ABSTRACT
In female animals, metabolic homoeostasis and reproductive fitness are critical to population expansion. The trade-off between lipid storage and reproduction inevitably occurs. However, most studies have focused on the complex network of relationships between reproductive and metabolic physiology at the transcriptional level. In this study, we identified a microRNA, miR-2b-2-5p, in a highly invasive quarantine pest, Bactrocera dorsalis. Knockdown of miR-2b-2-5p by antagomiR microinjection impaired ovarian development, reduced fecundity, and decreased triglyceride (TAG) storage in the fat body, whereas overexpression of miR-2b-2-5p by injection of its mimic caused reproductive defects similar to knockdown but increased TAG. Bioinformatics analysis and dual luciferase assay indicated that cyclic AMP response element (CRE)-binding protein (CREB) was the target gene of miR-2b-2-5p. RNAi-mediated knockdown of CREB led to excessive lipid storage and reproductive defects. Further starvation treatment revealed that miR-2b-2-5p functions by fine-tuning CREB expression in response to dietary stimuli. These results suggest that miR-2b-2-5p acts as a monitor to regulate CREB mRNA levels in the fat body, maintaining lipid homoeostasis and keeping the reproductive system on track. Thus, our study not only provides new insights into the interaction between metabolism and reproduction at the posttranscriptional level in B. dorsalis, but also providing a potential eco-friendly control strategy (RNAi-based biopesticides targeting essential miRNAs) for this notorious agricultural pest.
Introduction
Successful reproduction is essential for the propagation of most species. In most oviparous insects, vitellogenesis and ovarian development are the central events of female reproduction. During these processes, vitellogenin (Vg) is produced in the fat body, secreted into the haemolymph, transported through the follicular epithelial space to the surface of the oocyte, and ultimately captured and sequestered by the developing oocytes via receptor-mediated endocytosis [Citation1,Citation2–4]. In addition, Vg has also been reported to be synthesized in follicular cells, nurse cells, and haemocytes [Citation5–7]. Cumulative studies have demonstrated that female reproduction is regulated by numerous biological and abiotic factors, such as hormones, environmental temperature, nutrition, and metabolism [Citation8–10].
Energy homoeostasis is crucial for insects to maximize their survival in response to environmental constraints [Citation11]. The fat body of insects, equivalent to the liver and adipose tissue of vertebrates, is the central organ of metabolic activity [Citation11]. Lipid metabolism is the centre of energy homoeostasis in many organisms, providing energy for various physiological processes, such as development, migration, reproduction, and diapause. Therefore, the fat body can be described as the ‘power plant’ of insects [Citation12,Citation13]. In Culex pipiens, interruption of lipogenesis caused the failure of storage lipids for overwintering [Citation14]. Also, defects in lipolysis led to a series of physiological damages in Drosophila [Citation15–18].
In female animals, energy metabolism and fertility are tightly connected and reciprocally regulated. There is no doubt that lipid metabolism and reproduction are closely connected, and a trade-off between lipid storage and reproduction inevitably occurs [Citation19–22]. On the one hand, a reduction in lipid storage causes the failure of vitellogenesis and ovarian development and reduces fertility in Drosophila, Colaphellus bowringi and Nilaparvata lugens [Citation23,Citation24]. On the other hand, obesity and excessive accumulation of lipid content also cause reduced fertility in Drosophila and mammals [Citation21,Citation25–27]. However, most studies have focused on the complex network of relationships between reproductive and metabolic physiology at the transcriptional level.
MicroRNAs (miRNAs), a class of endogenous small noncoding RNAs approximately 22 nucleotides in length, play important roles in gene regulation at the posttranscriptional level [Citation28–30]. In general, miRNAs mainly bind to the 3‘UTR of the target mRNA in the “seed region” (2–8 bases at the 5’ end of the miRNA) according to the principle of base complementary pairing, complex with Argonaute (AGO) proteins and target mRNAs to inhibit their translation or promote their degradation [Citation31–33]. The interaction between miRNAs and mRNA is complicated. Single miRNAs can potentially target over 300 different transcripts, and mRNAs can be targeted by dozens of miRNAs [Citation34–36]. Therefore, miRNAs have proven to be important gene regulators in many biological processes in insects, such as development, metabolic homoeostasis, stress responses and reproduction [Citation12,Citation37,Citation38]. A significant number of miRNAs have been identified based on high-throughput sequencing, yet their potential biological functions are still poorly understood. In mosquitos, miR-277 has been proven to play an essential role in lipid metabolism and reproduction by targeting ilp7 and ilp8 [Citation39]. In particular, there are still few reports on the functional studies of miRNAs in metabolism and reproduction and their interconnections in other insects, especially in agricultural pests.
Bactrocera dorsalis is a highly invasive quarantine pest known for its high fecundity and short reproductive cycle. The normal operation of the reproductive system is critical for insect population multiplication, and metabolism is fundamental to the life of insects, providing energy for reproduction. Therefore, understanding the molecular mechanisms linking metabolism and reproduction is of particular importance. In this study, we demonstrated that miR-2b-2-5p played an important role in regulating lipid metabolism and reproduction in female B. dorsalis by agomiR/antagomiR microinjection. Cyclic AMP response element (CRE)-binding protein (CREB) was identified as the potential target gene of miR-2b-2-5p using bioinformatics software and a luciferase reporter assay. Further functional study found that miR-2b-2-5p targets CREB and that its regulatory axis is sensitive to nutritional status. Therefore, miR-2b-2–5p is promising as a new molecular target in the future pest management of B. dorsalis.
Results
Spatiotemporal expression profile of miR-2b-2-5p in female B. dorsalis
In our previous study, we identified a series of miRNAs differentially expressed during sexual maturation and potentially involved in female reproduction from different periods of the ovary and fat body using small RNA sequencing [Citation40]. After quantitative real-time PCR (qRT-PCR) verification and preliminary functional screening based on literature, we focused specifically on miR-2b-2-5p, whose function is still unknown in insect. shows the sequence logo of miR-2b-2-5p from different species available in miRbase. We first employed qRT–PCR to determine the distribution of mature miR-2b-2-5p among different tissues and developmental stages in female adult of B. dorsalis. The results showed that mature miR-2b-2-5p was highly expressed in the fat body, followed by the gut, thorax, head and ovary (). Then, the female fat body at 2-day intervals was used to quantify its time-course expression profile. The results showed that the expression level of miR-2b-2-5p was low on day 1–5 post eclosion (previtellogenic stage), gradually increased with sexual maturation, peaked at the 7th day post eclosion (vitellogenic deposition stage) and maintained a high expression level thereafter (). These results indicated that miR-2b-2-5p might play an important role in female reproduction of B. dorsalis.
