Abstract
The potential effects of the fungicide vinclozolin (VCZ) on the immune system were evaluated in F0 (dams) and F1 generations of Sprague Dawley rats exposed to a soy-free diet containing VCZ at 10, 150 and 750 ppm. In dams, exposure to VCZ at the highest concentration from gestation day 7 to postpartum day 51 (65 days total exposure) produced a significant increase in the numbers of splenocytes, B cells, T cells, helper T cells and cytotoxic T cells and a decrease in the percentage of NK cells. In F1 males, exposure to VCZ gestationally, lactationally and through feed from postnatal day 22 to 64 (78 days total exposure) produced no effect on spleen or thymus weights or splenocyte subsets. However, increases in the spleen IgM antibody-forming cell response to sheep red blood cells (150 and 750 ppm) and the activity of NK cells (150 ppm) were observed. In F1 females, exposure to VCZ produced a decrease in the activity of NK cells in all the treatment groups. Although decreases in the number of cytotoxic T cells (150 ppm) and the percentages of NK cells (10 ppm) and cytotoxic T cells (150 ppm) were also observed, the lack of a dose-related response suggested that these findings might not be biologically meaningful. In conclusion, these results demonstrate that exposure to VCZ at the concentrations tested modulates the immune responses in Sprague Dawley rats. Furthermore, the differential effect of VCZ in F1 male and female rats is consistent with the reported anti-androgenic properties of VCZ.
INTRODUCTION
Vinclozolin (VCZ) is a dicarboximide fungicide used on fruits, vegetables, ornamental plants, and vines (Ronis and Badger, Citation1995). There is evidence that VCZ [3-(3,5-dichlorophenyl)-5-methyl-5-vinyl-oxazolidine-2,4-dione] has antiandrogen activity and alters sex differentiation in male rats by inhibition of androgen receptor (AR)-mediated gene activation (Wong et al., Citation1995; Kelce et al., Citation1994). In male rat offspring perinatal exposure to VCZ causes hypospadias, ectopic testes, vaginal pouch formation, agenesis of the ventral prostate, and nipple retention, whereas female offspring are phenotypically normal (Gray et al., Citation1994). Furthermore, VCZ can produce these malformations at doses that have little or no reproductive effect in adults (Kelce and Wilson, Citation1997). It has been suggested that the two primary metabolites of VCZ–2-[[(3, 5-dichlorophenyl)-carbamoyl] oxy]-2-methyl-3-butenoic acid (M1) and 3′,5′dichloro-2-hydroxy-2-methylbut-3-enanlide (M2)—compete for AR androgen binding and inhibit androgen-induced transcriptional activation by blocking AR binding to androgen response element. The parent compound VCZ, on the other hand, is a poor inhibitor (Wong et al., Citation1995; Kelce et al., Citation1994).
In 1999, the National Research Council of the U.S. National Academy of Sciences articulated an “Endocrine Disruptor” hypothesis that environmental exposure to a variety of endocrine-active chemicals may contribute to a variety of adverse effects in wildlife and humans (1999). As a result of reports of transplacental effects of estrogenic compounds, and evidence that these chemicals can modify immune responses particularly when administered perinatally during lymphoid organ organogenesis (CitationDelclos and Newbold, 1997; Olsen and Kovacs, Citation1996; Holladay et al., Citation1993), the potential for perinatal exposure to VCZ to alter immune function was investigated. In our study, it was hypothesized that adult and developmental exposures to VCZ had the potential to adversely affect the immune system. Furthermore, developmental exposure to VCZ might also produce differential immunomodulation in F1 male and female rats since there is evidence that in utero exposure to estrogen or androgen has sex-dimorphic effects on the immune response (CitationLuster et al., 1978, 1979; Shelat et al., Citation1997). Thus, several quantitative measures and functional assays (e.g., splenic phenotypic analysis, IgM antibody forming cell responses and natural killer cell activity) were employed to evaluate immune effects.
