ABSTRACT
Mitochondrial noncoding RNAs (mt-ncRNAs) include noncoding RNAs inside the mitochondria that are transcribed from the mitochondrial genome or nuclear genome, and noncoding RNAs transcribed from the mitochondrial genome that are transported to the cytosol or nucleus. Recent findings have revealed that mt-ncRNAs play important roles in not only mitochondrial functions, but also other cellular activities. This review proposes a classification of mt-ncRNAs and outlines the emerging understanding of mitochondrial circular RNAs (mt-circRNAs), mitochondrial microRNAs (mitomiRs), and mitochondrial long noncoding RNAs (mt-lncRNAs), with an emphasis on their identification and functions.
Introduction
Mitochondria, the ‘powerhouse of the cell’, are ancient organelles that joined the cells over the course of evolution. The conventional view of the mammalian mitochondrial genome is that mitochondrial DNA (mtDNA) encodes only 2 rRNAs, 22 tRNAs, and 13 protein subunits of the oxidative phosphorylation system [Citation1]. Over the past 20 years, this view has been renewed by increasingly thorough examinations of RNA species in the mitochondria. These studies have revealed the fascinating complexity of the mitochondrial transcriptome, which exerts a variety of effects on mitochondrial function and human diseases [Citation2–5].
Noncoding RNAs have been identified as important players in epigenetic regulation [Citation6]. Mitochondria-residing noncoding RNAs, which are transcribed from either the mitochondrial genome or nuclear genome, play crucial roles in not only mitochondrial function, but also other cellular functions [Citation7,Citation8]. Among them, circular RNAs (circRNAs) in the mitochondria are the least understood, and their functions have not yet been elucidated. Mitochondria-located microRNAs (miRNAs), termed as ‘mitomiRs’, mainly mediate mitochondrial function, such as ROS production, via transcriptional or translational regulation [Citation7,Citation9]. Moreover, some mitomiRs are associated with various diseases [Citation10–12]. Long noncoding RNAs (lncRNAs) located in the mitochondria are involved in several types of cancer, regulating the metabolism and growth of tumour cells, and participating in mitochondrial gene regulation, inflammation resolution, and so on [Citation13–16]. Some noncoding RNAs, however, are transcribed from the mitochondrial genome and are located in the cytosol and nucleus. They are associated with multiple cancers, hopefully serving as a new therapeutic target for cancer therapy [Citation4,Citation17].
To classify noncoding RNAs related to the mitochondria and their corresponding genome clearly, we adopt the term ‘mitochondrial noncoding RNAs (mt-ncRNAs)’, which was previously used by Cavalcante et al [Citation8]; however, the definition of mt-ncRNAs is not the same. Here, mt-ncRNAs comprise noncoding RNAs residing in the mitochondria, which are transcribed from the mitochondrial genome or nuclear genome, as well as noncoding RNAs located in the cytosol, nucleus, and even plasma, which are transcribed from the mitochondrial genome. mt-ncRNAs can be classified into four types based on their location and genome origin ( and ). Alternatively, mt-ncRNAs can be categorized traditionally according to noncoding RNA species, which will be thoroughly discussed later.
Figure 1. Classification, location and genome origin of mitochondrial noncoding RNAs (mt-ncRNAs). Based on noncoding RNA species, mt-ncRNAs can be classified into three types: mitochondrial circular RNAs (mt-circRNAs), mitochondrial microRNAs (mitomiRs), and mitochondrial long noncoding RNAs (mt-lncRNAs). Alternatively, according to their location and genome origin, mt-ncRNAs can be categorized into four types: nuclear-encoded mitochondria-located mt-ncRNAs, mtDNA-encoded mitochondria-located mt-ncRNAs, mtDNA-encoded nucleus/cytosol-located mt-ncRNAs, and mtDNA-encoded extracellular-located mt-ncRNAs. In addition, plenty of ncRNAs participating in the regulation of mitochondrial function, termed as ‘mitochondria-associated noncoding RNAs’, do not belong to mt-ncRNAs because they are neither located in the mitochondria nor encoded by the mitochondrial genome. Communication between the mitochondria and the host nucleus through anterograde and retrograde signals are regulated by mt-ncRNAs as well as mitochondria-associated noncoding RNAs
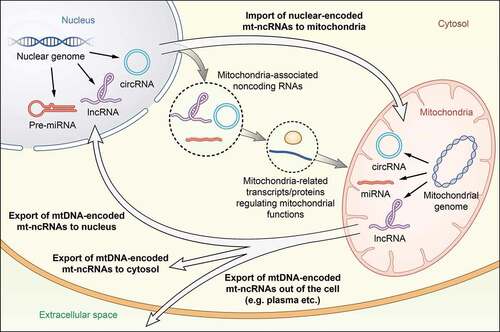
Table 1. Classification of mitochondrial noncoding RNAs (mt-ncRNAs) according to location and corresponding genome
In this review, we first provide a brief introduction of the origin of mt-ncRNAs and describe two types of classifications of mt-ncRNAs. We then focus on their functions in the mitochondria as well as in human diseases. Furthermore, we discuss the epitranscriptomics of mitochondrial transcripts and the latest approaches in manipulating the mitochondrial genome and transcripts.
The origin and classification of mt-ncRNAs
According to the widely accepted endosymbiotic theory, mitochondria originated from the integration of an endosymbiotic alpha proteobacterium into a host cell related to Asgard Archaea, and the origin of the mitochondrial protein import apparatus allowed the host and symbiont compartments to mix genes and proteomes [Citation18,Citation19]. The mitochondrial genome is mostly maternally inherited in mammals [Citation20]. Maternal mitochondrial inheritance is probably an active process of paternal mitochondria and mtDNA elimination, which is achieved by autophagy and the ubiquitin-proteasome system after fertilization [Citation21–23]. However, evidence also suggests that it may be a passive process of prefertilization paternal mtDNA elimination, and uneven mitochondrial distribution in embryos [Citation24]. Mammalian mitochondria contain a 16.5-kb circular, double-stranded genome. The two strands of mtDNA are distinguished as the heavy strand and light strand, and mtDNA encodes 2 rRNAs, 22 tRNAs, and 13 protein subunits [Citation1]. Transcription of mtDNA originates from LSP (light strand promoter) and two HSP (heavy strand promoter), respectively. It requires mitochondrial RNA polymerase, TFAM, and TFB2M for initiation; TEFM for elongation; and MTERF1 for termination, finally producing long polycistronic transcripts [Citation25–28]. Mitochondria own their unique ribosomes (namely mitoribosomes); and their translation is totally dependent on nuclear-encoded proteins. It requires IF2mt and IF3mt for initiation; EF-Tumt, EF-Tsmt, and EF-G1mt for elongation; mtRF1a for termination; and EF-G2mt as well as RRFmt for recycling of mitoribosomes [Citation29–33]. Recently, accumulating evidence has shown the presence of noncoding RNAs transcribed from mtDNA, opening a new dimension to our understanding of mitochondrial transcripts.
According to their location and genome origin, mt-ncRNAs can be classified into four types.
Nuclear-encoded mitochondria-located mt-ncRNAs: This type contains the largest number of mt-ncRNAs. The majority of mt-ncRNAs seem to be transcribed from the nuclear genome, matured in the cytoplasm, and then translocate into the mitochondria, where they perform their functions.
mtDNA-encoded mitochondria-located mt-ncRNAs: This type accounts for a considerable fraction of mt-ncRNAs.
mtDNA-encoded nucleus/cytosol-located mt-ncRNAs: Only a few mt-ncRNAs belong to this type.
mtDNA-encoded extracellular-located (e.g. plasma etc.) mt-ncRNAs: Only a few mt-ncRNAs belong to this type.
On the other hand, based on noncoding RNA species, mt-ncRNAs are categorized into three types: mitochondrial circular RNAs (mt-circRNAs), mitochondrial microRNAs (mitomiRs), and mitochondrial long noncoding RNAs (mt-lncRNAs). The functions of these three types of mt-ncRNAs are summarized in and will be discussed in detail below.
Table 2. Mitochondrial noncoding RNAs (mt-ncRNAs) identified in mammals
In addition, there are numerous noncoding RNAs involved in the regulation of mitochondrial function, but they are neither located in the mitochondria nor encoded by the mitochondrial genome. We propose the term ‘mitochondria-associated noncoding RNAs’ to describe such noncoding RNAs. A plethora of reviews has introduced the functions of mitochondria-associated noncoding RNAs [Citation8,Citation34]. They broadly affect mitochondrial metabolism [Citation10,Citation13,Citation14,Citation35], apoptosis, cell senescence [Citation13,Citation14], and a variety of human diseases, such as cancers [Citation7,Citation12,Citation13], cardiovascular diseases [Citation11,Citation36] and diabetes [Citation10].
In addition, PIWI-interacting RNAs (piRNAs), an animal-specific class of small silencing RNAs, silence transposable elements and regulate gene expression [Citation37]. A few studies have reported the presence of piRNAs in the mitochondria, but their exact function remains poorly understood (reviewed by Cavalcante et al. [Citation8]).
Mitochondrial circular RNAs (mt-circRNAs)
circRNAs are endogenous biomolecules that are covalently closed at the 5´and 3´ ends and are mostly expressed from known protein-coding genes [Citation38]. circRNAs are produced by a non-canonical splicing event called backsplicing [Citation39]. They are more resistant to exonucleases than linear RNAs and perform important biological functions by acting as miRNAs or protein inhibitors (‘sponges’), regulating protein function, or being translated themselves [Citation40]. In addition, circRNAs have been implicated in diseases such as diabetes mellitus, neurological disorders, cardiovascular diseases and cancer [Citation40]. Although several circRNAs transcribed from mtDNA have been identified [Citation41,Citation42], the functions of mtDNA-encoded or mitochondria-located circRNAs (mt-circRNAs) are poorly understood. Among the three types of mt-ncRNAs, mt-circRNAs seem to be the most elusive. The identification of mt-circRNAs and elucidation of their functions in humans and mammals have not been reported until recently. Their mysterious veils are gradually unravelling, indicating their unique functions in human diseases.