Figure 1. Spatial-temporal expression pattern of miR-2b-2-5p in female B. dorsalis. (A) The picture was drawn using WebLogo 3. The x-axis indicates the length of bases, while the y-axis indicates the frequency of nucleic acids. (B) qRT–PCR to detect the relative expression levels of mature miR-2b-2-5p in different tissues isolated from 7-day-old female B. dorsalis. (C) The relative expression levels of mature miR-2b-2-5p in the fat body at 2-day intervals after the emergence of female B. dorsalis. Data in B and C are expressed as mean ± SEM of four independent replicates. Different letters indicate significant differences among each group determined by one-way ANOVA followed by Tukey’s LSD test (P < 0.05).
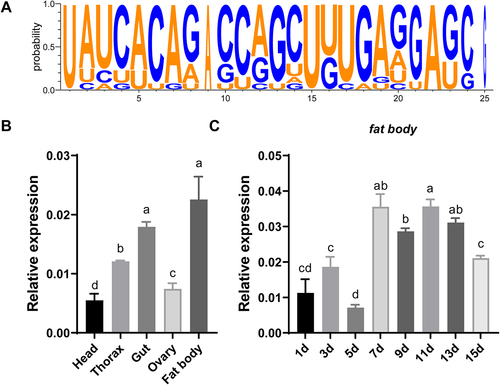
Suppression of miR-2b-2-5p results in ovarian development defects
To explore the roles of miR-2b-2-5p during sexual maturation, we employed a chemically synthesized antagomiR to inhibit its endogenous expression by microinjection, which is widely used for miRNA loss-of-function studies. qRT–PCR results indicated that the relative expression of miR-2b-2-5p decreased by approximately 40.6% and 43.37% in the fat body and ovary at 24 h post injection, respectively, compared with the antagomiR negative control (Ant-NC) ().
Figure 2. Knockdown of miR-2b-2-5p using antagomiR impaired female fecundity in B. dorsalis. (A) Effects of antagomiR treatment on the relative expression of miR-2b-2-5p in the ovary and fat body, and Ant-NC as a negative control. (B-F) Microinjection of antagomiR affected female vitellogenesis and fecundity. (B) Ovaries were dissected at 7, 10, and 13 days post microinjection, and Ant-NC was used as a negative control (n = 30). Scale bar: 200 µm. (C) The ovarian proportions (n = 30). (D) The average number of eggs laid daily per female during the oviposition period (n = 10). X-axis indicates the number of days after the first mating. (E) The cumulative eggs laid. (F) The relative expression level of Vg1 and Vg2 in the fat body was detected after 2 days of antagomiR treatment compared to Ant-NC. Data represent three biological replicates with three technical replicates and are shown as the mean ± SEM. * P < 0.05, ** P < 0.01, ***P < 0.001.
We then evaluated the effect of miR-2b-2-5p knockdown on ovarian development. Ovaries can be divided into five stages based on the degree of development in B. dorsalis [Citation41]. Tissue dissection revealed that the percentage of ovaries at stages III, IV, and V in the Ant-2b-2 group was significantly lower than that in the Ant-NC control group on the 7th, 10th and 13th days, suggesting that knockdown of miR-2b-2-5p resulted in a delay in ovarian development (). Next, we evaluated the fecundity by counting the number of eggs laid. These results showed that the average number of eggs laid daily in the Ant-2b-2 group was significantly lower than those of the Ant-NC control in the first six counts, and the cumulative egg number was also significantly lower than that of the control group (P < 0.01) (). However, knockdown of miR-2b-2-5p had no effect on the mating rate or progeny viability (Fig. S1).
Vitellogenesis is the process by which the ovary accumulates vitellogenin [Citation4]. Although the ovary of B. dorsalis can synthesize some amount of vitellogenin by itself, most is synthesized in the fat body, subsequently released into the haemolymph and finally taken up by developing oocytes via receptor-mediated endocytosis [Citation4,Citation42]. We then quantified the expression levels of vitellogenin genes (Vg1 and Vg2) in the fat body. qRT–PCR results also showed that the expression levels of both genes significantly decreased in the Ant-2b-2 group compared with the Ant-NC control group at 24 h post microinjection (). Taken together, these results suggested that miR-2b-2-5p might regulate the vitellogenesis in the fat body and then contribute to ovarian development and fecundity.
Knockdown of miR-2b-2-5p impairs lipid biogenesis
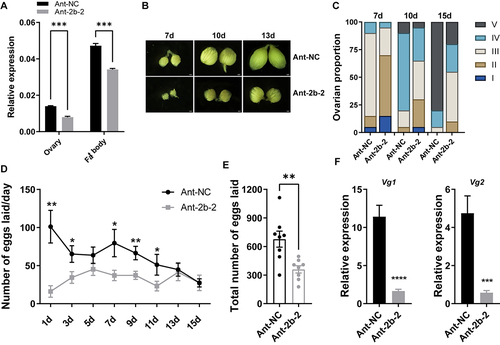
In addition to the intake of yolk protein, the developing oocyte also reserves large amounts of lipids, which make up 30–40% of the dry weight of the insect oocyte [Citation43]. Although ovarian follicular cells are capable of synthesizing a certain amount of TAG, this represents only approximately 1% of the total oocyte. More is derived from the fat body via the receptor-mediated endocytic pathway [Citation20]. Therefore, we next measured the energy storage substances in the fat body. Metabolic assays showed that knockdown of miR-2b-2-5p significantly reduced the content of TAG compared with the Ant-NC control, whereas the content of glycogen was significantly elevated (). TAG is mainly stored in the fat body in the form of lipid droplets, and we then visualized the size of lipid droplets using Nile Red staining. Consistent with the metabolic assay results, suppression of miR-2b-2-5p significantly reduced the size of lipid droplets at 72 h ().
Figure 3. Knockdown of miR-2b-2-5p using antagomiR affected energy metabolism in female B. dorsalis. (A-B) Whole bodies of flies were homogenized in PBST solution, and then the contents of TAG (A) and glycogen (B) were detected. The results were normalized to the total protein content. Boxplots show the data of seven independent biological replicates (5 flies for each replicate). Asterisks indicate significant differences by Student’s t test (** P < 0.01; * P < 0.05). (C) Nile Red staining of the lipid droplets in the fat body of females (Scale bar: 5 μm), and the lipid droplet size was quantified using ImageJ (C’). (D) Heatmap illustrating the relative expression levels of genes involved in the lipid metabolism pathway (lipogenesis and lipolysis). Data are shown as mean ± SEM, Three biological replicates were performed with three technical replicates * P < 0.05, ** P < 0.01, *** P < 0.001.
Reducing TAG biosynthesis or increasing TAG degradation can lead to a decrease in TAG content. We further performed qRT–PCR to quantify the relative expression levels of genes involved in the lipogenesis and lipolysis pathways. These results indicated that the expression of genes involved in lipogenesis (fat acid synthase [Citation44], Diacylglycerolacyltransferase-1 (DGAT1), Lipid Storage Droplet 2 (LSD2), lipin 3 (LPIN3), Preheparin lipoprotein lipase 2 (PLPL2) , Acetyl-CoA carboxylase (ACC) and glycerol phosphate acyltransferase (GPAT)) were significantly decreased in the Ant-2b-2 group compared with the Ant-NC control group, while the expression of genes involved in lipolysis were not significantly different between these two groups (). These results suggest that knockdown of miR-2b-2-5p affects TAG biosynthesis, resulting in metabolic defects.
