition on the TGF-β1 signalling pathway.
It is encouraging to see the numerous studies in recent years demonstrating various circRNAs associated with MI and I/R injury offering several potential therapeutic targets with circHIPK3 being independently validated to act in oxidative stress. However, further independent replication in large, well-controlled studies and cohorts to prioritize circRNA candidates for future study will be needed.
CircRNAs in heart failure/hypertrophy/cardiac fibrosis
Once the damage has occurred through MI or chronic hypertension, the heart undergoes macroscopic and microscopic remodelling [Citation136,Citation137]. An early infiltrative inflammatory response is followed by replacement of infarcted myocardium with non-elastic fibrotic tissue. Diffuse interstitial fibrosis may also affect areas of the heart remote from the initial injury. The architecture of the left ventricle exhibits mechanically disadvantageous changes, including overall ventricular dilatation and alteration from an efficient elliptical shape to a spherical chamber morphology. In vulnerable
patients, these processes can lead to subclinical myocardial dysfunction and subsequent symptomatic heart failure (HF) [Citation136,Citation137,Citation139]. Cardiac fibrosis can be caused by abnormal deposition of extracellular matrix in the myocardium. It can be the result of scarring after MI but can be more widespread and common in heart failure. The scarring is detrimental by either stiffening the myocardium thereby reducing the pumping ability of the heart by impairing electrical conductance [Citation140].
represents nine studies on circRNAs that have been demonstrated to act in hypertrophy and cardiac fibrosis contributing to heart failure. All nine studies suggest a circRNA-miRNA-protein axis. Only one study conducted by Han et al [Citation141] was carried out in humans, with the remaining studies carried out in mice. Using RNA pulldown experiments, Han et al showed circ_0097435 could bind multiple miRNAs and promote apoptosis although the protein targets remain unknown. They then extracted exosomes from patients with heart failure and controls and showed that hsa_circ_0097435 expression was significantly higher in patients suggesting a biomarker role. Interestingly, two studies suggest yet a further role of the circHIPK3. CircHIPK3 was associated with two different miRNA/protein axes – miR-17-3p/Adenylyl cyclase type 6 (ADCY6) by Deng et al [Citation142] with downregulation of circHIPK3 reducing cardiac fibrosis and maintaining cardiac function post MI, and miR-29b-3p/α-smooth muscle actin (α-SMA), Collagen type I alpha I (COL1A1, COL3A1) by Ni et al [Citation143], who saw reduced cardiac fibroblast proliferation with circHIPK3 silencing. However, they did not show direct evidence for a circHIPK3/miRNA interaction and only demonstrated cytoplasmic co-localization by RNA FISH. Neither miRNA replicated the previous circHIPK3 study by Bai et al [Citation134].
Table 5. circRNAs associated with heart failure/hypertrophy and cardiac fibrosis
Two studies suggest the same circRNA, circ_000203, exacerbates cardiac fibrosis and hypertrophy. Tang et al [Citation144] showed circRNA_000203 inhibiting the anti-fibrosis effect of miR-26b-5p in cardiac fibroblasts via collagen alpha 2(I) (Col1a2) and connective tissue growth factor (CTGF); Li et al [Citation142] showed circRNA_000203 could sponge miR-26b-5p along with miR-140-3p in mouse ventricular cardiomyocytes which enhances Gata4 levels to exacerbate cardiac hypertrophy. This is the first time two studies implicate the same circRNA acting upon the same miRNA, albeit with differing downstream targets.
Lim et al [Citation63] built on earlier studies that demonstrated a role of miR-133a and cardiac hypertrophy and heart failure – inhibition of miR-133a induces whereas overexpression inhibits cardiac hypertrophy [Citation146,Citation147]. Lim et al demonstrated an interaction between circSlc8a1 and miR-133a and undertook both knockdown and overexpression of circSlc8a1 in vivo and showed that reducing endogenous circSlc8a1 (thereby increasing miR-133a levels) inhibited hypertrophy and forced cardiomyocyte overexpression resulted in heart failure thereby proposing inhibition of circSlc8a1 as a novel therapeutic target.