Recently, we have identified an mtDNA-encoded circRNA (hsa_circ_0089762) named steatohepatitis-associated circRNA ATP5B regulator (SCAR) and provided insights into its function [Citation2]. Liver fibroblasts from patients with nonalcoholic steatohepatitis (NASH) exhibited elevated mitochondrial ROS (mROS) production and efflux into the cytosol, mediating the pro-inflammatory phenotype of fibroblasts. Through circRNA expression profile analysis of liver fibroblasts from patients with NASH, we found that SCAR was downregulated. By constructing a mitochondria-targeting nanoparticle (mito-NP) platform with the ability to overexpress circRNA in the mitochondria, we confirmed that SCAR was located in the mitochondria and inhibited fibroblast activation. Mechanistically, SCAR directly bound to ATP5B of ATP synthase in the mPTP complex and blocked the interaction between CypD and mPTP during the resting state. Lipid-induced ER stress reduced PGC-1α-mediated circRNA SCAR expression by CHOP, relieved the inhibition of CypD-mPTP binding, and facilitated mPTP opening. Additionally, SCAR alleviated high fat diet-induced cirrhosis in mice and was closely associated with steatosis-to-NASH progression and insulin resistance in patients. Therefore, targeting circRNA SCAR appears to be an attractive therapeutic strategy for immunometabolic diseases.
Interestingly, the same circRNA, hsa_circ_0089762, plays a crucial role in chronic lymphocytic leukaemia (CLL) [Citation43]. Wu et al. found a high abundance of this circRNA, which was termed mc-COX2, in the plasma exosomes of patients with CLL. Upregulation of mc-COX2 predicted poor prognosis in patients with CLL. Knockdown of mc-COX2 affected mitochondrial function, including reduced ATP production and induced apoptosis. Several mitochondria-related chemical compounds and inhibitors reduced mc-COX2 and inhibited proliferation of CLL cells, and this proliferation inhibition effect was enhanced by the cooperation of mc-COX2 interference, providing a potential target to improved drug sensitivity [Citation43]. Liu et al. reported abundant mitochondria-encoded circRNAs (mecciRNAs) in the mitochondria isolated from cells and tissues in humans and mice, respectively. Two mecciRNAs encoded by the mitochondrial gene ND1 (mecciND1) and ND5 (mecciND5) have been suggested to facilitate the transportation of proteins encoded by nuclear genome via interactions with some proteins involved in mitochondrial protein and RNA entry, such as TOM40 and PNPase (polynucleotide phosphorylase) [Citation44].
Gao et al. detected three circRNAs, which were mapped to the mitochondrial genome in cattle testes using ribo-depleted total RNA-Seq [Citation45]. Zhang et al. identified 118 circRNAs with low abundance in the mitochondrial fraction of HepG2 cells using whole-transcriptome sequencing and circRNA algorithms [Citation46]. Subsequently, by treating RNA with RNase R and then amplifying putative 3´-5´ junction sites, Mance et al. reported that approximately 105 circRNAs were derived from fragments of protein-coding regions in isolated mitochondrial mRNAs from HEK293 cells, comprising a small portion (~10%) of the total mRNA. Because coding regions the circRNAs carried were truncated, they were likely not translatable [Citation47]. Although these circRNAs have been detected, they are yet to be functionally deciphered.
Mitochondrial microRNAs (termed as ‘mitomiRs’)
miRNAs are short noncoding RNAs of approximately 22 nucleotides in length. The biogenesis of miRNAs is a multistep process. They are transcribed as structured primary miRNAs (pri-miRNAs) and processed into precursor miRNAs (pre-miRNAs) and finally into mature miRNAs [Citation48]. Mature miRNAs operate as functional units that include an Argonaute (AGO) protein, and AGO–miRNA binding to the 3´ UTR leads to gene silencing through translation repression and mRNA decay. miRNAs are involved in virtually every cellular process and are essential for animal development, cell differentiation, and homoeostasis, and deregulation of miRNA function is associated with numerous diseases [Citation49]. Mitochondria-located miRNAs, termed as ‘mitomiRs’ [Citation50], were detected approximately 10 years ago. They are mainly transcribed from the nuclear genome and translocated into the mitochondria, whereas some are transcribed from the mitochondrial genome [Citation51]. The functions of these mitomiRs are somewhat different from those of cytosol-located miRNAs.
There seems to be a strong association between mitomiRs and heart-related diseases. miR-181c, which showed mitochondrial localization in neonatal rat ventricular myocytes, bound to the 3ʹUTR of COX1. Overexpression of miR-181c dramatically decreased COX1 protein levels, without interfering with COX1 mRNA levels, while increasing mRNA and protein levels of COX2 and COX3, leading to complex IV remodelling and altering mitochondrial function, including augmented ROS production [Citation52]. Similarly, systematic administration of miR-181c in rats exhibited signs of heart failure by regulating mitochondrial genes, indicating its implications in heart disease [Citation53].
In mouse type 1 diabetic heart, redistribution of miR-378 to the interfibrillar mitochondria caused a loss of ATP6 in the presence of RISC components, AGO2 and FXR1. Manipulation of miR-378 in vivo restored functional ATP6 and cardiac pump function [Citation54]. In addition, in mouse type 2 diabetic heart, PNPase was suggested to interact with AGO2 to transport miRNA-378 into the mitochondria, which may mediate bioenergetics during type 2 diabetes mellitus [Citation55]. Two other mitomiRs critically involved in diabetic cardiomyopathy, miR-92a-2-5p and miR-7b-5p, were downregulated in the mitochondria of db/db mice heart. It is well established that miRNAs mediate translational repression and mRNA decay in the cytoplasm. Unexpectedly, miR-92a-2-5p and miR-7b-5p targeted Cytb and compensated for the reduction of Cytb by positive translational modulation. Interestingly, cardiac diastolic dysfunction in db/db mice was fully rescued via the administration of exogenous miR-92a-2-5p, but not miR-7b-5p, along with reduced ROS production and lipid deposition because let-7b-5p promoted lipid deposition via the cytosol gene [Citation56].
Similarly, miR-21 counteracted Cytb downregulation induced in spontaneous hypertensive rats (SHRs) by promoting Cytb translation. The plasma levels of miR-21 were positively associated with high blood pressure in human patients, and in vivo delivered miR-21 alleviated cardiac hypertrophy in SHRs [Citation57]. miR-1 is another example of enhancing mitochondrial translation by mitomiRs. The muscle-specific miR-1, which was induced during myogenesis, directly stimulated the translation of ND1 and COX1 in an AGO2 dependent and GW182-independent manner, while inhibiting nuclear DNA-encoded targets in the cytoplasm. Hence, a highly coordinated myogenic program was suggested to be mediated by miR-1 [Citation5].
Two mitomiRs were associated with chemoresistance in tongue squamous cell carcinoma (TSCC). Downregulation of miR-5787 [Citation58] and upregulation of miR-2392 [Citation59] reprogrammed glucose metabolism by shifting it from oxidative phosphorylation to aerobic glycolysis in cisplatin-resistant TSCC cells. However, their specific mechanisms are different. miR-5787 enhanced the translation of COX3, whereas miR-2392 partially repressed the transcription of ND4, Cytb, and COX1 in an AGO2-dependent manner. Low miR-5787 and COX3 expression, high miR-2392 expression, and low ND4, Cytb, and COX1 expression predicted poor survival outcomes in patients with TSCC.
In addition to the mitomiRs discussed above, a plethora of mitochondria-located miRNAs has been detected in humans and mammals, as reviewed by Gusic et al. and Ro et al. in detail [Citation34,Citation51]. However, their functions remain poorly elucidated. Moreover, several studies have suggested that mitomiRs can be transcribed from mtDNA.
Ro et al. identified thousands of mitochondria-located small noncoding RNAs (termed ‘mitosRNAs’) encoded from murine and human mitochondrial genomes. Given the evidence that DICER inactivation affected but did not completely suppress mitosRNA expression, an alternative pathway for the biogenesis of mitosRNA may exist [Citation51]. Overexpression of mitosRNAs upregulated expression of their host genes in vitro, suggesting that mitosRNAs may participate in controlling mitochondrial gene expression [Citation51].
Barrey et al. in silico discovered 33 pre-miRNAs and 25 miRNAs targeting mtDNA, and among them, pre-miR302a, let-7b, and miR-365 were verified to be localized in the mitochondria of human myoblasts. Potential miRNA targets in the mitochondrial genome were predicted by in silico analysis, revealing the potential involvement of these miRNAs in silencing mitochondrial mRNA [Citation60]. Sripada et al. found that miR-4461, miR-4463, miR-4484, and miR-4485, which were abundant in the purified mitochondria of HeLa and HEK293T cells, aligned to the mitochondrial genome and may process mitochondrial transcripts associated with ribosome assembly and electron transport chain [Citation61]. Bandiera et al. found that miR-1974, miR-1977, and miR-1978 were significantly enriched in the purified mitochondria of HeLa cells, and these miRNAs perfectly mapped to mitochondrial tRNA and rRNA genes [Citation50].
Of note, the presence of the miRNAs machinery in mitochondria remains controversial. So far, both Drosha and DGCR8 have not been detected in the mitochondria. Ro et al. validated that DICER was absent but thousands of small noncoding RNAs transcribed from the mitochondrial genomes were present in the mitochondria of HEK293T cells [Citation51]. Das et al. [Citation52] and Jagannathan et al. [Citation54] also demonstrated the absence of DICER in the mitochondria of rat or mice cardiomyocytes. But later Vargas et al. reported the co-localization of pre-miR-338 and DICER in the mitochondria of axons of SCG neurons [Citation62]. Considering this has been the only publication reporting the co-localization of pre-miRNAs and DICER in the mitochondria so far, the existence of a non-canonical biogenesis machinery of mtDNA-encoded miRNAs seems to be doubtful. Are these miRNAs truly encoded by mtDNA or just byproducts of contamination? As for RISC components, several studies have revealed the presence of AGO2 and FXR1 in the mitochondria [Citation5,Citation50,Citation52,Citation54,Citation59,Citation61], whereas the absence of AGO2 in the mitochondria of HEK293T cells was validated by Ro et al. [Citation51]. In addition, GW182, another critical component of translational repression mediated by miRNAs, was also proved to be absent in the mitochondria [Citation5,Citation51] and has not been detected up to now. This makes us wonder if there is another RNAi-like machinery in the mitochondria.