Overexpression of miR-2b-2-5p causes reproductive defects and lipid overaccumulation
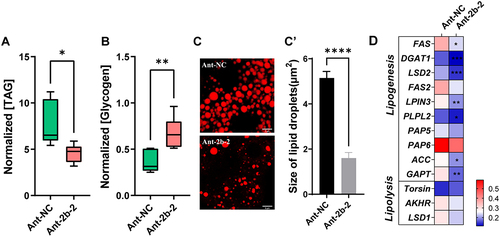
Next, microinjection of miR-2b-2-5p mimic was carried out to examine the related phenotypes as observed above. qRT–PCR confirmed the significantly increased expression of miR-2b-2-5p at 24 h post mimic injection (). Tissue dissection revealed that overexpression of miR-2b-2-5p also led to ovarian developmental defects, similar to those observed in the antagomiR treatment. The percentage of ovaries at III, IV and V was significantly lower in the mimic-2b-2-5p group than that in the mimic-NC control at the 7th, 10th and 13th days post eclosion (). In addition, the average number of eggs laid every two days after mating on the 1d, 3d, 5d, and 11d was significantly lower in the mimic-2b-2-5p-injected group than in mimic-NC control-injected group (). The total number of eggs laid was significantly reduced compared to the mimic-NC group (). Similar to the above results, decreased expression of Vg1 and Vg2 was also observed in the mimic-2b-2 group compared with the mimic-NC control group, but there were no differences in the hatching rate or mating rate between these two groups (; Fig. S2). Taken together, these results suggest that overexpression of miR-2b-2-5p is also capable of causing defects in ovarian development and impairing fertility.
Figure 4. Overexpression of miR-2b-2-5p using agomiR impaired the fecundity of female B. dorsalis. (A) Effects of agomiR treatment on the relative expression of miR-2b-2-5p in the fat body. (B-F) Microinjection of agomiR affected vitellogenesis and fecundity. (B) Ovaries were dissected at 7, 10, and 13 days post microinjection, and Ant-NC was used as the negative control (n = 30). (C) The ovarian proportions (n = 30). (D) The average number of eggs laid daily (n = 10). X-axis indicates the number of days after the first mating. (E) The cumulative number of eggs laid by each group. (F) The relative expression levels of Vg1 and Vg2 in the fat body after 2 days of mimic-2b-2-5p treatment compared to mimic-NC. Data represent three biological replicates with three technical replicates and are shown as the mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001.
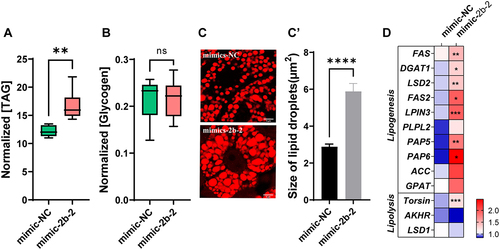
We next examined the effect of mimic injection on lipid metabolism. Notably, overexpression of miR-2b-2-5p significantly elevated the total TAG content, while glycogen content was not significantly different between the mimic-2b-2 and mimic-NC groups (). Nile Red staining results indicated that the average size of lipid droplets in the mimic-2b-2 group was significantly larger than that in the mimic-NC group (). qRT–PCR results indicated that genes involved in the lipid biosynthesis process (FAS, DGAT1, LSD2, FAS2, LPIN3, phosphatidate phosphatase 5 (PAP5), and PAP6) were significantly upregulated in the mimic-2b-2 group compared to the mimic-NC group, while the expression level of lipolysis genes (AKHR and LSD1) were not significantly different, except for Torsin, which showed mildly elevated expression in the mimic-2b-2 group (). All these results suggested that overexpression and knockdown of miR-2b-2-5p both affected metabolic homoeostasis, resulting in reproductive defects.
Figure 5. Overexpression of miR-2b-2-5p using agomiR affected the energy metabolism of female B. dorsalis. (A-B) Effects of miRNA agomiR injection on the contents of triglycerides (A) and glycogen (B) in the whole body. Results were normalized to the total protein content. Boxplots show the data of seven independent biological replicates (5 flies for each replicate). Asterisks indicate significant differences by Student’s t test (** P < 0.01; ns not signification). (C) Nile Red staining of lipid droplets in the fat body of females (Scale bar: 5 μm), and the lipid droplet size was quantified using ImageJ (C’). (D) Heatmap illustrating the relative expression levels of genes involved in the lipid metabolism pathway (lipogenesis and lipolysis). The asterisk in each cell indicated the statistically significant differences. * P < 0.05, ** P < 0.01, *** P < 0.001.
CREB is the target gene of miR-2b-2-5p
To identify potential targets of miR-2b-2-5p, we employed the bioinformatics software programs (miRanda [Citation45] and TargetScan [Citation46]) for target prediction analysis and identified a total of 13 potential target genes (Table S1). Since miRNA acts mainly by negatively regulating the transcription of target genes, we then quantified the gene expression changes in candidate target genes in knockdown or overexpression samples compared with corresponding controls. When miR-2b-2-5p was repressed, the expression levels of CREB, LCFA and HINT3 significantly increased in the Ant-2b-2 group compared with those in the control group, while PTP was significantly decreased, and the rest of the candidate target genes did not show any significant changes in expression (). When miR-2b-2-5p was overexpressed, the expression levels of CREB, LCFA and HINT3 were significantly decreased, whereas the expression levels of CYP3A4 and BRWD3 were significantly increased, and no significant alterations were seen in the others (). These results suggested that these three candidate target genes (CREB, LCFA and HINT3) are potential targets for miRNA action.
Figure 6. Target gene screening and dual-luciferase reporter assay. (A-B) qRT–PCR detected the expression levels of potential target genes after microinjection of miR-2b-2-5p antagomiR (A) or agomiR (B). Ant-NC or mimic-NC as negative controls. The asterisk in each cell indicated the statistically significant differences. * P < 0.05, ** P < 0.01, *** P < 0.001. (C) Luciferase reporter assays of candidate targets (CREB, LCFA and HINT3) in vitro. (D) Abolishment of the repression effect by binding site mutation (CREBM). The relative fluorescence intensity was calculated by dividing the Renilla luciferase fluorescence intensity by the firefly fluorescence intensity. Data represent three biological replicates and is shown as the mean ± SEM. ** P < 0.01.