Lastly, a study by Want et al [Citation148] suggested a cardioprotective of a circRNA role against fibrosis and hypertrophy. They showed that the circRNA they called heart-related circRNA (HRCR) acted as a sponge to the positive regulator of cardiac hypertrophy miR-223. By sequestering miR-223 they found less degradation of the protein apoptosis repressor with CARD domain (ARC).
Again, encouraging to see the numerous studies implicating various circRNAs in cardiac hypertrophy which are potentially therapeutic targets. It is also encouraging to start to see independent validation of the same circRNA such as circ_000203 implicated in the same disease phenotype, albeit with different mechanisms. The same circRNA may act upon more than one downstream target which is being suggested by the different studies. Further evidence for a significant cardiovascular circRNA – circHIPK3 is also provided. Again, a need for further studies with large sample sizes to support these findings is needed.
Summary
Growing evidence supports an important role of circRNAs in normal physiology and a range of disease states, including CVDs. The majority of studies have focussed on the circRNA/miRNA/mRNA axis which may be due to the first circRNAs being demonstrated as potent miRNA sponges and the relative ease of the in vitro methods that demonstrated this function. Technically challenging in vitro techniques demonstrating circRNA translation may have hindered initial studies, with ribosomal footprinting only detecting the fraction of reads that cross the back spliced junction. With circRNA library enrichment and the validation of proteins with mass spectrometry other circRNA functions are now emerging and whilst translated circRNAs have not been associated with CVD, evidence for circRNA translation in cancer biology [Citation81,Citation82] suggests it is only a matter of time before protein-coding cardiovascular circRNAs are identified as the technical challenges are overcome.
Several circRNA/miRNA/mRNA studies suffer from low cohort numbers and some show no direct evidence of the proposed circRNA/miRNA interaction. Many other studies use luciferase assays, which demonstrate an interaction with circRNA/miRNA mimics in vitro but these should be validated with more robust experimental methods such as circRNA/AGO2 immunoprecipitation.
As yet, there is a shortage of experimental replication confirming involvement of the same circRNA and consequently, there is little overlap of circRNAs within or between cardiovascular aetiologies. However, recent studies are beginning to validate the same circRNA involved in the same or different aetiologies. CircHIPK3 is commonly expressed in diverse tissues, such as the lung, heart, stomach, colon, brain and has been demonstrated to be a key circRNA in a variety of human cancers [Citation149]. Several studies highlighted here suggest a role in both myocardial ischaemia and cardiac hypertrophy for circHIPK3. It is encouraging that other circRNAs are being independently replicated such as MICRA, circ_000203 and circ_0010729 as we begin to clarify the lead candidate biomarkers and therapeutic targets for cardiovascular disease among this relatively newly identified class of non-coding RNA. It is surprising that so few studies have employed RNA sequencing for circRNA detection with most cardiovascular studies using microarray or RT-qPCR technologies, limiting detection of novel circRNAs. With the development of methods for circRNA enrichment in biofluids and more user-friendly software and bioinformatics pipelines future uptake of RNA sequencing technologies detection may be encouraged. Additionally, the only in silico tools for circRNA analysis in the studies appear to be for predicting circRNA/miRNA binding sites and again this may be due to the focus on this mechanism of function. highlights the bioinformatic tools that are available such as predicting potential interactions of circRNAs with RNA binding proteins as well as identification of circRNAs with protein-coding potential. The utilization of these bioinformatic tools may help to broaden the lens and validate other functions for circRNAs.
In recent years the physiological and pathological roles of cardiovascular circRNAs have begun to be identified. Additionally, several circRNAs have been found to be promising diagnostic and prognostic biomarkers as well as novel therapeutic targets. Precise mechanisms are still being elucidated but adding to traditional in vitro methods emerging biological and informatic techniques are advancing our understanding will be increased and clinical application of circRNAs will enhance our treatment for cardiovascular disease.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Nigro JM, Cho KR, Fearon ER, et al. Scrambled exons. Cell. 1991;64(3):607–613.