Blumental-Perry et al. discovered an mtDNA-encoded ncRNA, mito-ncR-805, which was initially recognized as a miRNA and later confirmed as a 70-nucleotide ncRNA, increased in AETII cells from mouse lungs following exposure to cigarette smoke (CS). CS exposure led to the redistribution of mito-ncR-805 from the mitochondria to the nucleus, where mito-ncR-805 promoted the expression of nuclear-encoded proteins, which resulted in positively regulated mitochondrial homoeostasis and function, and promoted mitochondrial bioenergetics, which helped cells to survive the stress [Citation63].
Mitochondrial Long Noncoding RNAs (mt-lncRNAs)
lncRNAs are defined as transcribed RNA molecules that are greater than 200 nucleotides in length. The molecular functions of lncRNAs can be summarized as four archetypes: signals, decoys, guides, and scaffolds [Citation64]. The expression or functional abnormality of lncRNAs is closely related to the occurrence of human diseases, such as tumours and cardiovascular diseases [Citation65]. Recent studies have revealed the significance of mt-lncRNAs. Based on their localization and genetic origin, four different classes of mt-lncRNAs can be distinguished: (1) nuclear-encoded mitochondria-located lncRNAs, (2) mtDNA-encoded mitochondria-located lncRNAs, (3) mtDNA-encoded nucleus/cytosol-located lncRNAs, and (4) putative mtDNA-encoded lncRNAs.
(1) Nuclear-encoded mitochondria-located lncRNAs
lncRNA RMRP
RMRP, the RNA component of the RNA processing endoribonuclease (RNase MRP), participates in rRNA processing [Citation66]. The presence of RMRP in the mitochondria was confirmed in 1994 [Citation67]. RMRP binds to two RNA-binding proteins, HuR and GRSF1, the former of which binds to RMRP in the nucleus and mediates its translocation into the cytosol [Citation66]. After importing into the mitochondrial matrix probably via PNPase [Citation68], GRSF1 binds to RMRP, increasing its abundance. Loss of GRSF1 causes a reduction in mitochondrial RMRP, oxygen consumption and mtDNA replication [Citation66]. Interestingly, GRSF1 is an important component of mitochondrial RNA granules (MRGs) [Citation69,Citation70], which comprise nascent mitochondrial RNA and various proteins, including RNase P. MRGs provide a platform for mitochondrial RNA processing [Citation71]. For example, the interaction between GRSF1 and RNase P is required for the processing of mitochondrial RNA precursors [Citation70].
SAMMON
The lncRNA SAMMON was discovered in human melanoma, primarily in the cytoplasm, but a fraction is co-localized in the mitochondria [Citation3]. p32 is a cytoplasmic and mitochondrial protein required for the maturation of mitochondrial rRNA [Citation72]. Normally, the RNA-binding protein CARF sequesters the exo-ribonuclease XRN2 in the nucleoplasm. In case of melanoma, however, upregulation of SAMMON facilitated p32 mitochondrial location and XRN2 nucleolar location and consequently increased protein synthesis, along with the promotion of tumour cell growth [Citation73]. Additionally, knockout of the SAMMON gene inhibits melanoma survival [Citation3].
SRA
Steroid receptor RNA activator (SRA) is an lncRNA that functions as an RNA coactivator of steroid receptors and non-steroid nuclear receptors, potentiating steroid receptors transcription activity and affecting myogenesis, steroidogenesis and so on [Citation74]. SRA is a target for two RNA-binding proteins: SHARP, which resides in the nucleus [Citation75], and SLIRP, which is predominantly located in the mitochondria [Citation76]. They both bind to SRA via RNA recognition motifs and compromise SRA-mediated transactivation. Despite the absence of experiments resolving SRA localization to the mitochondria, these data suggest that SLIRP and SRA are present in both the nucleus and cytosol and thus may exert effects in both compartments [Citation77].
MALAT1
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), an lncRNA normally expressesed in the nucleus, is primarily associated with tumours and metastasis [Citation78]. MALAT1 was enriched in the mitochondria isolated from HepG2 cells but was scarcely detectable in the mitochondria from normal hepatic HL7702 cells [Citation79]. MALAT1 interacts with multiple loci on mtDNA, including the D-loop, COX2, and ND3 genes, impacting mitochondrial epigenetic regulation. The binding of MALAT1 changed the status of DNA methylation at the CpG 3 site in front of CO1 [Citation80]. Knockdown of MALAT1 caused a range of aberrant mitochondrial metabolism and function, activation of mitochondrial apoptosis, and impaired cell invasion, revealing that MALAT1 mediated the regulation of mitochondrial metabolism in hepatoma cells [Citation80].
GAS5
Recently, Sang et al. identified and validated the enrichment of 21 lncRNAs from purified mitochondria, and focused on a nucleus-encoded lncRNA named GAS5. Under glucose-deficient conditions, GAS5 suppressed the metabolic tandem association of fumarate hydratase, malate dehydrogenase, and citrate synthase, thus reducing mitochondrial tricarboxylic acid (TCA) flux. High expression of GAS5 and low TCA flux benefit clinical outcomes in breast cancer [Citation16].
lncFAO
Nakayama et al. identified an lncRNA, lncFAO, in mouse macrophages at a later time after LPS stimulation. The β-subunit of mitochondrial tri-functional protein was activated by lncFAO via direct interaction during the resolution and reparative phases, resulting in the enhancement of its functions: fatty acid β-oxidation activation, suppression of proinflammatory cytokines, and regulation of inflammation resolution [Citation15].
(2) mtDNA-encoded nucleus/cytosol-located lncRNAs
A chimeric mtDNA-encoded lncRNA, consisting of the mitochondrial 16S rRNA covalently joined to a 121-nucleotide 5´-leader sequence derived from its complementary strand, was first discovered in mice cells [Citation81]. A similar transcript, known as sense noncoding mitochondrial RNA (SncmtRNA), was subsequently identified in proliferating human tumour cells but not in resting cells. It consists of a hairpin structure comprising the mitochondrial 16S rRNA linked to an 815-nucleotide 5´-leader sequence derived from its complementary strand and forms a double-stranded structure with a 40-nucleotide loop [Citation82]. Subsequently, two antisense noncoding mitochondrial RNAs (ASncmtRNA-1 and ASncmtRNA-2) containing similar stem-loop structures were discovered in normal proliferating human cells but were downregulated in tumour cells, indicating that ASncmtRNAs might function as unique tumour suppressors [Citation4]. SncmtRNA and ASncmtRNAs were reported to be localized outside the mitochondria, especially in the nucleus associated with heterochromatin, and therefore might be new components of the mitochondrial-nuclear communication pathway or retrograde signalling [Citation17], however, the specific trafficking mechanism, remains unexplored.
As a potential new cancer hallmark, the common downregulation of ASncmtRNAs has been assessed in multiple tumour tissues, including cervical cancer [Citation83–85], breast cancer [Citation86,Citation87], renal adenocarcinoma [Citation88], bladder cancer [Citation89,Citation90] and melanoma [Citation91,Citation92]. Knockdown of ASncmtRNAs with antisense oligonucleotides induced inhibition of cell proliferation and increased cell death in these tumour cells, along with activation of pro-apopototic factors and downregulation of anti-apoptotic factors including survivin. ASncmtRNA-2 was suggested to be a precursor of two miRNAs (miR-4485 and miR-1973) [Citation93], which probably mediated the downregulation of survivin at the translational level [Citation83]. Furthermore, in vivo treatment with antisense oligonucleotides significantly suppressed tumour growth and metastatic spread to other organs. Altogether, these results indicate the potential role of ASncmtRNAs serving as promising therapeutic targets for cancer therapy.
(3) mtDNA-encoded mitochondria-located lncRNAs
Three mtDNA-encoded mitochondrial lncRNAs, lncND5, lncND6 and lncCytb have been identified and confirmed in purified human mitochondria [Citation79,Citation94,Citation95]. These lncRNAs were 58%, 34%, and 14% as abundant as their complementary mRNAs (ND5, ND6, and Cytb), respectively. They formed intermolecular duplexes with respective complementary mRNAs, along with the variation in their abundance in different cell lines and tissues, suggesting that these lncRNAs may contribute to the regulation of mitochondrial gene expression and stabilization of their mRNA counterparts [Citation95]. However, lncCytb, showed aberrant localization in hepatoma cells (HepG2) compared with that in normal hepatic HL7702 cells, indicating its potential role as a mitochondria-nucleus communication mediator [Citation79]. In addition, using PacBio full-length transcriptome data, another mtDNA-encoded lncRNA, MDL1, which covered the antisense tRNAPro gene and D-loop region, and its antisense transcript, MDL1AS, have been identified in humans [Citation96].
(4) Putative mtDNA-encoded lncRNA
A putative mtDNA-encoded lncRNA, named long intergenic noncoding RNA predicting cardiac remodelling (LIPCAR), was identified in the plasma of heart failure patients [Citation97]. Aligning the the LIPCAR sequence to the human mtDNA revealed that LIPCAR sequence is discontinuous: the 5´ half maps to antisense of the mitochondrial Cytb gene, but the 3´ half maps to antisense of the mitochondrial COX2 gene. Mitochondrial genes lack introns and are not known to undergo splicing; therefore, LIPCAR seems to be a putative mtDNA-encoded lncRNA because of its discontinuity [Citation98]. Its circulating level increased at the late stages of left ventricular remodelling and patients with chronic heart failure; therefore, it could act as a prognostic indicator for cardiac remodelling and predict future death in patients with heart failure [Citation97,Citation99]. Moreover, the level of circulating LIPCAR was significantly upregulated in patients with coronary heart disease (CAD), suggesting its function as a novel biomarker for CAD [Citation100].
