ABSTRACT
RNA-based enzyme RNase P is a ribonucleoprotein complex responsible primarily for 5’-maturation of tRNAs. S. cerevisiae RNase P comprises a catalytic RNA component and nine proteins. The assembly and maturation of S. cerevisiae RNase P involves an abundant and catalytically active precursor form, which includes all components except for proteins Rpr2 and Pop3. Rpr2 and Pop3 are essential proteins, but their roles in RNase P were not clear. Here we use a step-wise in vitro assembly of yeast RNase P to show that the addition of proteins Rpr2 and Pop3 increases the activity and thermal stability of the RNase P complex, similar to the effects previously observed for archaeal RNases P.
Introduction
RNA-based RNase P [Citation1] is a ubiquitous site-specific endoribonuclease primarily responsible for 5’-maturation of tRNAs. The RNA component of RNase P is the catalytic moiety of the enzyme [Citation2]. The core elements of the RNA component responsible for the pre-tRNA substrate recognition and cleavage are structurally conserved in all domains of life, whereas the protein complement differs significantly [Citation3–5]. The S. cerevisiae RNase P holoenzyme includes RPR1 RNA (369 nucleotides-long) and 9 protein components: Pop1 (100.5 kDa), Pop3 (22.6 kDa), Pop4 (32.9 kDa), Pop5 (19.6 kDa), Pop6 (18.2 kDa), Pop7 (15.8 kDa), Pop8 (15.5 kDa), Rpp1 (32.2 kDa, 2 copies), and Rpr2 (16.3 kDa). Yeast RNase P shares all of its proteins except for Rpr2 with its sister enzyme, RNase MRP [Citation6]. All RNase P proteins are essential in yeast [Citation6].
A partial stepwise reconstruction of yeast RNase P RNP including all components except for proteins Rpr2 and Pop3 has been previously described [Citation7]. This reconstruction, combined with the cryo-EM structure of the enzyme [Citation8], has allowed the characterization of the structural and functional roles of most of the yeast RNase P proteins. The largest yeast RNase P protein Pop1 serves as the major structural brace stabilizing the global fold of RNase P RNA and is required for the catalytic activity of RNA in vitro [Citation7–9]. Proteins Pop6 and Pop7 form a heterodimer that mediates additional interactions between Pop1 and RNA [Citation7–10]. Protein Pop4 bridges the catalytic and specificity domains of RNase P RNA [Citation8] and dramatically increases the activity of the RNP in vitro [Citation7]. Proteins Pop5, Pop8, and 2 copies of Rpp1 form a heterotetramer that extensively interacts with RNase P RNA and contributes to the formation of the substrate-binding pocket; Pop5 and Rpp1 are required for the RNP activity, while the presence of Pop8 considerably increases it [Citation7,Citation8]. Rpr2 and Pop3 bind at the periphery of the complex; they were absent in the RNase P reconstruction experiments [Citation7] and their effect on the activity of the yeast enzyme was unknown.
The assembly and maturation of yeast RNase P involves a precursor that is missing peripheral protein components Rpr2 and Pop3 [Citation11] (). The precursor form constitutes 10–20% of all cellular RNase P and is catalytically active [Citation11]. The roles of yeast Rpr2 and Pop3 (which are essential for the viability of the cell [Citation6]) were not clear. Here we used in vitro reconstruction of RNase P to investigate the effects of the addition of Rpr2 and Pop3 on the activity and thermal stability of S. cerevisiae RNase P.
Figure 1. Formation of the mature form of yeast RNase P from the precursor molecule involves the binding of proteins Rpr2 and Pop3. RNA is shown in grey. Figure is based on 6ah3.pdb [Citation8].
![Figure 1. Formation of the mature form of yeast RNase P from the precursor molecule involves the binding of proteins Rpr2 and Pop3. RNA is shown in grey. Figure is based on 6ah3.pdb [Citation8].](/cms/asset/2254ddcc-5523-4708-b318-cc12b7480f02/krnb_a_2201110_f0001_oc.jpg)
Materials and methods
The components of RNase P were produced as previously described [Citation7]. All proteins were overexpressed in Escherichia coli ().