- Cocquerelle C, Daubersies P, Majérus MA, et al. Splicing with inverted order of exons occurs proximal to large introns. Embo J. 1992;11(3):1095–1098.
- Zhang XO, Wang H-B, Zhang Y, et al. Complementary sequence-mediated exon circularization. Cell. 2014;159(1):134–147.
- Jeck WR, Sorrentino JA, Wang K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. Rna. 2013;19(2):141–157.
- Yuan C, Luo X, Zhan X, et al. EMT related circular RNA expression profiles identify circSCYL2 as a novel molecule in breast tumor metastasis. Int J Mol Med. 2020;45(6):1697–1710.
- Fu X, Zhang J, He X, et al. Circular RNA MAN2B2 promotes cell proliferation of hepatocellular carcinoma cells via the miRNA-217/MAPK1 axis. J Cancer. 2020;11(11):3318–3326.
- Akhter R. Circular RNA and Alzheimer’s disease. Adv Exp Med Biol. 2018;1087:239–243.
- Rybak-Wolf A, Stottmeister C, Glažar P, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58(5):870–885.
- Li Z, Huang C, Bao C, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22(3):256–264.
- Zhang Y, Zhang X-O, Chen T, et al. Circular intronic long noncoding RNAs. Mol Cell. 2013;51(6):792–806.
- Bachmayr-Heyda A, Reiner AT, Auer K, et al. Correlation of circular RNA abundance with proliferation–exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci Rep. 2015;5:8057.
- Vilades D, Martínez‐Camblor P, Ferrero‐Gregori A, et al. Plasma circular RNA hsa_circ_0001445 and coronary artery disease: performance as a biomarker. Faseb J. 2020;34(3):4403–4414.
- Song Z, Zhang Q, Zhu J, et al. Identification of urinary hsa_circ _0137439 as potential biomarker and tumor regulator of bladder cancer. Neoplasma. 2020;67(1):137–146.
- Fanale D, et al. Circular RNA in exosomes. Adv Exp Med Biol. 2018;1087:109–117.
- Zhang Y, Xue W, Li X, et al. The biogenesis of nascent circular RNAs. Cell Rep. 2016;15(3):611–624.
- Starke S, Jost I, Rossbach O, et al. Exon circularization requires canonical splice signals. Cell Rep. 2015;10(1):103–111.
- Ashwal-Fluss R, Meyer M, Pamudurti N, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56(1):55–66.
- Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32(5):453–461.
- Ivanov A, Memczak S, Wyler E, et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10(2):170–177.
- Huang C, Liang D, Tatomer DC, et al. A length-dependent evolutionarily conserved pathway controls nuclear export of circular RNAs. Genes Dev. 2018;32(9–10):639–644.
- Conn SJ, Pillman K, Toubia J, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160(6):1125–1134.
- You X, Conrad TOF. Acfs: accurate circRNA identification and quantification from RNA-Seq data. Sci Rep. 2016;6(1):38820.
- Szabo L, Salzman J. Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet. 2016;17(11):679–692.
- Pandey PR, Rout PK, Das A, et al. RPAD (RNase R treatment, polyadenylation, and poly(A)+ RNA depletion) method to isolate highly pure circular RNA. Methods. 2019;155:41–48.
- Xiao MS, Wilusz JE. An improved method for circular RNA purification using RNase R that efficiently removes linear RNAs containing G-quadruplexes or structured 3ʹ ends. Nucleic Acids Res. 2019;47(16):8755–8769.
- Panda AC, De S, Grammatikakis I, et al. High-purity circular RNA isolation method (RPAD) reveals vast collection of intronic circRNAs. Nucleic Acids Res. 2017;45(12):e116–e116.
- Hansen TB, Venø MT, Damgaard CK, et al. Comparison of circular RNA prediction tools. Nucleic Acids Res. 2016;44(6):e58.
- Zeng X, Lin W, Guo M, et al. A comprehensive overview and evaluation of circular RNA detection tools. PLoS Comput Biol. 2017;13(6):e1005420.
