INTRODUCTION
Immunotoxicology may be broadly defined as the evaluation of effects on the structure and function of the immune system and its components following exposure to chemical, biological, or physical agents; these effects may be either temporary or permanent. It may be argued that the discipline was inaugurated by J. G. Vos with the publication of his seminal review in 1977 (Vos, Citation1977). For much of its history since that time, the term “immunotoxicology” has been almost synonymous with immunosuppression (Dean et al., Citation2001). This is due in large part to the initial development and standardization of the testing paradigms and functional and host resistance models for assessing immune status, particularly in the United States and Europe. Although many of these models could, in theory, be employed to assess immunostimulation, a review of the early literature reveals a limited application of the models for this purpose.
The Traditional Model of Immunotoxicology
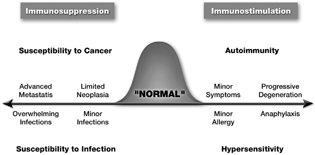
The “traditional” model of immunotoxicology (in the sense that it is the model most commonly invoked) is sometimes referred to as the continuum of immune response, and is illustrated in . In this model, the immune response is envisioned as a Poisson distribution, assuming that the immune response of a population falls within a narrow range of values; excursion from this range becomes pathological. For the purposes of experimental immunotoxicology (i.e., using purpose-bred, genetically similar/identical animals living in a microbially benign, stress-free, and highly controlled environment), this Poisson distribution may be accurate, but is unlikely to be very useful for realistic risk assessment. The result of perturbing this response in a negative fashion, generally termed immunosuppression, is straightforward: a diminished functional capability of 1 or several of the various components of the immune system. These perturbations, whether structural, functional, or a combination of the 2, can result in a graded decrease in the ability of the host to control infection or neoplasia. Based on the generally accepted notion that the purpose of the immune system is to differentiate self from nonself, we refer to immunity as homeostasis of identity.
Whereas the traditional model probably adequately explains a decreased control of infection and neoplasia, the obverse condition of increased immune function leading to either autoimmunity or hypersensitivity is not always consistent with either experimental or clinical evidence. The limitation with this model is its assumption of immune response linearity. Indeed, there are only limited data demonstrating that autoimmunity is a consequence of augmented normal immunity. Granted, in many cases autoimmune reactions are vigorous and do not appear to be under the autocrine feedback control that is evident in normal immunity. Rather, the defining characteristic of autoimmunity is that the response is directed toward antigens that are, teleologically speaking, inappropriate.
The placement of hypersensitivity in this continuum is also problematic because these types of reactions, although undesirable from the standpoint of human health, are arguably within the range of normal human immune function. The same humoral and cellular mechanisms that eliminate parasites or other pathogens can, in some individuals, recognize ragweed pollen or even perfumes as dangerous. Viewed in this way, hypersensitivity may be thought of as normal in its purpose, but sometimes inappropriately directed and even pathological in its intensity.
Another important consideration, but one that is often overlooked, is that assumptions built into this model limit the practicality of applying results of animal studies to human risk assessment. For example:
The traditional model does not account for multiplicity of effector and control mechanisms, but rather simplistically lumps multiple interrelated cellular networks together as the “immune system”;
The traditional model does not account for interaction of the immune network with other organ systems, particularly the nervous and endocrine systems;
The traditional model does not account for environmental and individual heterogeneity.
Thus, it is our contention that the 2-dimensional traditional model of immunotoxicology does not adequately explain the complexity of how the immune response is affected by encounters with the real world. For this reason, we propose the following model of immunotoxicology.
A New Model of Immunotoxicology
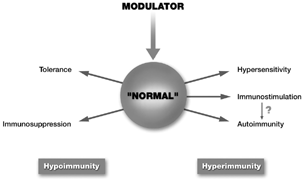
Our proposed alternative model is shown in . In this model, the immune response may be envisaged as a flexible “balloon” capable of expanding (corresponding to an increased level of activity following microbial challenge), shrinking (the resolution of an infection and a return to resting levels of activity), and being “squeezed” in different directions when acted on by an outside agent. (To avoid burdening the agent with positive or negative connotations, in the model we refer to this simply as a “modulator”.