Post-transcriptional modifications of mitochondrial transcripts
The transcription of mtDNA is quite different from that of the nuclear genome. It produces long polycistronic transcripts [Citation101,Citation102]. According to the widely accepted ‘tRNA punctuation model’, most of the coding regions of mitochondrial mRNAs and rRNAs are separated by tRNAs. Polycistronic transcripts are processed into mature individual RNA species via endonucleolytic cleavage [Citation102]. In humans, RNase P [Citation103] and ELAC2 (homolog of RNase Z) [Citation104] perform precursor-tRNA cleavage at the 5′ and 3′ends of tRNAs, respectively. After release, all mitochondrial tRNAs, rRNAs, and mRNAs need to undergo several stages of maturation and modification. tRNAs undergo chemical nucleotide modifications, CCA addition at the 3′-end synthesized by TRNT1, deadenylation catalysed by PDE12, and aminoacylation mediated by ARS2s. rRNAs undergo chemical nucleotide modifications, and mRNAs undergo 3′ polyadenylation by mtPAP. Finally, mitochondrial RNA decay, which occurs in distinct foci, is mediated by the PNPase-hSuv3 complex. These have been thoroughly reviewed by Souza et al. [Citation105]. In the following sections, we elaborate on the post-transcriptional modifications of mitochondrial transcripts and their effect on cellular function as well as human diseases.
To date, more than 160 types of cellular RNA molecule modifications and approximately 340 functionally associated proteins have been identified [Citation106]. Fewer modifications are present in mammalian mitochondrial RNAs than in their cytoplasmic counterparts [Citation106–108]. Modifications of mitochondrial tRNAs are the most varied and complex among all known mitochondrial RNA species. In 22 species of human mitochondrial tRNAs, 18 RNA modifications at 137 positions have been identified [Citation108]. There are no reports about post-transcriptional modifications of mt-ncRNAs yet.
Based on their main functions, mitochondrial tRNA modifications can be divided into two groups: anticodon loop modifications and core modifications. Positions 34 and 37 within the anticodon loop of tRNAs are hotspots of RNA modifications, containing various complex modifications. In mammalian mitochondria, 60 codons are deciphered by a minimum set of 22 mitochondrial tRNAs [Citation108]. Therefore, the non-canonical base pairing between position 34 (the wobble position) and the third base of the codon triplet, which expands codon recognition and achieves decoding flexibility, is of great significance [Citation109–112]. Modifications of position 34, which facilitate non-canonical base pairing include τm5U (5-Taurinomethyluridine), τm5s2U (5-Taurinomethyl-2-thiouridine) and f5C (5-Formylcytosine). Additionally, modifications of position 37 contribute to maintaining translation fidelity and accuracy by stable codon–anticodon interactions [Citation111–114]. They include i6A (N6-Isopentenyladenosine), ms2i6A (2-Methylthio-N6-isopentenyladenosine), t6A (N6-Threonylcarbamoyladenosine), and m1G (1-Methylguanosine).
Core modifications primarily benefit the structural stability and correct folding of mitochondrial tRNAs [Citation111,Citation112]. These modifications are basically small modifications, including base methylation and Ψ (pseudouridine). The corresponding enzymes involved in anticodon loop modifications and core modifications are listed in . Mutations in these enzymes and at or near mitochondrial RNA sites that carry modifications are associated with a wide range of human diseases, such as HSD10 disease, mitochondrial myopathy and sideroblastic anaemia, mitochondrial myopathy, myoclonus epilepsy associated with ragged-red fibres, Leber’s hereditary optic neuropathy, autosomal recessive intellectual disability and Alzheimer’s disease. Such mutations usually lead to defects in tRNA modifications, thereby hampering mitochondrial translation and function, and these may be the molecular basis of the human diseases mentioned above.
Table 3. Inventory of mammalian mitochondrial RNA modifications with known RNA-modifying enzymes
Mammalian mitoribosomes differ considerably from other ribosomes, primarily because of their high protein:RNA ratio [Citation115]. Because mitochondrial rRNAs serve as the scaffold of mitoribosomes, efficient mitoribosome biogenesis and functions rely heavily on correct folding and strong stability of mitochondrial rRNAs. Consistent with this view, modifications of mitochondrial rRNAs cluster in functionally important regions, such as the peptidyl transferase centre and decoding site [Citation111,Citation112]. In the 12S rRNA, only base methylation was observed at several positions. Modifications of the 16S rRNA include base methylation, Ψ, and 2´-O-methylation. The corresponding enzymes are shown in . Studies have shown links between mutations in these enzymes and several human diseases including type 2 diabetes and MELAS. Affected mitoribosome assembly and mitochondrial function may underlie these diseases. Furthermore, emerging evidence raise the possibility that post-transcriptional modifications, including Ψ and m1A, are present in mitochondrial mRNAs [Citation116–118]. LHON is associated with ND5 methylation levels [Citation118].
Taken together, post-transcriptional modifications of mitochondrial transcripts exert unique effects on mitochondrial function and have important implications on the pathogenesis of a variety of diseases.
The Art and Advances in Manipulating Mitochondrial Genome and Transcripts
The manipulation of the mitochondrial genome is challenging. In 2020, the Nobel prize in Chemistry was awarded to Emmanuelle Charpentier and Jennifer Doudna for the development of CRISPR/Cas9, which is useful for the manipulation of nuclear genomes. This system essentially consists of the Cas9 enzyme and a guide RNA (gRNA). The introduction of CRISPR/Cas9 into the cell nucleus causes site-specific double-strand breaks (DSBs). CRISPR/Cas9 stimulates gene disruption or deletion by non-homologous end joining, and gene correction or insertion by homology-directed repair (HDR) [Citation119]. However, robust approaches to import gRNA into mammalian mitochondria are currently lacking [Citation120,Citation121]. Therefore, CRISPR/Cas9 is still ineffective in mitochondrial genome editing. A previous study claimied that mtDNA editing is possible using CRISPR/Cas9 [Citation122]. Nevertheless, their conclusion seems doubtful because of the absence of definite proof.
Over the past decades, several mitochondria-targeted protein-only nucleases have been used to manipulate the mitochondrial genome especially in mtDNA diseases. They are mitochondrial restriction endonucleases (mtREs), mitochondrially targeted zinc finger nucleases (mtZFNs) and mitochondrially targeted transcription activator-like effector nucleases (mtTALENs). Mitochondria have a multi-copy genome, and mutant mtDNA can co-exist with wild-type mtDNA in cells, which is called heteroplasmy. Mitochondrial diseases become biochemically and clinically apparent only when the level of mutant mtDNA reaches a threshold [Citation120]. All these nucleases promote DSBs in mtDNA. Since mammalian mitochondria cannot repair DSBs [Citation123] or perform HDR [Citation124], mtDNA bearing DSBs can be degraded and the heteroplasmy shifts towards wild-type mtDNA content. Therefore, the pathologies can be resolved.
Several restriction endonucleases, such as PstI, SmaI and XmaI, have been used to manipulate mtDNA heteroplasmy, benefiting from the mitochondrial targeting sequence (MTS). They recognize and eliminate particular mtDNA haplotypes carrying a specific restriction site [Citation125–127]. In vivo experiments confirmed their mtDNA heteroplasmy-shifting ability in multiple tissues in both transgenic and adeno-associated virus-treated mice [Citation128–130]. However, this approach is limited by the presence of mutation-specific restriction sites. Subsequently, two new classes of mitochondria-targeted nucleases, mtZFNs and mtTALENs, have emerged. Engineered zinc-finger proteins in mtZFNs and engineered transcription activator-like effectors in mtTALENs are able to recognize specific DNA sequences. DSBs were subsequently performed by nuclease FokI. In vitro and in vivo data demonstrated that both mtZFNs [Citation105,Citation131–134] and mtTALENs [Citation135–139] efficiently eliminated mutant mtDNA and reversed disease phenotypes. However, none of these three nucleases allows precise single-base editing. In addition, when the mutation load of mtDNA is very high, significantly decreased mtDNA copy numbers may be harmful.
Recently, Mok et al. [Citation140] developed an innovative mtDNA editing tool called DddA-derived cytosine base editors (DdCBEs). They found that an interbacterial toxin, DddA, catalysed the deamination of cytidines within dsDNA. DdCBEs consisted of split-DddA halves, transcription activator-like effector array proteins, and a uracil glycosylase inhibitor. DdCBEs catalysed C•G-to-T•A conversions in human mtDNA with high target specificity and product purity without perturbing mtDNA copy numbers.
Over the past few decades, mitochondria-targeted nanomaterials have emerged. The development of mitochondria-targeted nanomedicines provides new dimensions for disease treatment. There are a variety of nanomaterials, such as DQAsomes, Mito-Poters, and transition metal complexes (comprehensively reviewed in [Citation141]). Nanomedicine has significantly advanced curative medicine by improving the pharmacokinetics and biodistribution profiles of various pharmacological products. Therefore, their pristine forms remained kept intact. They have been applied to a wide range of diseases, including mitochondria-associated diseases, cancers, cardiovascular diseases, neurodegenerative diseases and diabetes [Citation142].
Driven by the nanomaterial technologies mentioned above, new avenues are being explored to regulate mitochondrial genetics and epigenetics. Recently, we constructed a mito-NP platform [Citation2]. The mito-NP platform contained an endosomal pH-responsive polymer, encapsulated circRNA-expressing vectors and triphenylphosphonium-decorated amphiphilic cationic peptides, which specifically deliver encapsulated nucleic acids to the mitochondria. The specific capacity of mito-NP for targeting the mitochondria was verified. circRNAs were able to be efficiently expressed in mitochondria, but not in the cytosol. Therefore, the use of nanocarriers may offer a promising solution for the import of noncoding RNAs into mammalian mitochondria. This may offer implications for the development of mt-ncRNAs editing.