Figure 2. Isolation of protein components of yeast RNase P. Protein Pop1 was co-expressed with Pop4, but purified separately [Citation7,Citation9]; proteins Rpr2, Pop3, and Pop4 were expressed and purified individually [Citation7]; complexes Pop6/Pop7 and 2×Rpp1/Pop5/Pop8 were obtained using co-expression of corresponding protein components [Citation7,Citation10]. Lanes 1, 8: size marker; lane 2: Rpr2; lane 3: Pop 6/Pop7 complex; lane 4: Pop3; lane 5: Pop4; lane 6: 2×Rpp1/Pop5/Pop8 complex; lane 7: Pop1. Coomassie Blue-stained SDS-polyacrylamide gel.
![Figure 2. Isolation of protein components of yeast RNase P. Protein Pop1 was co-expressed with Pop4, but purified separately [Citation7,Citation9]; proteins Rpr2, Pop3, and Pop4 were expressed and purified individually [Citation7]; complexes Pop6/Pop7 and 2×Rpp1/Pop5/Pop8 were obtained using co-expression of corresponding protein components [Citation7,Citation10]. Lanes 1, 8: size marker; lane 2: Rpr2; lane 3: Pop 6/Pop7 complex; lane 4: Pop3; lane 5: Pop4; lane 6: 2×Rpp1/Pop5/Pop8 complex; lane 7: Pop1. Coomassie Blue-stained SDS-polyacrylamide gel.](/cms/asset/5a51065b-9265-4059-9c2b-30297e1c743e/krnb_a_2201110_f0002_b.gif)
Proteins Pop4, Pop3, Rpr2 were fused to the C-terminus of maltose-binding protein (MBP) via a linker containing a tobacco etch virus (TEV) protease cleavage site. The MBP tag was used for the purification of these proteins and removed using TEV protease.
Proteins Rpp1, Pop5, Pop8 were co-expressed from a single polycistronic construct and purified as a 2×Rpp1, Pop5, Pop8 tetramer [Citation7].
Proteins Pop6, Pop7 were co-expressed and purified as a heterodimer [Citation10].
To obtain protein Pop1, it was co-expressed with Pop4. Co-expression of the two proteins was required to obtain soluble Pop1; neither of the co-expressed proteins had any purification tags. The co-expressed Pop4 was separated from Pop1 in the process of Pop1 purification and discarded [Citation9].
Final isolated proteins did not include any purification tags; however, Pop4, Pop3, and Rpr2 had an additional glycine residue at their respective N-terminus due to the used purification scheme.
The RNA component of RNase P was produced using run-off in vitro transcription with a linearized plasmid pYRP2 [Citation7] as the template. pYRP2 carries a deletion of non-conserved nucleotides A24, U25 to facilitate proper RNA folding; the deletion did not affect the assembly and activity of the RNP [Citation7].
The assembly of the RNase P RNP complexes was performed in a stepwise fashion by the addition of proteins to an equimolar amount of refolded RNase P RNA as previously described [Citation7]. Concentrations of components were determined spectrophotometrically using NanoDrop ND-1000 spectrophotometer (Thermo Scientific). RNA concentration was calculated based on the absorbance at 260 nm and using the absorbance coefficient 0.025 (μg/ml)−1/cm. Concentrations of proteins were calculated based on absorbance at 280 nm; the absorbance coefficients were calculated using ExPASy ProtParam tool (http://www.expasy.org [Citation7,Citation12]). Proteins were added sequentially as follows: Pop6/Pop7, Pop1, Rpp1/Pop5/Pop8, Pop4, Rpr2, Pop3. Attempts to pre-incubate complexes with a substrate or at a higher temperature [Citation7] did not improve the result.
In vitro transcribed S. cerevisiae pre-tRNAThr(AGT) construct [Citation7] was used as the substrate in the cleavage assays. This pre-tRNA included a 15-nucleotide 5’-leader and a 10 nucleotide-long 3’-trailer; all sequences corresponded to the S. cerevisiae genome (chromosomal location chrIII:295469–295565, http://www.yeastgenome.org/cgi-bin/seqTools). In addition, a 5’-end GG was added to facilitate transcription [Citation7]. Prior to the use in cleavage assays, the substrate was annealed by heating to 50°C followed by slow cooling to 25°C.