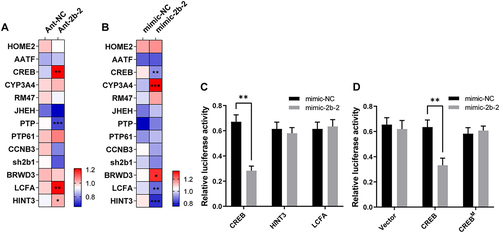
Next, we extracted the 3’UTR sequence of these target genes and predicted the binding site of miR-2b-2-5p using RNAhybrid [Citation47]. The approximate 400 bp sequence containing the binding site was subcloned into the psiCHECK2 vector, and dual luciferase assay was performed to verify their interaction in vitro. We found that the relative luciferase activity of the CREB reporter displayed a significant reduction, but there were no significant changes in the LCFA and HINT3 reporters compared with the control reporter (). When mutating the binding site of the miR-2b-2-5p seed region, the relative luciferase activity recovered to the mimic-NC control level (). Taken together, CREB is identified as the target of miR-2b-2-5p.
CREB plays essential roles in lipolysis and ovarian development.
CREB, named cyclic AMP response element (CRE)-binding protein, is one of the best characterized stimulus-induced transcription factors in response to a diverse array of stimuli [Citation48]. It is highly conserved from Aplysia to Drosophila to humans [Citation49] and is mainly activated by phosphorylation of serine 133. Studies on the biological roles of CREB have revealed that it is essential for multiple biological processes, such as cellular differentiation, proliferation, adaptive responses and metamorphosis [Citation48,Citation50].
To decipher the roles of CREB in regulating lipid metabolism and ovarian development, we first determined the distribution of CREB among different tissues and developmental stages in female B. dorsalis adult. The qRT–PCR results showed that CREB was highly expressed in the thorax, followed by the fat body, and its expression was relatively constant in the adult fat body after eclosion (Fig. S3). Then, we employed the RNAi to knockdown the expression of CREB. The abundance of CREB was significantly reduced by 46% in the dsCREB group compared with the dsEGFP control group (). We observed that the ovarian phenotypes in the dsCREB treatment group were similar to those in the Ant-2b-2-5p and mimic-2b-2-5p treatment groups. That is, ovarian development and fecundity were inhibited, and the expression levels of Vg1 and Vg2 were reduced compared with those in the dsEGFP control group ().
Figure 7. Effects of knocking down CREB on reproduction and energy metabolism in female B. dorsalis. (A) Effects of dsCREB injection on the relative expression of CREB in the fat body. (B-F) RNAi-based knockdown of CREB affected female fecundity. Ovarian phenotype at 7, 10, and 13 days post microinjection (n = 30) (B) and the ovarian proportions (n = 30) (C). (D) The average number of eggs laid daily (n = 10). X-axis indicates the number of days after the first mating. (E) and the cumulative number of eggs laid. (F) The relative expression levels of Vg1 and Vg2 in the fat body 2 days after dsRNA injection. (G-J) Effects of dsRNA treatment on the contents of TAG (G), glycogen (H), the morphology of lipid droplets (I) and quantified with ImageJ (I’) as well as expression level of genes involved in the lipid metabolism pathway (J). Data are expressed as the mean ± SEM. * P < 0.05; ** P < 0.01, *** P < 0.001 and **** P < 0.0001 (Student’s t test).
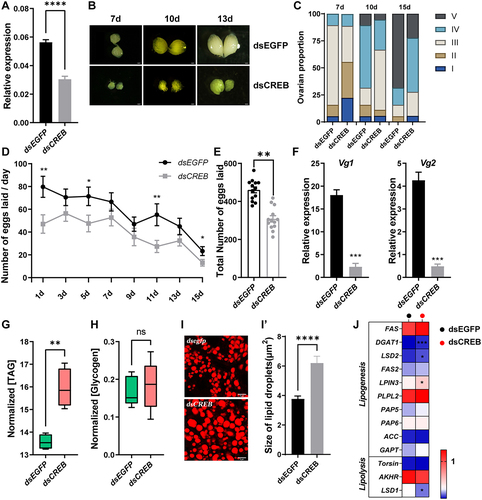
Next, we quantified the contents of TAG and glycogen. Similar to mimic-2b-2 treatment, the TAG content significantly increased in the dsCREB-treated group, while there was no effect on glycogen levels (). Lipid droplet staining results revealed that the average size of lipid droplets in the dsCREB-treated group was significantly larger than that of the dsEGFP-treated group, further confirming the above results ().
Moreover, at 24 h after injection, genes involved in lipogenesis (DGAT1, LSD2 and LPIN3) were significantly upregulated in the dsCREB-treated group compared with the dsEGFP-treated group. LSD1, which is involved in lipolysis, was significantly downregulated, while the other lipolysis genes did not show any significant changes (). In summary, CREB may act as a regulator of lipogenesis to maintain metabolic homoeostasis, ultimately controlling reproduction in B. dorsalis.
miR-2b-2-5p/CREB is under the control of dietary nutrition
A previous study indicated that Drosophila CREB transcriptional activity in the fat body is regulated by adipokinetic hormone (AKH), a homolog of mammalian glucagon [Citation51]. Under starvation conditions, AKH is secreted from corpora cardiaca (CC) endocrine cells and promotes nutrient utilization in the fat body, leading to increased circulating glycaemic levels [Citation52]. It is thus hypothesized that the miRNA-CREB axis is likely sensitive to the nutrient availability.
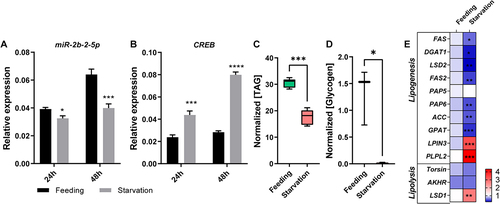
We next employed extreme dietary strategies to verify this hypothesis. Under starvation conditions, the expression level of miR-2b-2-5p was significantly downregulated at 24 h and more severely downregulated at 48 h compared with the well feeding group (). However, the target gene CREB displayed the reverse expression trends (). In addition, metabolic assay results demonstrated that the content of TAG and glycogen under starvation conditions were significantly lower than those under feeding conditions (). We next tested the effects of starvation on the expression levels of metabolism-related genes after 24 h of starvation. The results showed that FAS, DGAT1, LSD2, FAS2, PAP6, ACC and GPAT, which are involved in lipogenesis, were significantly downregulated. Only LSD1, which is involved in lipolysis, was significantly upregulated (). Furthermore, the expression levels of Vg1 and Vg2 dramatically decreased under the condition of water-only starvation (Fig. S4). The above results suggest that starvation causes the failure of lipogenesis, stimulation of lipolysis, and affects vitellogenesis in female B. dorsalis adults, and the miR-2b-2-5p/CREB axis may be the key regulation pathway in response to starvation.