Perspectives
Studies in the field of mt-ncRNAs are just beginning, with plenty of unsolved key scientific questions. (1) There are a huge number of unidentified mt-ncRNAs, and their functions remain elusive. (2) Although an increasing number of mt-ncRNAs are being identified, information on the canonical ncRNAs biogenesis machinery in the mitochondria is still lacking. For example, cytosol-located circRNAs are produced from precursor mRNA back-splicing of thousands of genes [Citation39,Citation143]. The question remains whether the same biogenesis mechanism can apply to mitochondrial counterparts. (3) Trafficking of ncRNAs between cellular compartments, including their entrance into and exit from the mitochondria, is still poorly understood. PNPase residing in the mitochondrial intermembrane space regulates the import of nuclear-encoded RNAs into the mitochondrial matrix [Citation68], and TOM/TIM components may regulate ncRNAs trafficking in and out of the mitochondria through the mitochondrial intermembrane and outermembrane [Citation66]. (4) Nuclear-encoded ncRNAs including lncRNA RMRP and MALAT1 can function as ‘anterograde signals’ to regulate mitochondrial gene expression and function, and mtDNA-encoded ncRNAs including ASncmtRNAs and mito-ncR-805 can function as ‘retrograde signals’ to regulate nuclear gene expression and protein activity. Communication between the mitochondria and the host nucleus through anterograde and retrograde signals regulated by mt-ncRNAs deserves further research. (5) The presence of post-transcriptional modifications in mt-ncRNAs requires further investigation. (6) Nuclear DNA has significant homology with mtDNA, and nuclear mtDNA sequences are called NUMTs [Citation144]. Therefore, differentiating the genome origin may be a problem. Although some studies have demonstrated that small mitochondrial RNAs are transcribed by the mitochondrial genome rather than by NUMTs using different methods [Citation51,Citation145], it is still an attractive and challenging question whether NUMTs can produce ncRNAs. (7) Nuclear genome editing is feasible at present; however, very little is known about mitochondrial genome editing. An interesting question is whether we can achieve mitochondrial genome editing using our previously constructed mito-NP platform? (8) Although mitochondria have an independent genome, the existence of multiple copies of mtDNA raises the question whether copy numbers have a potential effect on mitochondrial function.
Concluding remarks
The discovery of ncRNAs contributes a new layer of complexity to the molecular architecture of human diseases, because ncRNAs exhibit the ability to regulate numerous aspects of cellular biology and disease pathogenesis. Despite the fact that mt-ncRNAs which have been discovered are just a tip of the iceberg, their critical functions have emerged.
Several studies have found that mt-ncRNAs perform diverse functions, ranging from mitochondrial metabolism and mitochondrial function to tumour growth. However, there are many gaps in our current understanding the function of mt-ncRNAs. Further research is warranted to yield crucial insights into the molecular pathogenesis of numerous mt-ncRNAs-associated human diseases and promote efficient prevention and treatment of such diseases based on mt-ncRNAs-based targets.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Anderson S, Bankier AT, Barrell BG, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290(5806):457–465. .
- Zhao Q, Liu J, Deng H, et al. Targeting mitochondria-located circRNA SCAR alleviates NASH via reducing mROS output. Cell. 2020;183(1):76–93. e22.
- Leucci E, Vendramin R, Spinazzi M, et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature. 2016;531(7595):518–522. .
- Burzio VA, Villota C, Villegas J, et al. Expression of a family of noncoding mitochondrial RNAs distinguishes normal from cancer cells. Proc Natl Acad Sci U S A. 2009;106(23):9430–9434.
- Zhang X, Zuo X, Yang B, et al. MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell. 2014;158(3):607–619.
- Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338(6113):1435–1439.
- Vendramin R, Marine JC, Leucci E. Non-coding RNAs: the dark side of nuclear-mitochondrial communication. EMBO J. 2017;36(9):1123–1133.
- Cavalcante GC, Magalhães L, Ribeiro-dos-santos Â, et al. Mitochondrial epigenetics: non-Coding RNAs as a novel layer of complexity. Int J Mol Sci. 2020;21(5):5. .
- Bandiera S, Matégot R, Girard M, et al. MitomiRs delineating the intracellular localization of microRNAs at mitochondria. Free Radic Biol Med. 2013;64:12–19.
- Baradan R, Hollander JM, Das S. Mitochondrial miRNAs in diabetes: just the tip of the iceberg. Can J Physiol Pharmacol. 2017;95(10):1156–1162.
- Srinivasan H, Das S. Mitochondrial miRNA (MitomiR): a new player in cardiovascular health. Can J Physiol Pharmacol. 2015;93(10):855–861.
- Ortega MA, Fraile-Martínez O, Guijarro LG, et al. The regulatory role of mitochondrial microRNAs (MitomiRs) in breast cancer: translational implications present and future. Cancers (Basel). 2020;12(9):2443. .
- Zhao Y, Sun L, Wang RR, et al. The effects of mitochondria-associated long noncoding RNAs in cancer mitochondria: new players in an old arena. Crit Rev Oncol Hematol. 2018;131:76–82.
- Dong Y, Yoshitomi T, Hu J-F, et al. Long noncoding RNAs coordinate functions between mitochondria and the nucleus. Epigenetics Chromatin. 2017;10(1):41.
- Nakayama Y, Fujiu K, Yuki R, et al. A long noncoding RNA regulates inflammation resolution by mouse macrophages through fatty acid oxidation activation. Proc Natl Acad Sci U S A. 2020;117(25):14365–14375.
- Sang L, Ju H-Q, Yang Z, et al. Mitochondrial long non-coding RNA GAS5 tunes TCA metabolism in response to nutrient stress. Nat Metab. 2021;3(1):90–106.
- Landerer E, Villegas J, Burzio VA, et al. Nuclear localization of the mitochondrial ncRNAs in normal and cancer cells. Cell Oncol (Dordr). 2011;34(4):297–305.
- Fan L, Wu D, Goremykin V, et al. Phylogenetic analyses with systematic taxon sampling show that mitochondria branch within Alphaproteobacteria. Nat Ecol Evol. 2020;4(9):1213–1219.
- Roger AJ, Munoz-Gomez SA, Kamikawa R, et al. Diversification of mitochondria. Curr Biol. 2017;27(21):R1177–R1192.
- Hutchison CA 3rd, Newbold JE, Potter SS, et al. Maternal inheritance of mammalian mitochondrial DNA. Nature. 1974;251(5475):536–538.
- Sutovsky P, Moreno RD, Ramalho-Santos J, et al. Ubiquitin tag for sperm mitochondria. Nature. 1999;402(6760):371–372.
- Song WH, Yi Y-J, Sutovsky M, et al. Autophagy and ubiquitin-proteasome system contribute to sperm mitophagy after mammalian fertilization. Proc Natl Acad Sci U S A. 2016;113(36):E5261–70.
- Rojansky R, Cha MY, Chan DC. Elimination of paternal mitochondria in mouse embryos occurs through autophagic degradation dependent on PARKIN and MUL1. Elife. 2016;5. DOI:https://doi.org/10.7554/eLife.17896
- Luo SM, Ge Z-J, Wang Z-W, et al. Unique insights into maternal mitochondrial inheritance in mice. Proc Natl Acad Sci U S A. 2013;110(32):13038–13043.
- Clayton DA. Transcription and replication of mitochondrial DNA. Hum Reprod. 2000;15(Suppl 2):11–17.
- Morozov YI, Parshin AV, Agaronyan K, et al. A model for transcription initiation in human mitochondria. Nucleic Acids Res. 2015;43(7):3726–3735.
- Hillen HS, Parshin AV, Agaronyan K, et al. Mechanism of Transcription Anti-termination in Human Mitochondria. Cell. 2017;171(5):1082–1093. e13.
- Asin-Cayuela J, Schwend T, Farge G, et al. The human mitochondrial transcription termination factor (mTERF) is fully active in vitro in the non-phosphorylated form. J Biol Chem. 2005;280(27):25499–25505.
- Brown A, Amunts A, Bai X-C, et al. Structure of the large ribosomal subunit from human mitochondria. Science. 2014;346(6210):718–722.
- Khawaja A, Itoh Y, Remes C, et al. Distinct pre-initiation steps in human mitochondrial translation. Nat Commun. 2020;11(1):2932.
- Schwartzbach CJ, Spremulli LL. Interaction of animal mitochondrial EF-Tu.EF-Ts with aminoacyl-tRNA, guanine nucleotides, and ribosomes. J Biol Chem. 1991;266(25):16324–16330.
- Soleimanpour-Lichaei HR, Kühl I, Gaisne M, et al. mtRF1a is a human mitochondrial translation release factor decoding the major termination codons UAA and UAG. Mol Cell. 2007;27(5):745–757.
- Tsuboi M, Morita H, Nozaki Y, et al. EF-G2mt is an exclusive recycling factor in mammalian mitochondrial protein synthesis. Mol Cell. 2009;35(4):502–510.
- Gusic M, Prokisch H. ncRNAs: new players in mitochondrial health and disease? Front Genet. 2020;11:95.
- Geiger J, Dalgaard LT. Interplay of mitochondrial metabolism and microRNAs. Cell Mol Life Sci. 2017;74(4):631–646.
- Jusic A, Devaux Y, Action EU-C-C. Mitochondrial noncoding RNA-regulatory network in cardiovascular disease. Basic Res Cardiol. 2020;115(3):23.
- Ozata DM, Gainetdinov I, Zoch A, et al. PIWI-interacting RNAs: small RNAs with big functions. Nat Rev Genet. 2019;20(2):89–108.
- Zhang XO, Wang H-B, Zhang Y, et al. Complementary sequence-mediated exon circularization. Cell. 2014;159(1):134–147.
- Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016;17(4):205–211.
- Kristensen LS, Andersen MS, Stagsted LVW, et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–691.
- Jeck WR, Sorrentino JA, Wang K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19(2):141–157.
- Salzman J, Chen RE, Olsen MN, et al. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9(9):e1003777.
- Wu Z, Sun H, Wang C, et al. Mitochondrial genome-derived circRNA mc-COX2 functions as an oncogene in chronic lymphocytic leukemia. Mol Ther Nucleic Acids. 2020;20:801–811.