The nature of the modulator, and its interaction with the immune system, determines the consequence of this interaction. The modulator may be an antigen, an external agent such as an environmental chemical, a physical agent such as ultraviolet light, or a physiological action in a related organ system, such as psychogenic stress. As a consequence of this modulator's action, a condition of hypoimmunity may be induced in which immune function may be decreased either generally (i.e., immunosuppression) or in an antigen-specific manner (i.e., tolerance). Conversely, depending upon on the nature of the modulator, a condition of hyperimmunity may result; this may take the form of hypersensitivity (a strong immune reaction that is inappropriately directed and often pathological), immunostimulation (enhancement of a normal function that may or may not be pathological), or autoimmunity (in which the immune system mounts a defensive response against the host, generally pathological). To continue our analogy, hypoimmunity would limit the degree to which the balloon could expand in response to an immune stimulus, whereas hyperimmunity would cause the balloon to expand beyond its normal limits.
An additional advantage of the new model is that the magnitude of the normal immune response (i.e., the size of the balloon) is not constrained by artificial ranges; in the traditional model, the area under the curve is never specified as either resting or active and therefore is of limited value. In the new model, each individual animal (rodent, primate, or any other species) serves as its own internal reference. For laboratory animals of defined genetic background and maintained under optimal healthy conditions, one can assume limited variability and the possibility of performing statistical analyses. We consequently assume that similar animals will have a similar response to the same modulator. For primates (including humans), we cannot assume this homogeneity in response and therefore would use each individual animal as its own control. By taking this individuality into account, we can hopefully gain a more realistic perspective on the difficulties of predicting immunomodulatory events, as well as the challenge of designing a one-size-fits-all approach to therapeutic immunomodulation (Kahan, Citation2003).
An intriguing corollary of this model is that certain reactions often termed “immunotoxic” may be the result of dysregulation of normal regulatory pathways. Although certain toxicants act on the immune system by brute force (e.g., loss of immune cells via bone marrow ablation, loss of proliferating cells by cytostatic agents, etc.), there are few if any examples of agents that produce effects in the immune system that are abnormal per se. The brute force modulators would probably be identified using standard toxicological models (Hastings, Citation2002); it is the more subtle modulators that require sophisticated methods and techniques to identify. One example would be agents that influence the TH1/TH2 immunoregulatory circuit. This circuit, in highly simplistic terms, directs the immune system to mount either a humoral or cell-mediated response. Certain chemicals may influence this pattern, resulting in immune dysregulation (van Zijverden et al., Citation2000; Pieters et al., Citation2003). Admittedly, the new model still represents a black box (or rather, a black balloon) in that the details of how a modulator interacts with the immune system is not easily illustrated. However, viewing the immune system as elastic and capable of returning to its normal configuration in the absence of a modulating influence obviates the need for this complexity, at least from an initial evaluation standpoint.
Although illustrated here in 2 dimensions for the sake of simplicity, the new model does not presuppose that only 1 modulator acts on the immune system at any given time (which was a limitation of the traditional model). One can easily imagine multiple modulators acting on the immune system simultaneously, in effect vectoring the immune response. This may explain why immunostimulation is sometimes, although not always, associated with the development of autoimmunity. This vectoring, along with the concept of modulators changing the direction or intensity of an immune response without altering its inherent nature, suggest that immunotoxicology could more accurately be designated “multidimensional immunomodulation.” (Greater accuracy not withstanding, we are not advocating the general use of this unwieldy terminology.)
Practical Implications of the New Model
Unfortunately, the new model does not answer some of the most vexing questions in immunotoxicology. Primary among these is the question of at what point does suppression of a normal immune response become pathological or, more specifically, toxicological. This is an ongoing concern during routine immunotoxicology testing. For example, how does one relate statistically significant differences between test and control animals with ultimate biological consequences? More to the point, when does immune suppression translate to altered host defense? Unfortunately, there are no nomograms allowing one to precisely correlate a particular degree of immunosuppression with unacceptable outcomes.
In addition, if it is assumed that immunosuppression is necessarily bad, does it always follow that immunostimulation is good? Certainly, homeostasis of identity is necessary for any organism's survival, even phylogenetically primitive creatures. However, is more of a good thing necessarily better? This is the essential conundrum: what is a superoptimal immune response, and how does one recognize it? In the following sections, we will discuss some of the major issues in immunotoxicology and how the concept of multidimensional immunomodulation can help address them.