- Gao Y, Zhao F. Computational strategies for exploring circular RNAs. Trends Genet. 2018;34(5):389–400.
- Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21.
- Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–360.
- Kim D, Pertea G, Trapnell C, et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36.
- Horiuchi T, Aigaki T. Alternative trans-splicing: a novel mode of pre-mRNA processing. Biol Cell. 2006;98(2):135–140.
- Li X, Wu Y. Detecting circular RNA from high-throughput sequence data with de Bruijn graph. BMC Genomics. 2020;21(1):749.
- Vromman M, Vandesompele J, Volders PJ. Closing the circle: current state and perspectives of circular RNA databases. Brief Bioinform. 2021 Jan 18;22(1):288-297.
- Zhang XO, Dong R, Zhang Y, et al. Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res. 2016;26(9):1277–1287.
- Ma XK, Wang M-R, Liu C-X, et al. CIRCexplorer3: a CLEAR pipeline for direct comparison of circular and linear RNA expression. Genomics Proteomics Bioinformatics. 2019;17(5):511–521.
- Gao Y, Wang J, Zhao F. CIRI: an efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol. 2015;16:4.
- Gao Y, Zhang J, Zhao F. Circular RNA identification based on multiple seed matching. Brief Bioinform. 2018;19(5):803–810.
- Cheng J, Metge F, Dieterich C. Specific identification and quantification of circular RNAs from sequencing data. Bioinformatics. 2016;32(7):1094–1096.
- Wang K, Singh D, Zeng Z, et al. MapSplice: accurate mapping of RNA-seq reads for splice junction discovery. Nucleic Acids Res. 2010;38(18):e178.
- Hoffmann S, Otto C, Doose G, et al. A multi-split mapping algorithm for circular RNA, splicing, trans-splicing and fusion detection. Genome Biol. 2014;15(2):R34.
- Song X, Zhang N, Han P, et al. Circular RNA profile in gliomas revealed by identification tool UROBORUS. Nucleic Acids Res. 2016;44(9):e87.
- Jia GY, Wang D-L, Xue M-Z, et al. CircRNAFisher: a systematic computational approach for de novo circular RNA identification. Acta Pharmacol Sin. 2019;40(1):55–63.
- Szabo L, Morey R, Palpant NJ, et al. Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development. Genome Biol. 2015;16:126.
- Li X, Chu C, Pei J, et al. CircMarker: a fast and accurate algorithm for circular RNA detection. BMC Genomics. 2018;19(S6):572.
- Sekar S, Geiger P, Adkins J, et al. ACValidator: a novel assembly-based approach for in silico verification of circular RNAs. Biol Methods Protoc. 2020;5(1):bpaa010.
- Sun P, Li G. CircCode: a powerful tool for identifying circRNA coding ability. Front Genet. 2019;10(981). DOI:https://doi.org/10.3389/fgene.2019.00981
- Gaffo E, Bonizzato A, Kronnie GT, et al. CirComPara: a multi-method comparative bioinformatics pipeline to detect and study circRNAs from RNA-seq data. Noncoding RNA. 2017;3(1). DOI:https://doi.org/10.3390/ncrna3010008
- Zhong S, Wang J, Zhang Q, et al. CircPrimer: a software for annotating circRNAs and determining the specificity of circRNA primers. BMC Bioinformatics. 2018;19(1):292.
- Meng X, Chen Q, Zhang P, et al. CircPro: an integrated tool for the identification of circRNAs with protein-coding potential. Bioinformatics. 2017;33(20):3314–3316.
- Li L, Bu D, Zhao Y. CircRNAwrap - a flexible pipeline for circRNA identification, transcript prediction, and abundance estimation. FEBS Lett. 2019;593(11):1179–1189.
- Jakobi T, Uvarovskii A, Dieterich C. circtools-a one-stop software solution for circular RNA research. Bioinformatics. 2019;35(13):2326–2328.