Cleavage assays were performed in a buffer containing 50 mM HEPES-NaOH pH 7.8, 100 mM ammonium acetate, 10 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 0.5% (v/v) glycerol, 0.5 μg/ml BSA. An aliquot of the pre-tRNA substrate (above) was 5’-32P-labelled, mixed with the cold substrate, and added to the reaction mix to the final substrate concentration of 4 μM. The reactions were run at varying temperatures for varying time with RNase P RNPs of varying compositions and concentrations, and stopped by the addition of an equal volume of a stop/loading buffer containing 8 M urea and 25 mM Na-EDTA pH 8.0. The products of the reactions were resolved on 8% denaturing polyacrylamide gels and quantified using a PhosporImager (Molecular Dynamics). The assays were run in triplicates at 30°C and 45°C, and in duplicated at all other temperatures.
Control RNase P holoenzyme was isolated from S. cerevisiae using a tandem affinity tag fused to the C-terminus of protein Rpr2 as previously described [Citation7].
Results and discussion
We have assembled an RNP complex consisting of RNase P RNA and proteins Pop1, Pop4, Pop5, Pop6, Pop7, Pop8, and Rpp1 (2 copies) (termed RNP7 here) using our previously described approach [Citation7]. The protein composition of RNP7 matches that of the abundant precursor of yeast RNase P, while the mature enzyme contains additional proteins: Rpr2 and Pop3 [Citation11]. To elucidate the roles of Rpr2 and Pop3, we have added these proteins to the RNP7 complex and investigated their effect on the activity and thermal stability of the enzyme.
Consistent with the previously reported results [Citation7], the RNP7 complex is catalytically active ( and Appendix Fig. S1A). This observation is also consistent with the previously reported catalytic activity of the precursor form of RNase P that misses Rpr2 and Pop3 [Citation11].
Figure 3. (A) Time course of the cleavage of pre-tRNA substrate by reconstructed RNase P RNP complexes RNP7 (RNase P RNA, Pop1, Pop4, Pop5, Pop6, Pop7, Pop8, Rpp1) (lanes 5–13), RNP7 plus protein Rpr2 (lanes 14–22), and RNP9 (RNP7 with added Rpr2 and Pop3) (lanes 23–36). pre-tRNAThr substrate (at 4 μM) was cleaved by RNPs (at 40 nM) at the temperatures indicated above the corresponding lanes. Lanes 1–4: negative controls, incubation at 30°C for 15 min. Lane 1: substrate; lane 2: substrate in the cleavage buffer; lane 3: substrate with RNase P RNA; lane 4: substrate with all proteins. (B) Quantification of pre-tRNA cleavage (gels are shown in Appendix Fig. S1). pre-tRNAThr substrate (at 4 μM) was cleaved by of RNP7 and RNP9 (at 100 nM) at the indicated temperatures. Note that the cleavage by RNP7 at 37–45°C and by RNP9 at 50°C occurs only in the first two minutes of the incubation while the sample tube is warming to the set temperature and does not proceed further apparently due to the thermal inactivation of the enzyme.
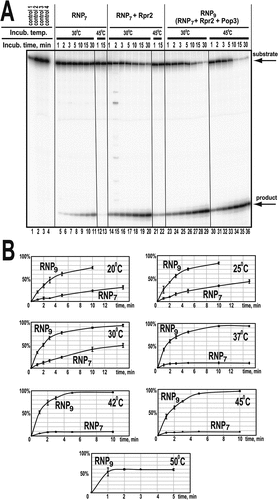
Interestingly, activity assays ( and Appendix Fig. S1A) show that while RNP7 is active at temperatures up to 30°C, the activity is lost at 37°C, indicating a reduced thermal stability of the complex with the protein composition matching that of the RNase P precursor. It was observed that incubation of RNP7 at 37°C and above resulted in visible precipitation of the complex. (It should be noted that in experiments under impermissible temperatures, about 10% of the substrate is cleaved in the first two minutes while the sample tube is warming to the set temperature, but the cleavage does not proceed further, .)
Whereas the assembly of RNP7 resulted in a quantitative binding of the components with the formation of a structurally homogeneous complex, the addition of Rpr2 and Pop3 did not result in a quantitative binding as judged by gel mobility shift and gel filtration experiments, suggesting that a stable binding of these proteins may require additional chaperoning activity [Citation7]. Nevertheless, here we detected a substantial effect of the addition of Rpr2 and Pop3 on the thermal stability and the observed activity of the RNase P RNP.