Inside the Balloon
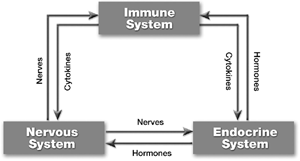
The most obvious consequence of using the new model is that it helps us consider the immune system as a dynamic interaction of multiple systems, any component of which may serve as a target for modulation. The most notable interactions of the immune system are with the nervous and endocrine systems; this tripartite system is commonly referred to as the neuroimmunoendocrine system (Raison and Miller, Citation2001) and is illustrated in . The neuroimmunoendocrine system has been acknowledged as being of potential importance for immunotoxicology (Fuchs and Sanders, Citation1994). However, the traditional model tended to obscure its role. In the new model, we must view the immune balloon not as a well-delineated entity (the Poisson curve of the old model), but rather as a fluid system with many interconnected components. In this way, we can now begin to consider not only how to design studies to comprehensively evaluate the total immune response, but also to begin devising ways in which the system may be therapeutically manipulated to vector the immune response in desired directions (Abo and Kawamura, Citation2002; Frieri, Citation2003; Pozo, Citation2003).
Immunostimulation
To effectively neutralize a variety of disruptions of self (whether an external invader or an internal traitor), the immune system must muster powerful cellular and biochemical responses. Naturally, such a system must employ feedback loops and other means of limiting and eventually halting these reactions and returning to a quiescent state. Slight immune deficiencies are usually compensated for; it is only when the immune system as a whole is damaged that pathology ensues. Although there are a plethora of modulators known to suppress the immune system, there are relatively fewer that have been demonstrated to be chronically stimulatory. As previously mentioned, the long-term consequences of such stimulation are currently unknown. However, in the short-term, there are certain situations where immunostimulation appears to be beneficial. One of these is therapeutic immunostimulation.
Therapeutic augmentation of the immune system is desirable in cases where the immune response is either congenitally deficient (severe combined immunodeficiency syndrome), too damaged to function properly (for example, in AIDS), or ablated either accidentally (radiation sickness) or clinically (bone marrow transplantation). As a sign of the times, a potentially important use for therapeutic stimulation may be the prophylactic activation of the innate immune system as a defense, and possibly as a treatment, for attack with biological weapons.
Host defense in vertebrates may be very roughly divided into nonspecific and specific arms (referring to a combination of interacting mechanisms). Nonspecific host defense, also termed innate immunity, includes cellular components such as natural killer cells and phagocytic cells such as macrophages and neutrophils, as well as soluble factors including complement and defensins. Following an activation signal (tissue damage, release of bacterial constituents), there is an almost immediate coordination of these cellular and soluble factors to nonspecifically combat a variety of nonself agents, without the development of a memory response. Conversely, specific immunity takes several days to evolve. Once this memory-based specificity has developed, secondary exposure to the threat results in a quicker, more targeted (anamnestic) response. It follows that a specific immune response to an immediate, relatively fast-acting, and highly dangerous threat (say, for example, germinating anthrax spores) could conceivably take long enough to establish that the host would succumb. In such cases, it may be beneficial to artificially accelerate the nonspecific response to protect the host until specific immunity can be established.
An obvious method for augmenting host defense (both specific and nonspecific) would be to use the body's own regulatory mechanisms, such as cytokines and other soluble mediators. Cytokines may be administered as recombinant versions of the natural molecules, although high systemic levels have been associated with toxicity (Winkler et al., Citation1999). Other approaches include versions of these molecules that have been genetically modified to limit their toxicity and enhance their efficacy (House, Citation2002). Another approach for therapeutic stimulation of the immune response is to use small molecule drugs to precisely modulate key events (Davies et al., Citation2001; Kumar et al., Citation2004). In the case of thalidomide, beneficial immunomodulatory effects may have been discovered inadvertently in studies designed to determine immunosuppressive activity (Karrow et al., Citation2000). Research into these various therapeutic modalities is making great advances.