- Liu X, Wang X, Li J, et al. Identification of mecciRNAs and their roles in the mitochondrial entry of proteins. Sci China Life Sci. 2020;63(10):1429–1449.
- Gao Y, Wu M, Fan Y, et al. Identification and characterization of circular RNAs in Qinchuan cattle testis. R Soc Open Sci. 2018;5(7):180413.
- Zhang J, Zhang X, Li C, et al. Circular RNA profiling provides insights into their subcellular distribution and molecular characteristics in HepG2 cells. RNA Biol. 2019;16(2):220–232.
- Mance LG, Mawla I, Shell SM, et al. Mitochondrial mRNA fragments are circularized in a human HEK cell line. Mitochondrion. 2020;51:1–6.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297.
- Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37.
- Bandiera S, Rüberg S, Girard M, et al. Nuclear outsourcing of RNA interference components to human mitochondria. PLoS One. 2011;6(6):e20746.
- Ro S, Ma H-Y, Park C, et al. The mitochondrial genome encodes abundant small noncoding RNAs. Cell Res. 2013;23(6):759–774.
- Das S, Ferlito M, Kent OA, et al. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res. 2012;110(12):1596–1603.
- Das S, Bedja D, Campbell N, et al. miR-181c regulates the mitochondrial genome, bioenergetics, and propensity for heart failure in vivo. PLoS One. 2014;9(5):e96820.
- Jagannathan R, Thapa D, Nichols CE, et al. Translational regulation of the mitochondrial genome following redistribution of mitochondrial microRNA in the diabetic heart. Circ Cardiovasc Genet. 2015;8(6):785–802.
- Shepherd DL, Hathaway QA, Pinti MV, et al. Exploring the mitochondrial microRNA import pathway through polynucleotide phosphorylase (PNPase). J Mol Cell Cardiol. 2017;110:15–25.
- Li H, Dai B, Fan J, et al. The different roles of miRNA-92a-2-5p and let-7b-5p in MITOCHONDRIAL TRANSLATION IN db/db Mice. Mol Ther Nucleic Acids. 2019;17:424–435.
- Li H, Zhang X, Wang F, et al. MicroRNA-21 lowers blood pressure in spontaneous hypertensive rats by upregulating mitochondrial translation. Circulation. 2016;134(10):734–751.
- Chen W, Wang P, Lu Y, et al. Decreased expression of mitochondrial miR-5787 contributes to chemoresistance by reprogramming glucose metabolism and inhibiting MT-CO3 translation. Theranostics. 2019;9(20):5739–5754.
- Fan S, Tian T, Chen W, et al. Mitochondrial miRNA Determines chemoresistance by reprogramming metabolism and regulating mitochondrial transcription. Cancer Res. 2019;79(6):1069–1084.
- Barrey E, Saint-Auret G, Bonnamy B, et al. Pre-microRNA and mature microRNA in human mitochondria. PLoS One. 2011;6(5):e20220.
- Sripada L, Tomar D, Prajapati P, et al. Systematic analysis of small RNAs associated with human mitochondria by deep sequencing: detailed analysis of mitochondrial associated miRNA. PLoS One. 2012;7(9):e44873.
- Vargas JNNS, Kar AN, Kowalak JA, et al. Axonal localization and mitochondrial association of precursor microRNA 338. Cell Mol Life Sci. 2016;73(22):4327–4340.
- Blumental-Perry A, Jobava R, Bederman I, et al. Retrograde signaling by a mtDNA-encoded non-coding RNA preserves mitochondrial bioenergetics. Commun Biol. 2020;3(1):626.
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–914.
- Zhang X, Hong R, Chen W, et al. The role of long noncoding RNA in major human disease. Bioorg Chem. 2019;92:103214.
- Noh JH, Kim KM, Abdelmohsen K, et al. HuR and GRSF1 modulate the nuclear export and mitochondrial localization of the lncRNA RMRP. Genes Dev. 2016;30(10):1224–39.
- Li K, Smagula CS, Parsons WJ, et al. Subcellular partitioning of MRP RNA assessed by ultrastructural and biochemical analysis. J Cell Biol. 1994;124(6):871–882.
- Wang G, Chen H-W, Oktay Y, et al. PNPASE regulates RNA import into mitochondria. Cell. 2010;142(3):456–467.
- Antonicka H, Sasarman F, Nishimura T, et al. The mitochondrial RNA-binding protein GRSF1 localizes to RNA granules and is required for posttranscriptional mitochondrial gene expression. Cell Metab. 2013;17(3):386–398.
- Jourdain AA, Koppen M, Wydro M, et al. GRSF1 regulates RNA processing in mitochondrial RNA granules. Cell Metab. 2013;17(3):399–410.
- Jourdain AA, Boehm E, Maundrell K, et al. Mitochondrial RNA granules: compartmentalizing mitochondrial gene expression. J Cell Biol. 2016;212(6):611–614.
- Fogal V, Richardson AD, Karmali PP, et al. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol Cell Biol. 2010;30(6):1303–1318.
- Vendramin R, Verheyden Y, Ishikawa H, et al. SAMMSON fosters cancer cell fitness by concertedly enhancing mitochondrial and cytosolic translation. Nat Struct Mol Biol. 2018;25(11):1035–1046.
- Sheng L, Ye L, Zhang D, et al. New Insights Into the Long Non-coding RNA SRA: physiological functions and mechanisms of action. Front Med (Lausanne). 2018;5:244.
- Shi Y, Downes M, Xie W, et al. Sharp, an inducible cofactor that integrates nuclear receptor repression and activation. Genes Dev. 2001;15(9):1140–1151. .
- Hatchell EC, Colley SM, Beveridge DJ, et al. SLIRP, a small SRA binding protein, is a nuclear receptor corepressor. Mol Cell. 2006;22(5):657–668.
- Colley SM, Leedman PJ. SRA and its binding partners: an expanding role for RNA-binding coregulators in nuclear receptor-mediated gene regulation. Crit Rev Biochem Mol Biol. 2009;44(1):25–33.
- Sun Y, Ma L. New Insights into long non-coding RNA MALAT1 in cancer and metastasis. Cancers (Basel. 2019;11(2):216.
- Zhao Y, Liu S, Zhou L, et al. Aberrant shuttling of long noncoding RNAs during the mitochondria-nuclear crosstalk in hepatocellular carcinoma cells. Am J Cancer Res. 2019;9(5):999–1008.
- Zhao Y, Zhou L, Li H, et al. Nuclear-Encoded lncRNA MALAT1 epigenetically controls metabolic reprogramming in HCC cells through the mitophagy pathway. Mol Ther Nucleic Acids. 2021;23:264–276.
- Villegas J, Zárraga AM, Muller I, et al. A novel chimeric mitochondrial RNA localized in the nucleus of mouse sperm. DNA Cell Biol. 2000;19(9):579–588.
- Villegas J, Burzio V, Villota C, et al. Expression of a novel non-coding mitochondrial RNA in human proliferating cells. Nucleic Acids Res. 2007;35(21):7336–7347.
- Vidaurre S, Fitzpatrick C, Burzio VA, et al. Down-regulation of the antisense mitochondrial non-coding RNAs (ncRNAs) Is a unique vulnerability of cancer cells and a potential target for cancer therapy. J Biol Chem. 2014;289(39):27182–27198.
- Villegas J. The mitochondrial antisense ncRNAs are down-regulated in early cervical carcinoma. J Cancer Sci Ther. 2012;01(S7). DOI:https://doi.org/10.4172/1948-5956.S7-004
- Dadlani K, Lopez C. Assessment of the expression of long noncoding mitochondrial RNAs (lncmtRNAs) during cervical cancer progression and cervical carcinoma. J Cancer Sci Ther. 2016;08(2). DOI:https://doi.org/10.4172/1948-5956.1000386
- Fitzpatrick C, Bendek MF, Briones M, et al. Mitochondrial ncRNA targeting induces cell cycle arrest and tumor growth inhibition of MDA-MB-231 breast cancer cells through reduction of key cell cycle progression factors. Cell Death Dis. 2019;10(6):423.
- Lobos-Gonzalez L, Bustos R, Campos A, et al. Exosomes released upon mitochondrial ASncmtRNA knockdown reduce tumorigenic properties of malignant breast cancer cells. Sci Rep. 2020;10(1):343.
- Borgna V, Villegas J, Burzio VA, et al. Mitochondrial ASncmtRNA-1 and ASncmtRNA-2 as potent targets to inhibit tumor growth and metastasis in the RenCa murine renal adenocarcinoma model. Oncotarget. 2017;8(27):43692–43708.
- Borgna V, Lobos-González L, Guevara F, et al. Targeting antisense mitochondrial noncoding RNAs induces bladder cancer cell death and inhibition of tumor growth through reduction of survival and invasion factors. J Cancer. 2020;11(7):1780–1791.
- Rivas A, Burzio V, Landerer E, et al. Determination of the differential expression of mitochondrial long non-coding RNAs as a noninvasive diagnosis of bladder cancer. BMC Urol. 2012;12:37.
- Lobos-Gonzalez L, Silva V, Araya M, et al. Targeting antisense mitochondrial ncRNAs inhibits murine melanoma tumor growth and metastasis through reduction in survival and invasion factors. Oncotarget. 2016;7(36):58331–58350.
- Varas-Godoy M, Lladser A, Farfan N, et al. In vivo knockdown of antisense non-coding mitochondrial RNAs by a lentiviral-encoded shRNA inhibits melanoma tumor growth and lung colonization. Pigment Cell Melanoma Res. 2018;31(1):64–72.
- Bianchessi V, Badi I, Bertolotti M, et al. The mitochondrial lncRNA ASncmtRNA-2 is induced in aging and replicative senescence in endothelial cells. J Mol Cell Cardiol. 2015;81:62–70.
- Mercer TR, Neph S, Dinger M, et al. The human mitochondrial transcriptome. Cell. 2011;146(4):645–658.