The addition of Rpr2 and Pop3 to RNP7 resulted in a considerable increase of the thermal stability of the complex: robust activity is observed at temperatures up to 45°C; the complex is inactivated by 50°C (, Appendix Fig. S1C). Incubation of the complex at 50°C resulted in a noticeable precipitation, indicative of thermal denaturation. Interestingly, this level of thermal stability appears to be somewhat excessive for a S. cerevisiae enzyme, given that S. cerevisiae strains typically do not grow at temperatures above 40°C [Citation13].
Also, the addition of proteins Rpr2 and Pop3 resulted in a considerable increase of the activity of the complex. Compared to the fully assembled RNase P isolated from yeast, the RNP7 complex had a reduced level of activity: kcat = 2.0 ± 1.0 min−1, Km = 3.5 ± 1.5 μM vs kcat = 40 ± 20 min−1, Km = 50 ± 20 nM (estimated at 30°C) [Citation7]. The non-quantitative character of the binding of Rpr2 and Pop3 does not allow for a meaningful detailed estimation of the kinetic parameters of the substrate cleavage in the presence of these proteins; however, the observed rate of cleavage at 20°-30°C and the concentration of the substrate of 4 μM was at least 5-fold higher than that for the RNP7 complex (, Appendix Fig. S1). The remaining difference between the rates of pre-tRNA cleavage by the isolated yeast RNase P versus the assembled complexes is consistent with the non-quantitative binding of Rpr2 and Pop3. Nevertheless, the substantial increase of the cleavage rate even with the incomplete Rpr2 and Pop3 binding indicates the role of these proteins.
The observed increase of the activity and thermal stability of yeast RNase P in the presence of Rpr2 and Pop3 is consistent with the available structural data and parallels the effects observed for archaeal RNase P [Citation14–18]. Both in yeast and archaeal enzymes, Rpr2 is sandwiched between Pop3 and the rest of the molecule (), forming extensive interactions with the part of RNase P RNA that is involved in the pre-tRNA substrate binding and is positioned to stabilize the fold of that part of RNA and to bridge it to the rest of the molecule. Additionally, Rpr2 interacts with the T-loop region of the pre-tRNA substrate, contributing to the proper positioning of the substrate. These roles can explain the effect of Rpr2 on the activity of RNase P.
Figure 4. In both yeast (panel A) and archaeal (panel B) RNases P, protein Rpr2 (red) is sandwiched between Pop3 (green) and the rest of the RNP and is involved in extensive interactions with the RNA component (grey) and protein Pop4 (magenta). Archaeal protein Pop3 is involved in interactions with the K-turn RNA motif (blue in panel B) that is absent in the yeast RNase P. Panel a is based on 6ah3.pdb [Citation8]. Panel B is based on 6k0a.pdb; the structure of Methanocaldococcus jannaschii RNase P is shown [Citation17]. Shown names of archaeal proteins correspond to the names of their yeast homologues.
![Figure 4. In both yeast (panel A) and archaeal (panel B) RNases P, protein Rpr2 (red) is sandwiched between Pop3 (green) and the rest of the RNP and is involved in extensive interactions with the RNA component (grey) and protein Pop4 (magenta). Archaeal protein Pop3 is involved in interactions with the K-turn RNA motif (blue in panel B) that is absent in the yeast RNase P. Panel a is based on 6ah3.pdb [Citation8]. Panel B is based on 6k0a.pdb; the structure of Methanocaldococcus jannaschii RNase P is shown [Citation17]. Shown names of archaeal proteins correspond to the names of their yeast homologues.](/cms/asset/091a158c-e073-4e19-9a6a-9c97b33f49c3/krnb_a_2201110_f0004_oc.jpg)
The structure of Rpr2 in the context of yeast RNase P () suggests the instability of the protein’s fold in the absence of Pop3, which is consistent with the observed propensity of isolated Rpr2 to aggregate. Pop3 in involved in extensive interactions with Rpr2, including the insertion of antiparallel N- and C-terminal beta-strands into the fold of Rpr2 () and is positioned to stabilize it. This stabilization is consistent with the role of Pop3 in the thermal stability of yeast RNase P.