Hypersensitivity
Hypersensitivity refers to a condition of reactivity to antigen that exceeds what would be considered “normal” since, in almost all cases, the result of this reactivity is deleterious rather than protective. (We are here referring to true immunological hypersensitivity, rather than including reactivity to irritants, pseudoallergy, or other conditions that share many of the same characteristics as immune-mediated hypersensitivity.) Although the particular cellular and molecular mechanisms involved in these responses are appropriate to an antigenic challenge, the vigor of the reactions results in consequences that can range from annoying (hay fever) to potentially pathological (poison ivy) to lethal (anaphylaxis). Although some of the antigens that induce these reactions are innocuous to most individuals (animal dander or pollen), there are many industrial and consumer care chemicals that can induce various degrees of hypersensitivity in humans. Of particular note is the observation that local allergic reactions can have distal effects due, at least in part, to circulating cytokines (Togias, Citation2004). Although long known to be an important basis of allergic contact dermatitis, these effects may also be important in other forms of allergy as well as immune tolerance.
Autoimmunity
For many years, one of the central dogmas of immunology was that the purpose of this system was to differentiate “self” from “nonself,” these terms being largely self-explanatory. During ontology, the immune system becomes educated to make this distinction, and after a certain point of no return, mounts a defense against anything foreign. This model, however, tends to break down in the condition known as autoimmunity, in which the immune system begins to recognize self as nonself. To a limited degree, autoimmunity appears to be part of the normal physiological process, possibly as a means of damping an active immune or inflammatory response (Bondanza et al., Citation2004; Nevo et al., Citation2004). However, when autoimmunity becomes pathological, damaging tissues or organ systems, it becomes autoimmune disease. A degree of comfort is obtained in that in autoimmune disease usually only a portion of self is recognized as foreign, although when that portion is a tissue such as Islet cells in the pancreas, it is cold comfort.
Using the self/nonself paradigm, numerous hypotheses have been advanced to account for autoimmunity. These hypotheses include similarity between self antigens and the inadvertent modification of self proteins, e.g., by modification following covalent binding of a chemical to self proteins, the breakdown of regulatory mechanisms that normally prevent recognition of self (e.g., suppression of autoreactivity, regulation of apoptosis, induction of anergy), or polyclonal lymphocyte activation, as by exposure to bacterial endotoxins or superantigens (Riminton et al., Citation2004; Wen et al., Citation2004). This latter possibility connects immunostimulation with autoimmunity as per the original paradigm, although this connection is by no means consistent. A more contemporary explanation for autoimmunity invokes the concept of danger. Paraphrased, this concept proposes that the immune system is unconcerned with notions of self and nonself; rather, the immune system is designed to recognize danger signals, and it is within this context that reactions consistent with autoimmunity (as well as hypersensitivity) are manifest (Matzinger, Citation1998).
Regardless of the ultimate mechanism(s) of autoimmunity, it is well established that susceptibility depends to a large degree on genetic predisposition. In this respect, it is similar to many types of hypersensitivity, in which certain individuals exhibit a greater propensity toward reactivity. It differs, however, in that predicting the potential of materials to induce reactivity is not as straightforward. In fact, predicting the potential of drugs to induce autoimmunity is currently one of the most problematic issues in preclinical immunotoxicology assessment.
Idiosyncratic Drug Reactions and Systemic Drug Hypersensitivity
We earlier mentioned how the development of immunotoxicology as a scientific discipline tended to focus on immunosuppression and its consequences. In pharmaceutical development, however, it has been the case that few drug candidates exhibit this activity, or at least to a degree that would cause concern. In comparison, hyperimmune reactions turn out to be rather common. This arena where the hyperimmune responses of hypersensitivity and autoimmunity appear to converge comprises the nonexclusive categories of idiosyncratic drug reactions and systemic drug hypersensitivity. According to Uetrecht (Citation1999) these reactions, often referred to as type B (bizarre) reactions, do not occur in all patients at any dose, and do not appear to involve the known pharmacologic properties of the drug itself. Given that the etiology of these responses is often unknown, predicting these reactions is quite difficult. Development of such assays is currently one of the most important goals in immunotoxicology (Naisbitt, Citation2004).