Figure 8. Starvation affects the expression levels of miR-2b-2-5p and CREB and further disturbs energy metabolism and vitellogenesis in B. dorsalis. (A-B) Detection of the expression levels of miR-2b-2-5p and CREB after 24 h and 48 h of starvation treatment. (C-D) Starvation influences the content of TAG and glycogen in the fat body. Results were normalized to the total protein content. Boxplots show the data of seven independent biological replicates (5 flies for each replicate). Asterisks indicate significant differences by Student’s t test (*** P < 0.001; * P < 0.05). (E) Heatmap illustrating the relative expression levels of genes involved in the lipid metabolism pathway (lipogenesis and lipolysis). The asterisk in each cell indicated the statistically significant differences. * P < 0.05, ** P < 0.01, *** P < 0.001.
Discussion
In the current study, we identified a functionally important miRNA involved in lipid metabolism and female reproduction. Our study revealed that knockdown and overexpression of miR-2b-2-5p resulted in ovarian developmental defects and abnormal lipid stores, which was mainly caused by altered regulation of lipid biosynthesis pathways. Moreover, we confirmed that miR-2b-2-5p functions by repressing CREB activity; CREB acts as a suppressive transcription factor and is sensitized to environmental signals. Under starvation conditions, CREB expression was significantly enhanced, which in turn repressed the transcription of lipid biosynthesis pathway genes. Therefore, the miRNA-CREB axis may be an important dimension of environmental signals acting on the gene expression network. To our knowledge, this is the first report of roles of miR-2b-2 in metabolic homoeostasis and female reproduction in insect.
In female reproduction, the most distinctive feature of ovarian development is the continuous extension and expansion of the ovarioles and the development of mature oocytes within the aligned oocytes, mainly through the deposition of vitellogenin and lipids [Citation4]. These components are mainly provided by the surrounding exogenous tissues, such as the fat body [Citation42]. Follicular cells in the ovary can also synthesize some of these components but in very limited amounts [Citation20]. Lipid metabolism is the centre of energy mobilization in insects, providing most of the large amount of energy required during reproduction [Citation22].
miRNA functional studies have broadened our understanding of the regulatory mechanisms of ovarian development and metabolic processes. For example, miR-14 is involved in regulating lipid synthesis in Drosophila, and the contents of TAG and DAG are significantly increased after miR-14 mutation [Citation53]. Deletion of miR-184-3p leads to termination of oocyte development [Citation54]. Our previous study also found that the miR-275/305 cluster negatively regulated the transcription of SLC2A1/GLIS2, eventually ensuring adult metabolic homoeostasis in B. dorsalis [Citation55]. In this study, depletion of miR-2b-2-5p led to failure of lipid storage and ovarian development, whereas overexpression of miR-2b-2-5p resulted in lipid overaccumulation but displayed a similar reproductive phenotype as miRNA inhibition in B. dorsalis. This means that increasing and decreasing lipid content can both lead to failure of ovarian development. Not surprisingly, similar phenomena have been reported among different insect species. For instance, in A. aegypti, miR-277 affects female mosquito reproduction by influencing lipid deposition and mobilization [Citation39]. Knockdown of lipin led to arrested ovarian development and hypertrophy of the fat bodies in cabbage beetles [Citation24]. Research in Drosophila also found that a high-fat diet induced maternal obesity (increased TAG levels) but also resulted in a significant decrease in the number of eggs laid [Citation26,Citation27]. These results indicated that metabolic homoeostasis is crucial for female reproduction. The development of reproductive organs is dependent on continuous energy supply from lipid lipolysis or mobilization, and miR-2b-2-5p affects reproductive development by regulating lipid metabolism in B. dorsalis.
In our study, we confirmed that CREB is the functional target of miR-2b-2-5p. CREB plays important roles in a wide range of cellular processes, including proliferation, differentiation, and adaptive responses, by regulating glycogen and lipid metabolism in mammals [Citation48]. In insects, CREB was reported to function in regulating metamorphosis, metabolism, learning and memory [Citation12,Citation50,Citation56,Citation57]. In addition, AKH can activate CREB through protein kinase A (PKA)‐mediated phosphorylation [Citation58,Citation59]. Downregulation of CREB specifically leads to TAG storage and obesity in the fat body in Drosophila, but glycogen levels are reduced [Citation60]. In Acyrthosiphon pisum, knockdown of CREB by RNAi significantly shortened lifespan and reduced the number of offspring [Citation61]. In A. aegypti, CREB functions as a repressor of yolk protein precursor gene expression at the time of vitellogenesis termination in the fat body in vitro [Citation62]. In the present study, RNAi-mediated knockdown of CREB displayed similar phenotypes as miR-2b-2-5p mimic treatment, that is, excessive lipid storage, reduced transcription of Vg and failure of ovarian development, suggesting that CREB is indispensable for lipid metabolism and female fecundity. The underlying mechanism of how CREB controls metabolic homoeostasis and reproduction needs further investigation. Knockdown of miR-2b-2-5p significantly elevated the content of glycogen, whereas its overexpression and knockdown of CREB has no effect on glycogen levels, indicating that the miR-2b-2-5p/CREB axis was possibly involved in regulation of sugar conversion to lipids, and the conversion was differential, depending on the expression levels of CREB.
Insects mobilize stored lipids during starvation to provide energy for basic physical activity, enhancing the activity of lipolysis [Citation63]. In Drosophila, starvation leads to significant upregulation of the expression of CREB and Foxo, which contribute to the hydrolysis of lipids for the body to survive [Citation60,Citation63,Citation64]. Therefore, we explored the relationship between miR-2b-2-5p/CREB and dietary status. Indeed, under starvation conditions, the expression of CREB was significantly enhanced. Meanwhile, the expression level of miR-2b-2-5p was relatively low under starvation conditions compared with under feeding conditions. In a previous study, we found that the miR-275/305 cluster is regulated by the insulin signalling pathway in a nutrient-dependent manner [Citation55]. Whether nutrient signalling, such as insulin or TOR signalling, is involved in the regulation of the miR-2b-2-5p/CREB axis needs further study.
Conclusion
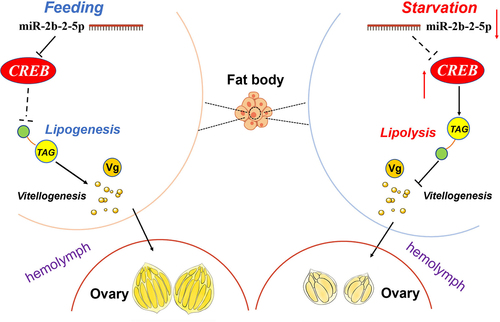
In conclusion, our study revealed the vital role of miR-2b-2-5p in lipid metabolism and reproduction in B. dorsalis. Depletion of this miRNA leads to failure of lipid storage and ovarian development, while overexpression causes excessive lipid synthesis but similar defects in reproduction. We identified CREB as the direct target of miR-2b-2-5p, and functional evidence demonstrated that fine-tuning of CREB by miR-2b-2-5p is required for lipid metabolism and reproduction in B. dorsalis. A starvation assay further confirmed that the miR-2b-2-5p/CREB axis played essential roles in regulating metabolic homoeostasis and reproductive progress ().