Both F0 (adult exposure only) and F1 generations (developmental and adult exposures) of Sprague Dawley rats were evaluated in these studies by exposing the animals to VCZ-containing food at dietary concentrations that altered the reproductive tract or other estrogen-sensitive organs of the offspring while causing only minimal maternal toxicity or other overt toxicity. VCZ is currently used on several U.S. and imported fruit and vegetable crops as well as on golf course turf grass, suggesting that human exposure to VCZ may occur by ingestion via pesticide residues on foods, inhalation via proximity to pesticide application, or dermal exposure via pesticide application and contact with turf and plants (U.S. EPA, Citation2001). Thus, human exposure can occur in utero, via breastfeeding and through the direct consumption of VCZ-contaminated food. Therefore, our initial study was performed in the animals that were exposed VCZ-containing food. Dams were fed a soy-free diet containing VCZ at the concentrations of 0, 10, 150, and 750 ppm from gestation day 7 to postpartum day 51. For pups, exposure occurred through dams gestationally and lactationally, and in the diet from feeding until postnatal day (PND) 64. The results demonstrated that exposure to VCZ modulateed the immune responses in Sprague Dawley rats. Furthermore, the differential modulation of VCZ in F1 male and female rats was consistent with the reported anti-androgenic effect of VCZ.
MATERIALS AND METHODS
Animals and Exposure
Time-mated primiparous Sprague Dawley rats (NCTR Strain 23CD) were obtained from the National Center for Toxicological Research (NCTR, Jefferson, AR) breeding colony (plug date = gestational day 0). Each dam was housed individually in a standard plastic cage containing hardwood chip bedding. The housing room was maintained on a 12:12 hour light-dark cycle, temperature at 23 ± 3°C, and humidity at 50 ± 10%.
Food and tap water were provided ad libitum. Two weeks prior to mating, dams (70–80 days old) were shifted from the standard autoclaved NIH-31 pellet diet to an irradiated soy- and alfalfa-free diet (5K96, purchased from Purina Mills, St. Louis, MO). This diet is based on the NIH-31 formula, except that casein replaces the protein contributed by soy and alfalfa, soy oil is replaced by corn oil, and the vitamin mix is adjusted for irradiation. The control diet was assayed for genistein and daidzein after hydrolysis of conjugates. The concentrations of both genistein and daidzein of this diet were determined by LC-ES/MS/MS to be approximately 0.5 ppm (Doerge et al., Citation2000). VCZ (purified from Ronilan WP, BASF Corp., Research Triangle Park, NC, by Battelle, Columbus, OH) was mixed into the standard 5K96 feed by the Diet Preparation Staff, Bionetics at the NCTR every three months. VCZ purity was > 99% as assessed by the Division of Chemistry at NCTR using HPLC analytic methods, mass spectrometry, and 1H NMR. Each dosed batch of feed was analyzed, and VCZ concentration was found to be stable for at least six months when stored refrigerated.
Dams were assigned to treatment groups based on their body weights at gestation day 0 such that groups had approximately equal weights. The dams (10 per dose group) consumed 5K96 diet containing 0, 10, 150, or 750 ppm VCZ for 65 days starting on day 7 of gestation. For a 250 g rat consuming 20 g chow every day, these concentrations are approximately equivalent to doses of 0.8, 12, and 60 mg VCZ/kg/day, respectively (Flynn et al., Citation2001). Litters remained with their biological dam whenever possible; fostering was done only to maintain a balanced sex ratio and then only within treatment groups. The day of birth was designated PND 1. On PND 2, litters were randomly culled to 8 pups, maintaining equal numbers of each sex. Offspring were weaned on PND 22 and housed 2 per cage with a same-sex sibling. Weaned pups continued on VCZ-containing 5K96 diet until sacrifice on PND 64 (78 days total exposure). The F0 and F1 rats were sacrificed at the NCTR, and the spleen and thymus were removed and cleaned of connective tissue before weighing. Spleens were placed into tubes containing gentamicin supplemented Earle's Balanced Salt Solution (EBSS). The tubes containing the whole spleens were packed on wet ice and shipped overnight to Virginia Commonwealth University (VCU, Richmond, VA) for assessment. The assays were performed on the same day the samples were received. The immunologic responses of spleens shipped to Richmond did not differ significantly from historical data from animals sacrificed at the VCU facility (Burns-Naas et al., Citation1998; Woolhiser and McCay, Citation1999).
All animal procedures were conducted under an animal protocol approved by the NCTR Institutional Animal Care and Use Committee (IACUC).
Enumeration of splenocytes
The quantification of splenocyte subsets was performed as previously described with modification (Bleavins et al., Citation1995). Briefly, splenocytes were prepared from rat spleens using a Stomacher 80 Lab Blender. After washing, the cells were resuspended in complete RPMI medium and counted using a Coulter Counter ZII with the red blood cells lysed using a ZAP-OGLOBIN II lytic reagent (Coulter Corporation, Miami, Florida).