The Role of Apoptosis in Immunomodulation
Another area to consider is apoptosis and the role of this phenomenon in immunomodulation. It is well known that most immature cells are eliminated via this mechanism to maintain immune tolerance for self and to prevent deleterious hyper reactivity of the immune system. What is important to understand, however, is that immune regulation via apoptosis involves the interplay of many cell types and their associated molecules (Rieux-Laucat et al., Citation2003). Compounds that eliminate or suppress certain immune cell types in a relatively discrete manner, such as certain therapeutic immunosuppressants, may actually activate certain immune functions. Probably the best-studied example is cyclosporine-induced autoimmunity. This phenomenon is associated with immune dysregulation, resulting in paradoxical upregulation of certain immune parameters (Jenkins et al., Citation1988; Damoiseaux et al., Citation1997; Zheng et al., Citation1998). Although the pattern of cyclosporine-associated autoimmune disease varies, autoimmune vasculopathy has been associated with chronic rejection of solid organ transplants (Chen et al., Citation2001). Exposure to cyclosporine has been demonstrated to produce autoimmune disease in neonatal animals (Sakaguchi and Sakaguchi, Citation1989). Infants born to female kidney transplant patients have been shown to develop signs of immune dysfunction: whether this results in increased susceptibility to immune dysfunction later in life remains controversial (Di Paolo et al., Citation2000).
It is also interesting to note that many drugs associated with allergies are given to patients with infections; thus, the immune system is exposed to potential haptens under conditions of heightened immune responsiveness (Park and Kitteringham, Citation1990). This could be part of the basis for hypersensitivity to antibiotics. Finally, the consequences of immune dysregulation and immune restoration have been observed in patients with HIV disease. These patients have long been known to be at increased risk for development of drug allergies, a seemingly paradoxical adverse effect (Pirmohamed and Park, Citation2001). Patients on highly active anti-retroviral therapy often develop signs of inflammatory disease as their immune status improves (Shelburne and Hamill, Citation2003). Thus, in clinical practice, the results of immunosuppression and normalization of immune function are often contrary to what might be expected based on simplistic assumptions about immune function.
The Way Ahead
Based on the preceding discussion, we propose that consideration be given to modifying the theoretical framework for immunotoxicology. It should be noted that much of this field began at a time when the molecular aspects of the immune response were still poorly understood by modern standards and techniques such as monoclonal antibody production, flow cytometry, genomics, proteomics, and so forth were in their infancy. We now understand that immunotoxicity, or, as we prefer here, inadvertent immunomodulation, can involve subtle mechanisms of toxicity. For example, consider contact hypersensitivity. As previously discussed, compounds that induce allergic contact dermatitis seem to share certain properties: they are reactive compounds, especially tending to bind to amino acid residues in proteins; they tend to be irritants, possibly a property related to their inherent reactivity; and, in situ reactivity tends to result in the release by epidermal cells of pro-inflammatory molecules such as TNF-α and IL-1, that prime the immune system (Kimber et al., Citation2002). Subsequent exposure elicits an enhanced, compound specific inflammatory reaction. Although we think of this as an adverse reaction, another way to think of this is that a protective system has been activated, with proteins acting as molecular scavengers that prevent reactive chemicals from binding to critical cellular targets and the immune response a normal reaction to such exposure.
CONCLUSION
In conclusion, assessing the ability of drugs, chemicals, or other modulators to produce unintended immunosuppression and determining the mechanisms whereby they do so remains an important goal for immunotoxicologists due to the well-recognized health consequences of a suboptimal immune response. However, it is important to recognize that a more global view of the immune response suggests that inappropriately directed and heightened immunity, a condition we have termed “hyperimmunity” could be at least as important in the context of human health. Many of the experimental paradigms and methodologies that have been developed to assess immunosuppression are increasingly being used to evaluate this other face of immunotoxicology, and, a comprehensive approach to safety assessment will eventually incorporate the panoply of immune responses.
The opinions expressed here are those of Dr. Hastings and do not reflect official FDA positions.