Figure 9. A model for the miR-2b-2-5p/CREB regulatory axis under different dietary stimuli in female B. dorsalis. Under starvation conditions, the expression level of miR-2b-2-5p was significantly downregulated, while the target gene CREB was upregulated. Accordingly, the mRNA level of lipolysis-related gene LSD1 significantly increased, and TAG level was lower. Also, the expression of Vg decreased. As a result, vitellogenesis and ovarian development was arrested. In contrast, under normal feeding conditions, miR-2b-2-5p reduced the expression of CREB, resulting in the lipogenesis and increased level of TAG. Therefore, vitellogenesis and ovarian development was promoted. Arrows and T-shaped symbols represent activation and inhibition, respectively. Dashed line and solid line denote low and strong activity, respectively.
Materials and methods
Insect rearing
B. dorsalis were obtained from the Institute of Horticultural and Urban Entomology at Huazhong Agricultural University (Wuhan, China) and had been cultured for more than 30 generations. The insectary environment was maintained at 27°C under a constant photoperiod (light:dark = 12 h:12 h). Adult flies were provided with a standard adult diet containing sucrose and yeast (at a 3:1 ratio) and water ad libitum. The hatched larvae were transferred to a plastic box containing adequate food until entering wandering stage and then transferred to moist vermiculite for pupation.
RNA extraction and qRT–PCR
Total RNA was extracted using TRIzol reagent (Takara, Japan). First-strand cDNA was synthesized from 1 μg of total RNA using a PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Japan). qRT–PCR for mature miRNA and mRNA quantification were performed using the 2×UNICON SYBR Green Master Mix (Low ROX) (Yeasen, Shanghai, China) according to the manufacturers’ instructions, with three technical replicates for each sample and U6 and Rpl32 as the internal references, respectively. Relative expression was calculated as 2−ΔΔCt.
agomiR/antagomiR microinjection
After eclosion, the 3-day-old females were anesthetized on ice and a total of 1 μL agomiR/antagomiR solution (20 μM) was injected into the ventral abdomen of female adults using an Eppendorf micromanipulation system (FemtoJet 5247; Eppendorf, Hamburg, Germany) as described previously [Citation65]. The same volume of random shuffled sequence was used as a negative control (NC). At 24 h post injection, flies were sacrificed to dissect the ovary and fat body for RNA extraction and the subsequent qRT–PCR analysis (10 flies in each treatment group). The miRNA mimic (agomiR), inhibitors (antagomiR) and negative control (NC) oligonucleotides were purchased from GenePharma (Shanghai, China). The sequences are listed in Table S2.
RNAi experiments
The double-stranded RNA (dsRNA) construct was prepared using a T7 RiboMAX Express RNAi Kit (Promega, USA) following the manufacturer’s protocols. The primers are designed through BLOCK-iT™ RNAi Designer (Thermo Fisher, USA) based on the gene sequence, and T7 promoter sequence have added to the 5ʹ ends of the amplification primers, and enhanced green fluorescent protein (EGFP) was used as the control. 1 μL of 2000 ng/μL dsRNA was injected into the ventral abdomen of 3-day-old female adults as described above. The fat body was dissected for RNA extraction to verify the RNAi efficiency using qRT-PCR 24 h after microinjection. The phenotypic analysis were performed 4 days later.
Reproductive phenotypic analysis
Different treated female B. dorsalis were anesthetized on ice and dissected in cold PBS to evaluate the ovary developmental stage on days 7, 10 and 13 post eclosion, with at least 30 flies for each treatment. Ovarian morphology was observed and imaged using an Olympus Stereo Microscope SZX16 (Olympus, Tokyo). The ovarian morphology was divided into five stages as prior literature [Citation41], including previtellogenic stage (I), vitellogenic deposition stage (II), expectant stage of mature eggs (III), peak stage of oviposition (IV), and last stage of oviposition (IV). The ovarian proportions were the ratio of the number of ovaries in corresponding stages to the number of all ovaries (n = 30). For the fecundity assay, different treated females were allowed to culture until sexual mature (12-day-old), and mated with virgin males of the same age in a self-developed egg counting device. Each group contained two couple of flies and ten replicates were performed. The number of laid eggs in each cup was counted at 1, 3, 5, 7, 9, 11, 13, and 15 days after mating, and the total number of laid eggs was calculated. To measure the hatching rate, about 200 embryos were collected and maintained on wet filter paper, and hatched larvae were counted 2 days later after hatching. Mating rate assays were performed according to our previous methods [Citation66].
Determination of TAG and glycogen levels
Metabolic assays were performed 3 days after antagomir/mimics injection. which have been optimized according to our previous study [Citation55]. Briefly, five adult flies were homogenized in 1 ml PBST buffer (0.05% Tween 20) and immediately incubated at 70°C for 5 min to inactivate endogenous enzymes. The homogenate was centrifuged for 5 min at 13,000 g to remove fly debris and the supernatant used for the subsequent metabolic determinations. TAG levels were measured using the Triglyceride (TAG) Content Assay kit (Beijing Boxbio Science & Technology Co., Ltd.). Glycogen levels were measured using the Glycogen Content Assay Kit (Beijing Boxbio Science & Technology Co., Ltd.) following the manufacturer’s instructions. Metabolite measurements were normalized with the total protein concentrations obtained by the BCA assay (TransGene Biotech, China). Seven biological replicates for each condition. Statistical significance was determined by unpaired two-tailed Student’s t-tests. Significance P values: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS P > 0.05.
Nile red staining
Nile Red staining was performed as described in our previous study [Citation55]. Briefly, fat bodies were fixed in 4% paraformaldehyde (Coolaber, China) and washed with 1×PBS two or three times. The tissues were then incubated for 2 hours in 1 μg/mL Nile Red (Sigma, USA) at room temperature. All samples were mounted on glass slides and imaged under identical excitation and detection conditions using a laser scanning confocal microscope (TCS SP8, Leica, Germany). The sizes of lipid droplets were measured using ImageJ software.
Target gene prediction and dual luciferase reporter assay
For the prediction of miR-2b-2-5p target genes in B. dorsalis, two different programs were used, TargetScan and miRanda. The target genes were screened according to the scoring criteria of each program. The 3’UTRs of target genes were amplified and introduced into the psiCHECK-2 vector (Promega, USA) by restriction enzyme digestion and ligation (XhoI and NotI). For the mutation vector, two pairs of primers amplify the upper and lower fragments of the mutation site, where the modifying sequences are incorporated into the 5’ ends of the primers. The PCR products were subsequently mixed and amplified by overlapping extension PCR [Citation67] and subcloned into the reporter plasmid. The primer sequences are listed in Table S2. Then, 100 ng of psiCHECK-2 recombinant vector and 50 nM mimic or the negative control (NC) were cotransfected into HEK293T cells using Lipofectamine 2000 (Thermo Fisher, USA) according to the manufacturer’s instructions. Cells were cultured at 37°C with 5% CO2. The activity of the two luciferase enzymes was measured using a dual luciferase reporter assay system (Promega, WI, USA) after 48 h of transfection. Three replicates were performed for each experiment. The results are expressed as the means of the ratio of Renilla luciferase activity/firefly luciferase activity.