- Andrés-León E, Núñez-Torres R, Rojas AM. miARma-Seq: a comprehensive tool for miRNA, mRNA and circRNA analysis. Sci Rep. 2016;6(1):25749.
- Chen CY, Chuang TJ. NCLcomparator: systematically post-screening non-co-linear transcripts (circular, trans-spliced, or fusion RNAs) identified from various detectors. BMC Bioinformatics. 2019;20(1):3.
- Zhao J, Li X, Guo J, et al. ReCirc: prediction of circRNA expression and function through probe reannotation of non-circRNA microarrays. Mol Omics. 2019;15(2):150–163.
- Humphreys DT, et al. Ularcirc: visualization and enhanced analysis of circular RNAs via back and canonical forward splicing. Nucleic Acids Res. 2019;47(20):e123.
- Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338.
- Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388.
- Guo JU, Agarwal V, Guo H, et al. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15(7):409.
- Geng HH, Li R, Su Y-M, et al. The circular RNA Cdr1as promotes myocardial infarction by mediating the regulation of miR-7a on its target genes expression. PLoS One. 2016;11(3):e0151753.
- Han D, Li J, Wang H, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66(4):1151–1164.
- Lim TB, et al. Targeting the highly abundant circular RNA circSlc8a1 in cardiomyocytes attenuates pressure overload induced hypertrophy. Cardiovasc Res. 2019;115(14):1998–2007.
- Xia P, et al. A circular RNA protects dormant hematopoietic stem cells from DNA sensor cGAS-mediated exhaustion. Immunity. 2018;48(4):688–701.e7.
- Holdt LM, Stahringer A, Sass K, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7:12429.
- Chen N, et al. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol. 2018;19(1):218.
- Zeng Y, Du WW, Wu Y, et al. A circular RNA binds to and activates AKT phosphorylation and nuclear localization reducing apoptosis and enhancing cardiac repair. Theranostics. 2017;7(16):3842–3855.
- Du WW, Fang L, Yang W, et al. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 2017;24(2):357–370.
- Du WW, Zhang C, Yang W, et al. Identifying and characterizing circRNA-protein interaction. Theranostics. 2017;7(17):4183–4191.
- Stagsted LV, Nielsen KM, Daugaard I, et al. Noncoding AUG circRNAs constitute an abundant and conserved subclass of circles. Life Sci Alliance. 2019;2(3):e201900398.
- Mo D, Li X, Raabe CA, et al. A universal approach to investigate circRNA protein coding function. Sci Rep. 2019;9(1):11684.
- Godet AC, David F, Hantelys F, et al. IRES trans-acting factors, key actors of the stress response. Int J Mol Sci. 2019;20(4):924.
- Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268(5209):415.
- Wang Y, Wang Z. Efficient backsplicing produces translatable circular mRNAs. Rna. 2015;21(2):172–179.
- Meyer KD, Patil D, Zhou J, et al. 5ʹ UTR m(6)A promotes cap-independent translation. Cell. 2015;163(4):999–1010.
- Yang Y, Fan X, Mao M, et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27(5):626–641.
- Legnini I, Di Timoteo G, Rossi F, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37.e9.
- Pamudurti NR, Bartok O, Jens M, et al. Translation of CircRNAs. Mol Cell. 2017;66(1):9–21.e7.
- Zhang M, Huang N, Yang X, et al. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene. 2018;37(13):1805–1814.
- Yang Y, Gao X, Zhang M, et al. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. JNCI. 2017;110(3):304–315.
- Liang W-C, Wong C-W, Liang -P-P, et al. Translation of the circular RNA circβ-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol. 2019;20(1):84.
- Zheng X, Chen L, Zhou Y, et al. A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol Cancer. 2019;18(1):47.
- Xia X, Li X, Li F, et al. A novel tumor suppressor protein encoded by circular AKT3 RNA inhibits glioblastoma tumorigenicity by competing with active phosphoinositide-dependent Kinase-1. Mol Cancer. 2019;18(1):131.