- Rackham O, Shearwood A-MJ, Mercer TR, et al. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA. 2011;17(12):2085–2093.
- Gao S, Tian X, Chang H, et al. Two novel lncRNAs discovered in human mitochondrial DNA using PacBio full-length transcriptome data. Mitochondrion. 2018;38:41–47.
- Kumarswamy R, Bauters C, Volkmann I, et al. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res. 2014;114(10):1569–75.
- Dorn GW 2nd. LIPCAR: a mitochondrial lnc in the noncoding RNA chain? Circ Res. 2014;114(10):1548–1550.
- Santer L, López B, Ravassa S, et al. Circulating long noncoding RNA LIPCAR predicts heart failure outcomes in patients without chronic kidney disease. Hypertension. 2019;73(4):820–828.
- Zhang Z, Gao W, Long -Q-Q, et al. Increased plasma levels of lncRNA H19 and LIPCAR are associated with increased risk of coronary artery disease in a Chinese population. Sci Rep. 2017;7(1):7491.
- Aloni Y, Attardi G. Symmetrical in vivo transcription of mitochondrial DNA in HeLa cells. Proc Natl Acad Sci U S A. 1971;68(8):1757–1761.
- Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290(5806):470–474.
- Holzmann J, Frank P, Löffler E, et al. RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell. 2008;135(3):462–474.
- Brzezniak LK, Bijata M, Szczesny RJ, et al. Involvement of human ELAC2 gene product in 3ʹ end processing of mitochondrial tRNAs. RNA Biol. 2011;8(4):616–626.
- D’Souza AR, Minczuk M, Garone C. Mitochondrial transcription and translation: overview. Essays Biochem. 2018;62(3):309–320.
- Boccaletto P, Machnicka MA, Purta E, et al. MODOMICS: a database of RNA modification pathways. Nucleic Acids Res. 2018;46(D1):D303–D307.
- Laptev I, Dontsova O, Sergiev P. Epitranscriptomics of mammalian mitochondrial ribosomal RNA. Cells. 2020;9(10):2181.
- Suzuki T, Yashiro Y, Kikuchi I, et al. Complete chemical structures of human mitochondrial tRNAs. Nat Commun. 2020;11(1):4269.
- Lusic H, Gustilo EM, Vendeix FAP, et al. Synthesis and investigation of the 5-formylcytidine modified, anticodon stem and loop of the human mitochondrial tRNAMet. Nucleic Acids Res. 2008;36(20):6548–6557.
- Cantara WA, Murphy FV, Demirci H, et al. Expanded use of sense codons is regulated by modified cytidines in tRNA. Proc Natl Acad Sci U S A. 2013;110(27):10964–10969.
- Bohnsack MT, Sloan KE. The mitochondrial epitranscriptome: the roles of RNA modifications in mitochondrial translation and human disease. Cell Mol Life Sci. 2018;75(2):241–260.
- Rebelo-Guiomar P, Powell CA, Van Haute L, et al. The mammalian mitochondrial epitranscriptome. Biochim Biophys Acta Gene Regul Mech. 2019;1862(3):429–446.
- Stuart JW, Gdaniec Z, Guenther R, et al. Functional anticodon architecture of human tRNALys3 includes disruption of intraloop hydrogen bonding by the naturally occurring amino acid modification, t6A. Biochemistry. 2000;39(44):13396–13404. .
- Urbonavicius J, Qian Q, Durand JM, et al. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 2001;20(17):4863–4873. .
- Rorbach J, Gao F, Powell CA, et al. Human mitochondrial ribosomes can switch their structural RNA composition. Proc Natl Acad Sci U S A. 2016;113(43):12198–12201.
- Antonicka H, Choquet K, Lin Z-Y, et al. A pseudouridine synthase module is essential for mitochondrial protein synthesis and cell viability. EMBO Rep. 2017;18(1):28–38.
- Li X, Xiong X, Zhang M, et al. Base-resolution mapping reveals distinct m(1)A methylome in nuclear- and mitochondrial-encoded transcripts. Mol Cell. 2017;68(5):993–1005. e9.
- Safra M, Sas-Chen A, Nir R, et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551(7679):251–255.
- Doudna JA, Charpentier E, Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096.
- Gammage PA, Moraes CT, Minczuk M. Mitochondrial genome engineering: the revolution may not be CRISPR-Ized. Trends Genet. 2018;34(2):101–110.
- Loutre R, Heckel A-M, Smirnova A, et al. Can mitochondrial DNA be CRISPRized: pro and contra. IUBMB Life. 2018;70(12):1233–1239.
- Jo A, Ham S, Lee GH, et al. Efficient mitochondrial genome editing by CRISPR/Cas9. Biomed Res Int. 2015;2015:305716.
- Moretton A, Morel F, Macao B, et al. Selective mitochondrial DNA degradation following double-strand breaks. PLoS One. 2017;12(4):e0176795.
- Hagstrom E, Freyer C, Battersby BJ, et al. No recombination of mtDNA after heteroplasmy for 50 generations in the mouse maternal germline. Nucleic Acids Res. 2014;42(2):1111–1116.
- Srivastava S, Moraes CT. Manipulating mitochondrial DNA heteroplasmy by a mitochondrially targeted restriction endonuclease. Hum Mol Genet. 2001;10(26):3093–3099.
- Tanaka M, Borgeld H-J, Zhang J, et al. Gene therapy for mitochondrial disease by delivering restriction endonuclease SmaI into mitochondria. J Biomed Sci. 2002;9(6 Pt 1):534–541.
- Alexeyev MF, Venediktova N, Pastukh V, et al. Selective elimination of mutant mitochondrial genomes as therapeutic strategy for the treatment of NARP and MILS syndromes. Gene Ther. 2008;15(7):516–523.
- Srivastava S, Moraes CT. Double-strand breaks of mouse muscle mtDNA promote large deletions similar to multiple mtDNA deletions in humans. Hum Mol Genet. 2005;14(7):893–902.
- Bacman SR, Williams SL, Garcia S, et al. Organ-specific shifts in mtDNA heteroplasmy following systemic delivery of a mitochondria-targeted restriction endonuclease. Gene Ther. 2010;17(6):713–720.
- Bacman SR, Williams SL, Duan D, et al. Manipulation of mtDNA heteroplasmy in all striated muscles of newborn mice by AAV9-mediated delivery of a mitochondria-targeted restriction endonuclease. Gene Ther. 2012;19(11):1101–1106.
- Gammage PA, Gaude E, Van Haute L, et al. Near-complete elimination of mutant mtDNA by iterative or dynamic dose-controlled treatment with mtZFNs. Nucleic Acids Res. 2016;44(16):7804–7816.
- Gammage PA, Rorbach J, Vincent AI, et al. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol Med. 2014;6(4):458–466.
- Gammage PA, Viscomi C, Simard M-L, et al. Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat Med. 2018;24(11):1691–1695.
- Minczuk M, Papworth MA, Miller JC, et al. Development of a single-chain, quasi-dimeric zinc-finger nuclease for the selective degradation of mutated human mitochondrial DNA. Nucleic Acids Res. 2008;36(12):3926–3938.
- Bacman SR, Kauppila JHK, Pereira CV, et al. MitoTALEN reduces mutant mtDNA load and restores tRNA(Ala) levels in a mouse model of heteroplasmic mtDNA mutation. Nat Med. 2018;24(11):1696–1700.
- Bacman SR, Williams SL, Pinto M, et al. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat Med. 2013;19(9):1111–1113.
- Hashimoto M, Bacman SR, Peralta S, et al. MitoTALEN: a general approach to reduce mutant mtDNA loads and restore oxidative phosphorylation function in mitochondrial diseases. Mol Ther. 2015;23(10):1592–1599.
- Reddy P, Ocampo A, Suzuki K, et al. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell. 2015;161(3):459–469.
- Yang Y, Wu H, Kang X, et al. Targeted elimination of mutant mitochondrial DNA in MELAS-iPSCs by mitoTALENs. Protein Cell. 2018;9(3):283–297.
- Mok BY, De Moraes MH, Zeng J, et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature. 2020;583(7817):631–637.
- Liew SS, Qin X, Zhou J, et al. Smart design of nanomaterials for mitochondria-targeted nanotherapeutics. Angew Chem Int Ed Engl. 2021;60(5):2232–2256.
- Oladimeji O, Akinyelu J, Singh M. Nanomedicines for subcellular targeting: the mitochondrial perspective. Curr Med Chem. 2020;27(33):5480–5509.
- Li X, Yang L, Chen LL, et al. Functions, and challenges of circular RNAs. Mol Cell. 2018;71(3):428–442.
- Woischnik M, Moraes CT. Pattern of organization of human mitochondrial pseudogenes in the nuclear genome. Genome Res. 2002;12(6):885–893.
- Pozzi A, Dowling DK, Sloan D. The genomic origins of small mitochondrial RNAs: are they transcribed by the mitochondrial DNA or by mitochondrial pseudogenes within the nucleus (NUMTs)? Genome Biol Evol. 2019;11(7):1883–1896.
- Colley SM, Iyer KR, Leedman PJ. The RNA coregulator SRA, its binding proteins and nuclear receptor signaling activity. IUBMB Life. 2008;60(3):159–164.
- Vilardo E, Rossmanith W. Molecular insights into HSD10 disease: impact of SDR5C1 mutations on the human mitochondrial RNase P complex. Nucleic Acids Res. 2015;43(10):5112–5119.
- Metodiev MD, Thompson K, Alston C, et al. Recessive mutations in TRMT10C cause defects in mitochondrial RNA processing and multiple respiratory chain deficiencies. Am J Hum Genet. 2016;98(5):993–1000.
- Davarniya B, Hu H, Kahrizi K, et al. The role of a novel TRMT1 gene mutation and rare GRM1 gene defect in intellectual disability in two Azeri families. PLoS One. 2015;10(8):e0129631.
- Najmabadi H, Hu H, Garshasbi M, et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478(7367):57–63.