Given that yeast Pop3 does not participate in extensive interactions with components of RNase P other than Rpr2 [Citation17], its role in the yeast enzyme is likely limited to the stabilization of Rpr2 and facilitation of its binding to the complex. Consistent with that, we did not observe any effects of Pop3 on the activity of the complex in the absence of Rpr2. It should be noted that in archaeal RNase P, the addition of the constitutive homologue of Pop3 (L7Ae) increases the activity and thermal stability of the enzyme as well [Citation14,Citation15]. In archaeal RNase P, Pop3 (L7Ae) contributes to the stability of the enzyme through its interactions with K-turn motif(s) of the RNA component as well as through extensive interactions with the homologue of Rpr2 [Citation17–19] (). Yeast RNase P has lost the K-turn RNA motifs (), thus minimizing interactions between Pop3 and RNA, but it has retained Pop3-Rpr2 interactions and the overall role of Pop3 in the stability of the enzyme.
Author contributions
A.P., I.B., A.S.K. designed and performed experiments, A.S.K. wrote the paper.
Supplemental Material
Download MS Word (4.9 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15476286.2023.2201110.
Additional information
Funding
References
- Altman S. A view of RNase P. Mol Biosyst. 2007;3:604–607.
- Guerrier-Takada C, Gardiner K, Marsh T, et al. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857.
- Jarrous N, Gopalan V. Archaeal/Eukaryal RNase P: subunits, functions and RNA diversification. Nucleic Acids Res. 2010;38:7885–7894.
- Esakova O, Krasilnikov AS. Of proteins and RNA: the RNase P/MRP family. RNA. 2010;16:1725–1747.
- Phan H-D, Lai LB, Zahurancik WJ, et al. The many faces of RNA-based RNase P, an RNA-world relic. Trends Biochem Sci. 2021;46:976–991.
- Chamberlain JR, Lee Y, Lane WS, et al. Purification and characterization of the nuclear RNase P holoenzyme complex reveals extensive subunit overlap with RNase MRP. Genes Dev. 1998;12:1678–1690.
- Perederina AI, Berezin I, Krasilnikov AS. In vitro reconstitution and analysis of eukaryotic RNase P RNPs. Nucleic Acids Res. 2018;46:6857–6868.
- Lan P, Tan M, Zhang Y, et al. (2018) Structural insight into precursor tRNA processing by yeast ribonuclease P. Science, 362, pii: eaat6678.
- Fagerlund RD, Perederina A, Berezin I, et al. Footprinting analysis of interactions between the largest eukaryotic RNase P/MRP protein Pop1 and RNase P/MRP RNA components. RNA. 2015;21:1591–1605.
- Perederina A, Esakova O, Koc H, et al. Specific binding of a Pop6/Pop7 heterodimer to the P3 stem of the yeast RNase MRP and RNase P RNAs. RNA. 2007;13:1648–1655.
- Srisawat C, Houser-Scott F, Bertrand E, et al. An active precursor in assembly of yeast nuclear ribonuclease P. RNA. 2002;8:1348–1360.
- Gasteiger E, Hoogland C, Gattiker A, et al. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. The Proteomics Protocols Handbook. Totowa, NJ, USA: Humana Press; 2005. pp. 571–607.
- Walsh RM, Martin PA. Growth of Saccharomyces cerevisiae and Saccharomyces uvarum in a temperature gradient incubator. J Inst Brew. 1977;83:169–172.
- Fukuhara H, Kifusa M, Watanabe M, et al. A fifth protein subunit Ph1496p elevates the optimum temperature for the ribonuclease P activity from Pyrococcus horikoshii OT3. Biochem Biophys Res Commun. 2006;343:956–964.
- Cho I-M, Lai LB, Susanti D, et al. Ribosomal protein L7Ae is a subunit of archaeal RNase P. Proc Natl Acad Sci, USA. 2010;107:14573–14578.
- Chen W-Y, Pulukkunat DK, Cho I-M, et al. Dissecting functional cooperation among protein subunits in archaeal RNase P, a catalytic ribonucleoprotein complex. Nucleic Acids Res. 2010;38:8316–8327.
- Wan F, Wang Q, Tan J, et al. Cryo-electron microscopy structure of an archaeal ribonuclease P holoenzyme. Nat Commun. 2019;10:2617.
- Phan HD, Norris AS, Du C, et al. Elucidation of structure-function relationships in Methanocaldococcus jannaschii RNase P, a multi-subunit catalytic ribonucleoprotein. Nucleic Acids Res. 2022;50:8154–8167.
- Lai LB, Tanimoto A, Lai SM, et al. A novel double kink-turn module in euryarchaeal RNase P RNAs. Nucleic Acids Res. 2017;45:7432–7440.