Enumeration of Leukocyte Subpopulations in the Spleen
For analysis of leukocyte subpopulations, erythrocytes were lysed with ammonium chloride and splenocytes were labeled with the appropriate monoclonal antibody (mAb), conjugated with a fluorescent molecule for visualization. Specific mAbs were used for staining as described previously (Guo et al., Citation2002): anti-CD5 (clone OX19) conjugated to phycoerythrin (PE) for enumeration of pan T cells, anti-CD4 (clone OX38) conjugated to fluorescein isothiocyanate (FITC) for enumeration of CD4+ cells, and anti-CD8a (clone OX8) conjugated to FITC for enumeration of CD8+ cells. For both the CD4+ and CD8+ cells, a double label with OX19 was used. Anti-CD45RA (clone OX33) conjugated to FITC was used to enumerate CD45+ B cells. NK cells were enumerated using a FITC conjugated NKR-P1A antibody (clone 10/78) and OX8 conjugated to PE. Splenic macrophages were enumerated using HIS36 conjugated to PE. An appropriate isotype control was run for each of the antibodies used. All the antibodies were obtained from PharMingen (San Diego, CA). Following the initial staining with antibody and washing with staining buffer, propidium iodide (PI) solution was added as a viability stain. Viability was at least 85%. Samples were run on a Becton Dickinson FACScan. Nonviable cells were eliminated through setting a live gate on red fluorescence. Data for all samples were then acquired in list mode with no other gating, 5,000 events were counted for each sample.
Spleen IgM Antibody-Forming Cell Response to the T-dependent Antigen, sRBC
The primary IgM antibody-forming cell response to sheep red blood cells (sRBC) was measured using a modified hemolytic plaque assay of Jerne and Nordin (Citation1963). The F0 and F1 rats were sensitized with 2 × 108 sRBC (i.v.; Rockland, Gilbertsville, PA) four days prior to sacrifice. Spleen cell suspensions were prepared as described above, and an aliquot of cells was added to a test tube containing guinea pig complement (1:4; GIBCO, Gaithersburg, MD), sRBC and warm agar (45°C). The mixture was plated in a petri dish, covered with a microscope cover slip, and incubated at 37°C for 3 h. Antibody forming cells (AFC) were counted using a Bellco plaque viewer. The data were expressed as AFC/106 spleen cells and AFC/ spleen.
Natural Killer (NK) Cell Activity
The activity of NK cells was assayed by the chromium release assay as described (Bleavins et al., Citation1995). Single cell suspensions were adjusted to six concentrations: 2×107, 107, 5×106, 2.5 × 106,, 1.25 ×106 and 0.625 × 106 cells/ml. The target cells (106/ml), 51Cr-YAC-1 cells (ATCC, Rockville, MD), were added to each well of a 96-well plate in a volume of 0.1 ml. The effector cells were added in a volume of 0.1 ml to each of two replicate wells of target cells to obtain effector: target ratios of 200:1, 100:1, 50:1, 25:1, 12.5:1 and 6.25:1. The spontaneous release and the maximum release of chromium were determined by adding 0.1 ml of medium and Triton X-100 (0.1%) to each of 12 replicate wells containing the target cells, respectively. Following a 4-hour incubation, the plates were centrifuged, and 0.1 ml of the supernatant was removed from each well and counted using a gamma counter. The mean percent cytotoxicity at each effector concentration was determined.
Statistical Analysis
The Bartlett's Test for homogeneity was used to select the type of analysis to be conducted. Homogeneous data were analyzed using a one-way analysis of variance, and Dunnett's t test was used to determine differences between the experimental and the control groups. For non-homogeneous data, a non-parametric analysis of variance was used, and the difference between the control group and the experimental groups determined by the Wilcoxon Rank Test. The Jonckheere's Test was used to evaluate dose-related trends across the vehicle and VCZ treatment groups. Statistical significance was set at P ≤ 0.05.
RESULTS
F0 Generation Female Rats (Dams)
The F0 Sprague Dawley female rats were treated with VCZ for 65 days as described above. Exposure to VCZ at the concentrations tested had no significant effect on terminal body weight (). There was no change in the weights of spleen and thymus when the data were expressed as either an absolute or a relative value ().
Effect of vinclozolin on the body and organ weights of Sprague Dawley rats
Exposure to VCZ produced no changes in the percentages of splenic B cells, T cells, T-cell subsets and macrophages as compared to control animals (). However, a 22% decrease in the percentage of splenic NK cells was observed at the VCZ level of 750 ppm (). Exposure to VCZ produced a dose-related increase in the number of total splenocytes, and this increase reached the level of statistical significance at the highest VCZ level (). Correspondingly, when the data were expressed as absolute values for splenocyte subpopulations, a significant increase was also observed for splenic B cells, T cells and T-cell subsets at the highest VCZ level ().