- Dewe JM, Fuller BL, Lentini JM, et al. TRMT1-catalyzed tRNA modifications are required for redox homeostasis to ensure proper cellular proliferation and oxidative stress survival. Mol Cell Biol. 2017;37(21). DOI:https://doi.org/10.1128/MCB.00214-17.
- Fernandez-Vizarra E, Berardinelli A, Valente L, et al. Nonsense mutation in pseudouridylate synthase 1 (PUS1) in two brothers affected by myopathy, lactic acidosis and sideroblastic anaemia (MLASA). J Med Genet. 2007;44(3):173–180.
- Bykhovskaya Y, Casas K, Mengesha E, et al. Missense mutation in pseudouridine synthase 1 (PUS1) causes mitochondrial myopathy and sideroblastic anemia (MLASA). Am J Hum Genet. 2004;74(6):1303–1308.
- Patton JR, Bykhovskaya Y, Mengesha E, et al. Mitochondrial myopathy and sideroblastic anemia (MLASA): missense mutation in the pseudouridine synthase 1 (PUS1) gene is associated with the loss of tRNA pseudouridylation. J Biol Chem. 2005;280(20):19823–19828.
- Villarroya M, Prado S, Esteve JM, et al. Characterization of human GTPBP3, a GTP-binding protein involved in mitochondrial tRNA modification. Mol Cell Biol. 2008;28(24):7514–7531.
- Kirino Y, Goto Y-I, Campos Y, et al. Specific correlation between the wobble modification deficiency in mutant tRNAs and the clinical features of a human mitochondrial disease. Proc Natl Acad Sci U S A. 2005;102(20):7127–7132.
- Yasukawa T, Suzuki T, Ishii N, et al. Defect in modification at the anticodon wobble nucleotide of mitochondrial tRNA(Lys) with the MERRF encephalomyopathy pathogenic mutation. FEBS Lett. 2000;467(2–3):175–178.
- Kopajtich R, Nicholls T, Rorbach J, et al. Mutations in GTPBP3 cause a mitochondrial translation defect associated with hypertrophic cardiomyopathy, lactic acidosis, and encephalopathy. Am J Hum Genet. 2014;95(6):708–720.
- Chen D, Zhang Z, Chen C, et al. Deletion of Gtpbp3 in zebrafish revealed the hypertrophic cardiomyopathy manifested by aberrant mitochondrial tRNA metabolism. Nucleic Acids Res. 2019;47(10):5341–5355.
- Ghezzi D, Baruffini E, Haack T, et al. Mutations of the mitochondrial-tRNA modifier MTO1 cause hypertrophic cardiomyopathy and lactic acidosis. Am J Hum Genet. 2012;90(6):1079–1087.
- Baruffini E, Dallabona C, Invernizzi F, et al. MTO1 mutations are associated with hypertrophic cardiomyopathy and lactic acidosis and cause respiratory chain deficiency in humans and yeast. Hum Mutat. 2013;34(11):1501–1509.
- Guan MX, Yan Q, Li X, et al. Mutation in TRMU related to transfer RNA modification modulates the phenotypic expression of the deafness-associated mitochondrial 12S ribosomal RNA mutations. Am J Hum Genet. 2006;79(2):291–302.
- Umeda N, Suzuki T, Yukawa M, et al. Mitochondria-specific RNA-modifying enzymes responsible for the biosynthesis of the wobble base in mitochondrial tRNAs. Implications for the molecular pathogenesis of human mitochondrial diseases. J Biol Chem. 2005;280(2):1613–1624.
- Zeharia A, Shaag A, Pappo O, et al. Acute infantile liver failure due to mutations in the TRMU gene. Am J Hum Genet. 2009;85(3):401–407.
- Haag S, Sloan KE, Ranjan N, et al. NSUN3 and ABH1 modify the wobble position of mt-tRNAMet to expand codon recognition in mitochondrial translation. EMBO J. 2016;35(19):2104–2119.
- Nakano S, Suzuki T, Kawarada L, et al. NSUN3 methylase initiates 5-formylcytidine biogenesis in human mitochondrial tRNA(Met). Nat Chem Biol. 2016;12(7):546–551.
- Kawarada L, Suzuki T, Ohira T, et al. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017;45(12):7401–7415.
- Boland C, Hayes P, Santa-Maria I, et al. Queuosine formation in eukaryotic tRNA occurs via a mitochondria-localized heteromeric transglycosylase. J Biol Chem. 2009;284(27):18218–18227.
- Khalique A, Mattijssen S, Haddad AF, et al. Targeting mitochondrial and cytosolic substrates of TRIT1 isopentenyltransferase: specificity determinants and tRNA-i6A37 profiles. PLoS Genet. 2020;16(4):e1008330.
- Yarham JW, Lamichhane TN, Pyle A, et al. Defective i6A37 modification of mitochondrial and cytosolic tRNAs results from pathogenic mutations in TRIT1 and its substrate tRNA. PLoS Genet. 2014;10(6):e1004424.
- Kernohan KD, Dyment DA, Pupavac M, et al. Matchmaking facilitates the diagnosis of an autosomal-recessive mitochondrial disease caused by biallelic mutation of the tRNA isopentenyltransferase (TRIT1) gene. Hum Mutat. 2017;38(5):511–516.
- Wei F-YF-Y, Zhou B, Suzuki T, et al. Cdk5rap1-mediated 2-methylthio modification of mitochondrial tRNAs governs protein translation and contributes to myopathy in mice and humans. Cell Metab. 2015;21(3):428–442.
- Lin H, Miyauchi K, Harada T, et al. CO2-sensitive tRNA modification associated with human mitochondrial disease. Nat Commun. 2018;9(1):1875.
- Zhou JB, Wang Y, Zeng QY, et al. Molecular basis for t6A modification in human mitochondria. Nucleic Acids Res. 2020;48(6):3181–3194.
- Powell CA, Kopajtich R, D’Souza AR, et al. TRMT5 mutations cause a defect in post-transcriptional modification of mitochondrial tRNA associated with multiple respiratory-chain deficiencies. Am J Hum Genet. 2015;97(2):319–328.
- Tarnopolsky MA, Brady L, Tetreault M, et al. TRMT5 mutations are associated with features of complex hereditary spastic paraparesis. Neurology. 2017;89(21):2210–2211.
- Zaganelli S, Rebelo-Guiomar P, Maundrell K, et al. The pseudouridine synthase RPUSD4 is an essential component of mitochondrial RNA granules. J Biol Chem. 2017;292(11):4519–4532.
- Shinoda S, Kitagawa S, Nakagawa S, et al. Mammalian NSUN2 introduces 5-methylcytidines into mitochondrial tRNAs. Nucleic Acids Res. 2019;47(16):8734–8745.
- Van Haute L, Hendrick AG, D’Souza AR, et al. METTL15 introduces N4-methylcytidine into human mitochondrial 12S rRNA and is required for mitoribosome biogenesis. Nucleic Acids Res. 2019;47(19):10267–10281.
- Powell CA, Minczuk M. TRMT2B is responsible for both tRNA and rRNA m(5)U-methylation in human mitochondria. RNA Biol. 2020;17(4):451–462.
- Laptev I, Shvetsova E, Levitskii S, et al. Mouse Trmt2B protein is a dual specific mitochondrial metyltransferase responsible for m(5)U formation in both tRNA and rRNA. RNA Biol. 2020;17(4):441–450.
- Chujo T, Suzuki T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA. 2012;18(12):2269–2276.
- Sekar S, McDonald J, Cuyugan L, et al. Alzheimer’s disease is associated with altered expression of genes involved in immune response and mitochondrial processes in astrocytes. Neurobiol Aging. 2015;36(2):583–591.
- Kim J, Kwon J, Kim M, et al. Low-dielectric-constant polyimide aerogel composite films with low water uptake. Polym J. 2016;48(7):829–834.
- Chen H, Shi Z, Guo J, et al. The human mitochondrial 12S rRNA m(4)C methyltransferase METTL15 is required for mitochondrial function. J Biol Chem. 2020;295(25):8505–8513.
- Metodiev MD, Spåhr H, Loguercio Polosa P, et al. NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 2014;10(2):e1004110. .
- Seidel-Rogol BL, McCulloch V, Shadel GS. Human mitochondrial transcription factor B1 methylates ribosomal RNA at a conserved stem-loop. Nat Genet. 2003;33(1):23–24.
- Koeck T, Olsson AH, Nitert MD, et al. A common variant in TFB1M is associated with reduced insulin secretion and increased future risk of type 2 diabetes. Cell Metab. 2011;13(1):80–91.
- Sharoyko VV, Abels M, Sun J, et al. Loss of TFB1M results in mitochondrial dysfunction that leads to impaired insulin secretion and diabetes. Hum Mol Genet. 2014;23(21):5733–5749.
- Bar-Yaacov D, Frumkin I, Yashiro Y, et al. Mitochondrial 16S rRNA is methylated by tRNA methyltransferase TRMT61B in all vertebrates. PLoS Biol. 2016;14(9):e1002557. .
- Couch FJ, Kuchenbaecker KB, Michailidou K, et al. Identification of four novel susceptibility loci for oestrogen receptor negative breast cancer. Nat Commun. 2016;7(1):11375.
- Lee KW, Bogenhagen DF. Assignment of 2ʹ-O-methyltransferases to modification sites on the mammalian mitochondrial large subunit 16 S ribosomal RNA (rRNA). J Biol Chem. 2014;289(36):24936–24942.
- Lee KW, Okot-Kotber C, LaComb JF, et al. Mitochondrial ribosomal RNA (rRNA) methyltransferase family members are positioned to modify nascent rRNA in foci near the mitochondrial DNA nucleoid. J Biol Chem. 2013;288(43):31386–31399.
- Rorbach J, Boesch P, Gammage PA, et al. MRM2 and MRM3 are involved in biogenesis of the large subunit of the mitochondrial ribosome. Mol Biol Cell. 2014;25(17):2542–2555.
- Garone C, D’Souza AR, Dallabona C, et al. Defective mitochondrial rRNA methyltransferase MRM2 causes MELAS-like clinical syndrome. Hum Mol Genet. 2017;26(21):4257–4266.