Starvation treatment
After eclosion, 3-day-old female adults were transferred and cultured in a transparent plastic box (19 cm × 11 cm × 11 cm) (n = 60). For starvation treatments, only water was provided, whereas the control group was provided an adult artificial diet and water ad libitum. After 24 h of treatment, downstream experiments were conducted, such as metabolic measurement and gene expression quantification analysis.
Statistical analysis
GraphPad Prism 7.0 was used for statistical analysis. All statistical values are presented as the mean ± SEM. Means were compared using Student’s t test at the following significance levels: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Supplemental Material
Download Zip (5.4 MB)Acknowledgments
The authors would like to thank Dr. Lin Ling in Southeast University and Dr. Wen Liu in Huazhong Agricultural University for their helpful feedback on this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15476286.2023.2204579.
Additional information
Funding
References
- Roy S, Saha T, Zou Z, et al. Regulatory pathways controlling female insect reproduction. Annu Rev Entomol. 2018;63(1):489–511. DOI:10.1146/annurev-ento-020117-043258
- Engelmann F. Reproductive biology of invertebrates. volume xii, part a: progress in vitellogenesis. Q Rev Biol. 2004;79(1):77–78.
- Wu Z, Yang L, He Q, et al. Regulatory mechanisms of vitellogenesis in insects. Front Cell Dev Biol. 2020;8:593613.
- Raikhel AS, Dhadialla TS. Accumulation of yolk proteins in insect oocytes. Annu Rev Entomol. 1992;37:217–251.
- Gilbert LI, Serafin RB, Watkins NL, et al. Ecdysteroids regulate yolk protein uptake by drosophila melanogaster oocytes. J Insect Physiol. 1998;44(7–8):637–644. DOI:10.1016/S0022-1910(98)00020-1
- Matsumoto T, Yamano K, Kitamura M, et al. Ovarian follicle cells are the site of vitellogenin synthesis in the pacific abalone Haliotis discus hannai. Comp Biochem Physiol Part A: Mol Integr Physiol. 2008;149(3):293–298. DOI:10.1016/j.cbpa.2008.01.003
- Huo Y, Yu Y, Chen L, et al. Insect tissue-specific vitellogenin facilitates transmission of plant virus. PLOS Pathog. 2018;14(2):e1006909. DOI:10.1371/journal.ppat.1006909
- Shi XX, Zhang H, Quais MK, et al. Knockdown of sphingomyelinase (NlSmase) causes ovarian malformation of brown planthopper, Nilaparvata lugens (Stål). Insect Mol Biol. 2022;31(4):391–402. DOI:10.1111/imb.12767
- Song J, Zhou S. Post-transcriptional regulation of insect metamorphosis and oogenesis. Cell Mol Life Sci. 2020;77(10):1893–1909.
- Lu K, Xia C, Liu WT, et al. TOR pathway-mediated juvenile hormone synthesis regulates nutrient-dependent female reproduction in nilaparvata lugens (Stål). Int J Mol Sci. 2016;17(4):438. DOI:10.3390/ijms17040438
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55(1):207–225.
- Toprak U, Hegedus D, Doğan C, et al. A journey into the world of insect lipid metabolism. Arch Insect Biochem Physiol. 2020;104(2):e21682. DOI:10.1002/arch.21682
- Skowronek P, Wójcik Ł, Strachecka A. Fat body-multifunctional insect tissue. Insects. 2021;12(6):547.
- Sim C, Denlinger DL. Transcription profiling and regulation of fat metabolism genes in diapausing adults of the mosquito Culex pipiens. Physiol Genomics. 2009;39(3):202–209.
- Grönke S, Mildner A, Fellert S, et al. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005;1(5):323–330. DOI:10.1016/j.cmet.2005.04.003
- Grönke S, Müller G, Hirsch J, et al. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 2007;5(6):e137. DOI:10.1371/journal.pbio.0050137
- Gáliková M, Diesner M, Klepsatel P, et al. Energy homeostasis control in drosophila adipokinetic hormone mutants. Genetics. 2015;201(2):665–683. DOI:10.1534/genetics.115.178897
- Baumbach J, Xu Y, Hehlert P, et al. Gαq, Gγ1 and Plc21C control drosophila body fat storage. J Genet Genomics. 2014;41(5):283–292. DOI:10.1016/j.jgg.2014.03.005
- Pang R, Qiu J, Li T, et al. The regulation of lipid metabolism by a hypothetical P-loop NTPase and its impact on fecundity of the brown planthopper. Biochim Biophys Acta Gen Subj. 2017;1861(7):1750–1758. DOI:10.1016/j.bbagen.2017.03.011
- Ziegler R, Van Antwerpen R. Lipid uptake by insect oocytes. Insect Biochem Mol Biol. 2006;36(4):264–272.
- Fontana R, Della Torre S. The deep correlation between energy metabolism and reproduction: a view on the effects of nutrition for women fertility. Nutrients. 2016;8(2):87.
- Hansen M, Flatt T, Aguilaniu H. Reproduction, fat metabolism, and life span: what is the connection? Cell Metab. 2013;17(1):10–19.
- Buszczak M, Lu X, Segraves W, et al. Mutations in the midway gene disrupt a Drosophila acyl coenzyme A: diacylglycerol acyltransferase. Genetics. 2002;160(4):1511–1518. DOI:10.1093/genetics/160.4.1511
- Guo S, Tian Z, Zhu F, et al. Lipin modulates lipid metabolism during reproduction in the cabbage beetle. Insect Biochem Mol Biol. 2021;139:103668.
- Della Torre S, Benedusi V, Fontana R, et al. Energy metabolism and fertility: a balance preserved for female health. Nat Rev Endocrinol. 2014;10(1):13–23. DOI:10.1038/nrendo.2013.203
- Brookheart RT, Swearingen AR, Collins CA, et al. High-sucrose-induced maternal obesity disrupts ovarian function and decreases fertility in Drosophila melanogaster. Biochim Biophys Acta Mol Basis Dis. 2017;1863(6):1255–1263. DOI:10.1016/j.bbadis.2017.03.014
- Liao S, Amcoff M, Nässel D. Impact of high-fat diet on lifespan, metabolism, fecundity and behavioral senescence in Drosophila. Insect Biochem Mol Biol. 2021;133:103495.
- Belles X. MicroRNAs and the Evolution of Insect Metamorphosis. Annu Rev Entomol. 2017;62(1):111–125.
- Zhang Q, Dou W, Pan D, et al. Genome-wide analysis of microRNAs in relation to pupariation in oriental fruit Fly. Front Physiol. 2019;10:301.
- Neshat S, Tzeng S, Green J. Gene delivery for immunoengineering. Curr Opin Biotechnol. 2020;66:1–10.
- Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–524.
- Reinhart B, Slack F, Basson M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901–906. DOI:10.1038/35002607
- Vella M, Choi E, Lin S, et al. The C. elegans microRNA let-7 binds to imperfect let-7 complementary sites from the lin-41 3‘UTR. Genes Dev. 2004;18(2):132–137. DOI:10.1101/gad.1165404
- Hussain M, Asgari S. Functional analysis of a cellular microRNA in insect host-ascovirus interaction. J Virol. 2010;84(1):612–620.
- Skalsky R, Cullen B. Viruses, microRnas, and host interactions. Annu Rev Microbiol. 2010;64(1):123–141.
- Zhou R, Rana T. RNA-based mechanisms regulating host-virus interactions. Immunol Rev. 2013;253(1):97–111.
- Qiao J, Du Y, Yu J, et al. MicroRNAs as potential biomarkers of insecticide exposure: a review. Chem Res Toxicol. 2019;32(11):2169–2181. DOI:10.1021/acs.chemrestox.9b00236
- Zhang Q, Dou W, Song ZH, et al. Identification and profiling of Bactrocera dorsalis microRnas and their potential roles in regulating the developmental transitions of egg hatching, molting, pupation and adult eclosion. Insect Biochem Mol Biol. 2020;127:127.
- Ling L, Kokoza VA, Zhang C, et al. MicroRNA-277 targets insulin-like peptides 7 and 8 to control lipid metabolism and reproduction in Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2017;114(38):E8017–8024. DOI:10.1073/pnas.1710970114
- Zhang R, Zhang S, Li T, et al. RNA sequencing identifies an ovary-enriched microRNA, miR-311-3p, involved in ovarian development and fecundity by targeting Endophilin B1 in Bactrocera dorsalis. Pest Manag Sci. 2022;79(2):688–700. DOI:10.1002/ps.7236 .
- Chou MY, Mau RF, Jang EB, et al. Morphological features of the ovaries during oogenesis of the Oriental fruit fly, Bactrocera dorsalis, in relation to the physiological state. J Insect Sci. 2012;12(144):1–12. DOI:10.1673/031.012.14401
- Hagedorn HH, Fallon AM. Ovarian control of vitellogenin synthesis by the fat body in Aedes aegypti. Nature. 1973;244(5411):103–105.
- Rolf Z, Rik VA. Lipid uptake by insect oocytes. Insect Biochem Mol Biol. 2006;36(4):264–272.
- Reichholf B, Herzog VA, Fasching N, et al. Time-resolved small RNA sequencing unravels the molecular principles of MicroRNA homeostasis. Mol Cell. 2019;75(4):756–768 e757. DOI:10.1016/j.molcel.2019.06.018
- Enright AJ, John B, Gaul U, et al. MicroRNA targets in Drosophila. Genome Biol. 2003;5(1):R1. DOI:10.1186/gb-2003-5-1-r1
- Lewis BP, Shih IH, Jones-Rhoades MW, et al. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. DOI:10.1016/S0092-8674(03)01018-3
- Kruger J, Rehmsmeier M. Rnahybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34(Web Server issue):W451–454.
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861.
- Frank DA, Greenberg ME. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994;79(1):5–8.
- Gaddelapati SC, Dhandapani RK, Palli SR. CREB-binding protein regulates metamorphosis and compound eye development in the yellow fever mosquito, Aedes aegypti. Biochim Biophys Acta, Gene Regul Mech. 2020;1863(8):194576.
- Song W, Cheng D, Hong S, et al. Midgut-derived activin regulates glucagon-like action in the fat body and glycemic control. Cell Metab. 2017;25(2):386–399. DOI:10.1016/j.cmet.2017.01.002
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431(7006):316–320.
- Xu PZ, Vernooy SY, Guo M, et al. The Drosophila MicroRNA mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol. 2003;13(9):790–795. DOI:10.1016/S0960-9822(03)00250-1
- Iovino N, Pane A, Gaul U. MiR-184 has multiple roles in Drosophila female germline development. Dev Cell. 2009;17(1):123–133.
- Xie J, Chen H, Zheng W, et al. MiR-275/305 cluster is essential for maintaining energy metabolic homeostasis by the insulin signaling pathway in Bactrocera dorsalis. PLoS Genet. 2022;18(10):e1010418. DOI:10.1371/journal.pgen.1010418
- Lin H, Chen C, de Belle J, et al. CREBA and CREBB in two identified neurons gate long-term memory formation in Drosophila. Proc Natl Acad Sci U S A. 2021;118(37):e2100624118. DOI:10.1073/pnas.2100624118.
- Qin D, Zhou Y, Zhang P, et al. Azadirachtin downregulates the expression of the CREB gene and protein in the brain and directly or indirectly affects the cognitive behavior of the Spodoptera litura fourth-instar larvae. Pest Manag Sci. 2021;77(4):1873–1885. DOI:10.1002/ps.6212
- Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12(3):141–151.
- Yang H, He X, Yang J, et al. Activation of Camp-response element-binding protein is positively regulated by PKA and calcium-sensitive calcineurin and negatively by PKC in insect. Insect Biochem Mol Biol. 2013;43(11):1028–1036. DOI:10.1016/j.ibmb.2013.08.011
- Iijima K, Zhao L, Shenton C, et al. Regulation of energy stores and feeding by neuronal and peripheral CREB activity in Drosophila. PLoS ONE. 2009;4(12):e8498. DOI:10.1371/journal.pone.0008498
- Kirfel P, Vilcinskas A, Skaljac M. Acyrthosiphon pisumLysine Acetyltransferase p300/CBP plays an important role in reproduction, embryogenesis and longevity of the pea aphid. Insects. 2020;11(5):265.
- Dittmer N, Sun G, Wang S, et al. CREB isoform represses yolk protein gene expression in the mosquito fat body. Mol Cell Endocrinol. 2003;210(1–2):39–49. DOI:10.1016/j.mce.2003.08.010
- Koo S, Flechner L, Qi L, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437(7062):1109–1111. DOI:10.1038/nature03967
- Kramer J, Davidge J, Lockyer J, et al. Expression of Drosophila FOXO regulates growth and can phenocopy starvation. BMC Dev Biol. 2003;3(1):5. DOI:10.1186/1471-213X-3-5
- Zhang J, Zhang Z, Zhang R, et al. Identification of COP9 signalosome subunit genes in bactrocera dorsalis and functional analysis of csn3 in female fecundity. Front Physiol. 2019;10:162.
- Cai Z, Yao Z, Li Y, et al. Intestinal probiotics restore the ecological fitness decline of Bactrocera dorsalis by irradiation. Evol Appl. 2018;11(10):1946–1963. DOI:10.1111/eva.12698
- Urban A, Neukirchen S, Jaeger KE. A rapid and efficient method for site-directed mutagenesis using one-step overlap extension PCR. Nucleic Acids Res. 1997;25(11):2227–2228.