- World Health Organization. Cardiovascular diseases; 2020. Available from: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1
- Santer L, Bär C, Thum T. Circular RNAs: a novel class of functional RNA molecules with a therapeutic perspective. Mol Ther. 2019;27(8):1350–1363.
- Zhang Y, Chen Y, Yao H, et al. Elevated serum circ_0068481 levels as a potential diagnostic and prognostic indicator in idiopathic pulmonary arterial hypertension. Pulm Circ. 2019;9(4):2045894019888416.
- Chen C, Shen H, Huang Q, et al. The circular RNA CDR1as regulates the proliferation and apoptosis of human cardiomyocytes through the miR-135a/HMOX1 and miR-135b/HMOX1 axes. Genet Test Mol Biomarkers. 2020;24(9):537–548.
- Bahn JH, Zhang Q, Li F, et al. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem. 2015;61(1):221–230.
- Wang Y, Liu J, Ma J, et al. Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol Cancer. 2019;18(1):116.
- Li Y, Zheng Q, Bao C, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984.
- Hulstaert E, et al. Charting extracellular transcriptomes in the human biofluid RNA atlas. Cell Rep. 2020:33(13):108552.
- Zhang H, Zhu L, Bai M, et al. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int J Cancer. 2019;144(10):2501–2515.
- Baruah J, Wary KK. Exosomes in the regulation of vascular endothelial cell regeneration. Front Cell Dev Biol. 2019;7:353.
- Rios-Colon L, Arthur E, Niture S, et al. The role of exosomes in the crosstalk between adipocytes and liver cancer cells. Cells. 2020;9(9):1988.
- Zhang J, Zhang X, Li C, et al. Circular RNA profiling provides insights into their subcellular distribution and molecular characteristics in HepG2 cells. RNA Biol. 2019;16(2):220–232.
- Vausort M, Salgado-Somoza A, Zhang L, et al. Myocardial infarction-associated circular rna predicting left ventricular dysfunction. J Am Coll Cardiol. 2016;68(11):1247–1248.
- Salgado-Somoza A, Zhang L, Vausort M, et al. The circular RNA MICRA for risk stratification after myocardial infarction. IJC Heart Vasculature. 2017;17:33–36.
- Wang L, Shen C, Wang Y, et al. Identification of circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as novel biomarkers for coronary artery disease. Atherosclerosis. 2019;286:88–96.
- Wu WP, Pan Y-H, Cai M-Y, et al. Plasma-derived exosomal circular RNA hsa_circ_0005540 as a novel diagnostic biomarker for coronary artery disease. Dis Markers. 2020;2020:3178642.
- Wang Y, et al. Exosomal CircHIPK3 released from hypoxia-induced cardiomyocytes regulates cardiac angiogenesis after myocardial infarction. Oxid Med Cell Longev. 2020;2020:8418407.
- Zhao Z, Li X, Gao C, et al. Peripheral blood circular RNA hsa_circ_0124644 can be used as a diagnostic biomarker of coronary artery disease. Sci Rep. 2017;7(1):39918.
- Song CL, Wang J-P, Xue X, et al. Effect of circular ANRIL on the inflammatory response of vascular endothelial cells in a rat model of coronary atherosclerosis. Cell Physiol Biochem. 2017;42(3):1202–1212.
- Holdt LM, Teupser D. Recent studies of the human chromosome 9p21 locus, which is associated with atherosclerosis in human populations. Arterioscler Thromb Vasc Biol. 2012;32(2):196–206.
- Holdt LM, Beutner F, Scholz M, et al. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler Thromb Vasc Biol. 2010;30(3):620–627.
- Burd CE, Jeck WR, Liu Y, et al. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6(12):e1001233–e1001233.
- Yang L, Yang F, Zhao H, et al. Circular RNA circCHFR facilitates the proliferation and migration of vascular smooth muscle via miR-370/FOXO1/Cyclin D1 pathway. Mol Ther Nucleic Acids. 2019;16:434–441.