REFERENCES
- Abo T., Kawamura T.. 2002. Immunomodulation by the autonomic nervous system: Therapeutic approach for cancer, collagen diseases, and inflammatory bowel diseases. Ther. Apher.. 6: 348–357. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Bondanza A., Zimmermann V. S., Dell'Antonio G., Cin E. D., Balestrieri G., Tincani A., Amoura Z., Piette J. C., Sabbadini M. G., Rovere-Querini P., Manfredi A. A.. 2004. Requirement of dying cells and environmental adjuvants for the induction of autoimmunity. Arthritis Rheum.. 50: 549–1560
- Chen W., Thoburn C. J., Miura Y., Sommer M., Hruban R., Qian Z., Baldwin W., Hess A. D.. 2001. Autoimmune-mediated vasculopathy. Clin. Immunol.. 100: 57–70. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Damoiseaux J. G. M., Beijleveld L. J. J., Van, Breda Vriesman P. J. C.. 1997. Multiple effects of cyclosporin A on the thymus in relation to T-cell development and autoimmunity. Clin. Immunol. Immunopathol.. 82: 197–202. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Davies F. E., Raje N., Hideshima T., Lentzsch S., Young G., Tai Y. T., Lin B., Podar K., Gupta D., Chauhan D., Treon S. P., Richardson P. G., Schlossman R. L., Morgan G. J., Muller G. W., Stirling D. I., Anderson K. C.. 2001. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 98: 210–216. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Dean J. H., House R. V., Luster M. I.. 2001. Immunotoxicology: Effects of, and response to, drugs and chemicals, In. Principles and Methods of Toxicology, Fourth Edition, A. W., Hayes, pp. 1415–1450, Taylor & Francis, London
- Di, Paolo S., Schena A., Morrone L. F., Manfredi G., Stallone G., Derosa C., Procino A., Schena F. P.. 2000. Immunologic evaluation during the first year of life of infants born to cyclosporine-treated female kidney transplant recipients. Transplantation. 69: 2049–2054. [PUBMED], [INFOTRIEVE]
- Friedman E. M., Lawrence D. A.. 2002. Environmental stress mediates changes in neuroimmunological interactions. Toxicol. Sci.. 67: 4–10. [PUBMED], [INFOTRIEVE]
- Frieri M.. 2003. Neuroimmunology and inflammation: Implications for therapy of allergic and autoimmune diseases. Ann. Allergy Asthma Immunol.. 90(6 Supp. 3)34–40. [PUBMED], [INFOTRIEVE]
- Fuchs B. A., Sanders V. M.. 1994. The role of brain-immune interactions in immunotoxicology. Crit. Rev. Toxicol.. 24: 151–176. [PUBMED], [INFOTRIEVE]
- Hastings K. L.. 2002. Implications of the new FDA/CDER immunotoxicology guidance for drugs. Int. Immunopharmacol.. 2: 1613–1618. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Hilts P. J.. 2003; Protecting America's Health. The FDA, Business, and One Hundred Years of Regulation. Alfred A. Knopf, New York
- House R. V.. 2002. Preclincial immunotoxicity assessment of cytokine therapeutics. Biotechnology and Safety Assessment, Third Edition, J. A., Thomas, R. L., Fuchs, pp. 191–231, Academic Press, San Diego
- Jenkins M. K., Schwartz R. H., Pardoll D. M.. 1988. Effects of cyclosporine A on T cell development and clonal deletion. Science. 241: 1655–1658. [PUBMED], [INFOTRIEVE]
- Kahan B. D.. 2003. Individuality: The barrier to optimal immunosuppression. Nat. Rev. Immunol.. 3: 831–838. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Karrow N. A., McCay J. A., Brown R. D., Musgrove D. L., Pettit D. A., Munson A. E., Germolec D. R., White K. L., Jr.. 2000. Thalidomide stimulates splenic IgM antibody response and cytotoxic T lymphocyte activity and alters leukocyte subpopulation numbers in female B6C3F1 mice. Toxicol. Appl. Pharmacol.. 165: 237–244. [PUBMED], [INFOTRIEVE]
- Kimber I, Basketter D. A., Gerberick G. F., Dearman R. A.. 2002. Allergic contact dermatitis. Int. Immunopharmacol.. 2: 201–211