- Zhuang JB, Li T, Hu X-M, et al. Circ_CHFR expedites cell growth, migration and inflammation in ox-LDL-treated human vascular smooth muscle cells via the miR-214-3p/Wnt3/beta-catenin pathway. Eur Rev Med Pharmacol Sci. 2020;24(6):3282–3292.
- Zhang S, Song G, Yuan J, et al. Circular RNA circ_0003204 inhibits proliferation, migration and tube formation of endothelial cell in atherosclerosis via miR-370-3p/TGFbetaR2/phosph-SMAD3 axis. J Biomed Sci. 2020;27(1):11.
- Liu H, Ma X, Mao Z, et al. Circular RNA has_circ_0003204 inhibits oxLDL-induced vascular endothelial cell proliferation and angiogenesis. Cell Signal. 2020;70:109595.
- Shen L, Hu Y, Lou J, et al. CircRNA0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR107. Mol Med Rep. 2019;19(5):3923–3932.
- Kong P, Yu Y, Wang L, et al. circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019;47(7):3580–3593.
- Mao YY, Wang J-Q, Guo -X-X, et al. Circ-SATB2 upregulates STIM1 expression and regulates vascular smooth muscle cell proliferation and differentiation through miR-939. Biochem Biophys Res Commun. 2018;505(1):119–125.
- Sun J, Zhang Z, Yang S. Circ_RUSC2 upregulates the expression of miR-661 target gene SYK and regulates the function of vascular smooth muscle cells. Biochem Cell Biol. 2019;97(6):709–714.
- Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol. 2004;164(6):1875–1882.
- Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012;32(7):1552–1562.
- Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106(3):360–368.
- Jin Q, Chen Y. Silencing circular RNA circ_0010729 protects human cardiomyocytes from oxygen-glucose deprivation-induced injury by up-regulating microRNA-145-5p. Mol Cell Biochem. 2019;462(1–2):185–194.
- Chen L, Luo W, Zhang W, et al. circDLPAG4/HECTD1 mediates ischaemia/reperfusion injury in endothelial cells via ER stress. RNA Biol. 2020;17(2):240–253.
- Ji X, Ding W, Xu T, et al. MicroRNA-31-5p attenuates doxorubicin-induced cardiotoxicity via quaking and circular RNA Pan3. J Mol Cell Cardiol. 2020;140:56–67.
- Lei D, Wang Y, Zhang L, et al. Circ_0010729 regulates hypoxia-induced cardiomyocyte injuries by activating TRAF5 via sponging miR-27a-3p. Life Sci. 2020;262:118511.
- Zhang J, Gao C, Zhang J, et al. Circ_0010729 knockdown protects cardiomyocytes against hypoxic dysfunction via miR-370-3p/TRAF6 axis. Excli J. 2020;19:1520–1532.
- Wang K, Gan T-Y, Li N, et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 2017;24(6):1111–1120.
- Li M, Ding W, Tariq MA, et al. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics. 2018;8(21):5855–5869.
- Gan J, Yuan J, Liu Y, et al. Circular RNA_101237 mediates anoxia/reoxygenation injury by targeting let‑7a‑5p/IGF2BP3 in cardiomyocytes. Int J Mol Med. 2020;45(2):451–460.
- Sun LY, Zhao J-C, Ge X-M, et al. Circ_LAS1L regulates cardiac fibroblast activation, growth, and migration through miR-125b/SFRP5 pathway. Cell Biochem Funct. 2020;38(4):443–450.
- Cai L, Qi B, Wu X, et al. Circular RNA Ttc3 regulates cardiac function after myocardial infarction by sponging miR-15b. J Mol Cell Cardiol. 2019;130:10–22.
- Zhao B, Li G, Peng J, et al. CircMACF1 attenuates acute myocardial infarction through miR-500b-5p-EMP1 axis. J Cardiovasc Transl Res. 2020. DOI:https://doi.org/10.1007/s12265-020-09976-5
- Zhang Q, Sun W, Han J, et al. The circular RNA hsa_circ_0007623 acts as a sponge of microRNA-297 and promotes cardiac repair. Biochem Biophys Res Commun. 2020;523(4):993–1000.
- Shao Y, Zhong P, Sheng L, et al. Circular RNA circDENND2A protects H9c2 cells from oxygen glucose deprivation-induced apoptosis through sponging microRNA-34a. Cell Cycle. 2020;19(2):246–255.
- Du WW, Yang W, Chen Y, et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J. 2017;38(18):1402–1412.
- Zhou LY, Zhai M, Huang Y, et al. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/FAM65B pathway. Cell Death Differ. 2019;26(7):1299–1315.
- Huang S, Li X, Zheng H, et al. Loss of super-enhancer-regulated circRNA Nfix induces cardiac regeneration after myocardial infarction in adult mice. Circulation. 2019;139(25):2857–2876.
- Garikipati VNS, Verma SK, Cheng Z, et al. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat Commun. 2019;10(1):4317.
- Bai M, Pan C-L, Jiang G-X, et al. CircHIPK3 aggravates myocardial ischemia-reperfusion injury by binding to miRNA-124-3p. Eur Rev Med Pharmacol Sci. 2019;23(22):10107–10114.
- Bai M, Pan C-L, Jiang G-X, et al. CircRNA 010567 improves myocardial infarction rats through inhibiting TGF-β1. Eur Rev Med Pharmacol Sci. 2020;24(1):369–375.
- Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128(4):388–400.
- Wu QQ, et al. Mechanisms contributing to cardiac remodelling. Clin Sci (Lond). 2017;131(18):2319–2345.
- Werfel S, Nothjunge S, Schwarzmayr T, et al. Characterization of circular RNAs in human, mouse and rat hearts. J Mol Cell Cardiol. 2016;98:103–107.
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35(3):569–582.
- Hinderer S, Schenke-Layland K. Cardiac fibrosis - A short review of causes and therapeutic strategies. Adv Drug Deliv Rev. 2019;146:77–82.
- Han J, Zhang L, Hu L, et al. Circular RNA-expression profiling reveals a potential role of Hsa_circ_0097435 in heart failure via sponging multiple MicroRNAs. Front Genet. 2020;11:212.
- Deng Y, Wang J, Xie G, et al. Circ-HIPK3 strengthens the effects of adrenaline in heart failure by MiR-17-3p - ADCY6 axis. Int J Biol Sci. 2019;15(11):2484–2496.
- Ni H, Li W, Zhuge Y, et al. Inhibition of circHIPK3 prevents angiotensin II-induced cardiac fibrosis by sponging miR-29b-3p. Int J Cardiol. 2019;292:188–196.
- Tang CM, Zhang M, Huang L, et al. CircRNA_000203 enhances the expression of fibrosis-associated genes by derepressing targets of miR-26b-5p, Col1a2 and CTGF, in cardiac fibroblasts. Sci Rep. 2017;7:40342.
- Li H, Xu J-D, Fang X-H, et al. Circular RNA circRNA_000203 aggravates cardiac hypertrophy via suppressing miR-26b-5p and miR-140-3p binding to Gata4. Cardiovasc Res. 2020;116(7):1323–1334.
- Danowski N, Manthey I, Jakob HG, et al. Decreased expression of miR-133a but Not of miR-1 is associated with signs of heart failure in patients undergoing coronary bypass surgery. Cardiology. 2013;125(2):125–130.
- Carè A, Catalucci D, Felicetti F, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13(5):613–618.
- Wang K, Long B, Liu F, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016;37(33):2602–2611.
- Wen J, Liao J, Liang J, et al. Circular RNA HIPK3: a Key Circular RNA in a Variety of Human Cancers. Front Oncol. 2020;10(773). DOI:https://doi.org/10.3389/fonc.2020.00773
- Zhou B, Yu JW. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-β1. Biochem Biophys Res Commun. 2017;487(4):769–775.
- Zhu Y, Pan W, Yang T, et al. Upregulation of circular RNA CircNFIB attenuates cardiac fibrosis by sponging miR-433. Front Genet. 2019;10:564.