- Kumar S, Jones T. R., Oakley M. S., Zheng H., Kuppusamy S. P., Taye A., Krieg A. M., Stowers A. W., Kaslow D. C., Hoffman S. L.. 2004. CpG oligodeoxy-nucleotide and montanide ISA 51 adjuvant combination enhanced the protective efficacy of a subunit malaria vaccine. Infect. Immun.. 72: 949–957. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Matzinger P.. 1998. An innate sense of danger. Sem. Immunol.. 10: 399–415
- Naisbitt D. J.. 2004. Drug hypersensitivity reactions in skin: Understanding mechanisms and the development of diagnostic and predictive tests. Toxicology. 194: 179–196. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Nevo U., Golding I., Neumann A. U., Schwartz M., Akselrod S.. 2004. Autoimmunity as an immune defense against degenerative processes: A primary mathematical model illustrating the bright side of autoimmunity. J. Theor. Biol.. 227: 583–592. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Park B. K., Kitteringham N. R.. 1990. Drug-protein conjugation and its immunological consequences. Drug Metab. Rev.. 22: 87–144. [PUBMED], [INFOTRIEVE]
- Pieters R., Ezendam J., Nierkens S.. 2003. Chemical-specific properties co-determine the type of adverse immune response. Autoimmun. Rev.. 2: 25–29. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Pirmohamed M., Park B. K.. 2001. HIV and drug allergy. Curr. Opin. Allergy Clin. Immunol.. 1: 311–316. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Pozo D.. 2003. VIP- and PACAP-mediated immunomodulation as prospective therapeutic tools. Trends Mol. Med.. 9: 211–217. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Raison C. L., Miller A. H.. 2001. The neuroimmunology of stress and depression. Semin. Clin. Neuropsychiatry. 6: 277–294. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Rieux-Laucat F., Fischer A., Le, Deist F.. 2003. Cell-death signaling and human disease. Curr. Opin. Immunol.. 15: 325–331. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Riminton D. S., Kandasamy R., Dravec D., Basten A., Baxter A. G.. 2004. Dermal enhancement: Bacterial products on intact skin induce and augment organ-specific autoimmune disease. J. Immunol.. 172: 302–309. [PUBMED], [INFOTRIEVE]
- Sakaguchi S., Sakaguchi N.. 1989. Organ-specific autoimmune disease induced in mice by elimination of T-cell subsets. V. Neonatal administration of cyclosporin A causes autoimmune disease. J. Immunol.. 142: 471–480. [PUBMED], [INFOTRIEVE]
- Shelburne S. A., III, Hamill R. J.. 2003. The immune reconstitution inflammatory syndrome. AIDS Rev.. 5: 67–79. [PUBMED], [INFOTRIEVE]
- Togias A.. 2004. Systemic effects of local allergic disease. J. Allergy Clin. Immunol.. 113: S8–S14. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Uetrecht J. P.. 1999. New concepts in immunology relevant to idiosyncratic drug reactions: The “Danger Hypothesis” and innate immune system. Chem. Res. Toxicol.. 12: 387–395. [CROSSREF], [PUBMED], [INFOTRIEVE]
- van, Zijverden M., van der, Pijl A., Bol M., van, Pinxteren F. A., de, Haar C., Penninks A. H., van, Loveren H., Pieters R.. 2000. Diesel exhaust, carbon black, and silica particles display distinct Th1/Th2 modulating activity. Toxicol. Appl. Pharmacol.. 168: 131–139. [PUBMED], [INFOTRIEVE]
- Vos J. G.. 1977. Immune suppression as related to toxicology. CRC Crit. Rev. Toxicol.. 5: 67–101. [PUBMED], [INFOTRIEVE]
- Wen L., Peng J., Li Z., Wong F. S.. 2004. The effect of innate immunity on autoimmune diabetes and the expression of Toll-like receptors on pancreatic islets. J. Immunol.. 172: 3173–3180. [PUBMED], [INFOTRIEVE]
- Winkler U., Jensen M., Manzke O., Schulz H., Diehl V., Engert A.. 1999. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (Rituximab, IDEC-C2B8). Blood. 94: 2217–2224. [PUBMED], [INFOTRIEVE]
- Zheng L., Trageser C. L., Willerford D. M., Lenardo M. J.. 1998. T-Cell growth cytokines cause the superinduction of molecules mediating antigen-induced T-lymphocyte death. J. Immunol.. 160: 763–769. [PUBMED], [INFOTRIEVE]