Effect of vinclozolin on the number and percentage of splenocyte subpopulations in F0 female Sprague Dawley rats
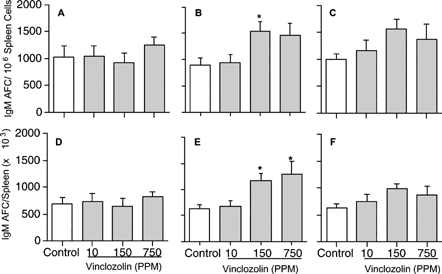
Exposure to VCZ had no effect on the IgM AFC response when the data were expressed as either the specific activity (AFC/106 cells; ) or the total activity (AFC/spleen; ).
1 Effect of vinclozolin on IgM antibody-forming cell (AFC) response in F0 (A, D), and F1 male (B, E) and female (C, F) rats. Rats were exposed to vinclozolin, and the number of IgM antibody-forming cells to sRBC in splenocytes determined as described. Data are presented as AFC/106 cells (the specific activity) and AFC/Spleen. All data are homogeneous except for the AFC/Spleen in F1 generation male rats. Values represent the mean ± SE derived from ten animals. *, p ≤ 0.05.
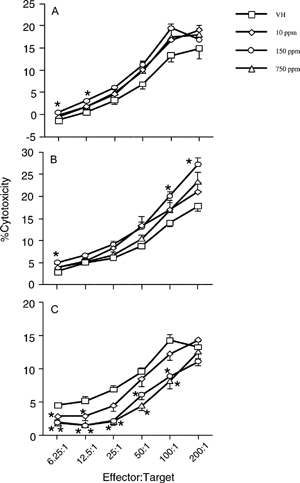
A slight, albeit statistically significant, increase in the percent cytotoxicity was observed at the effector:target ratios of 6.25:1 and 12.5:1 for the 150 ppm VCZ treatment group (). These increases might not be biologically meaningful since there were no significant differences between treatment groups and the vehicle controls at other E:T ratios.
2 Effect of vinclozolin on NK cell activity in F0 (A), and F1 male (B) and female (C) rats. Rats were exposed to vinclozolin, and the activity of NK cells determined as described. All the data in F0 are homogeneous except for those at the E:T ratios of 25:1 and 50:1. The data in F1 male rats are homogeneous at the E:T ratios of 12.5:1, 25:1 and 200:1, and non-homogeneous at the E:T ratios of 6.25:1, 50:1 and 100:1. All the data in F1 female rats are homogeneous. Values represent the mean ± SE derived from ten animals. *, p ≤ 0.05.
F1 Generation Male Rats
The F1 male rats were exposed to VCZ transplacentally and lactationally from gestation day 7 to PND 22; and exposure continued through feeding until PND 64. No effect on terminal body weight and selected organ weights was observed ().
Exposure to VCZ had no effect on the total number of splenocytes, and the percentages of splenic B cells, T cells, T-cell subsets, NK cells and macrophages as compared to control animals (). When the data were expressed as absolute values, a significant increase was observed for NK cells (25%) at the 10 ppm VCZ level but not for other subpopulations at any treatment levels evaluated ().
Effect of vinclozolin on the number and percentage of splenocyte subpopulations in F1 generation male Sprague Dawley rats
Exposure to VCZ produced an increase in the splenic IgM AFC response to T-dependent antigen sRBC (). When the data were expressed as IgM AFC/106 spleen cells, a significant increase (72%) was observed at the 150 ppm VCZ level (). When the data were expressed as IgM AFC/spleen, significant increases were observed at the 150 and 750 ppm VCZ levels (); the percentage of increase was 85% and 106%, respectively.
When the effect of VCZ on NK cell activity was evaluated, increased cytotoxicity was observed at all the treatment levels as compared to the control animals. However, the increases only reached the level of statistical significance at the 150 ppm VCZ level ().
F1 Generation Female Rats
Exposure to VCZ had no effect on the terminal body weight and thymus weights in F1 females (). A decrease (15%) in the absolute weight of spleen was observed at the 750 ppm VCZ level (). However, the decrease did not reach a statistically significant level when the data were expressed as the relative spleen weight.
Exposure to VCZ had no effect on the total number of splenocytes (). However, a significant decrease of cytotoxic T cells at the 150 ppm VCZ level was observed for both the absolute and percent values (); the percentage of decrease was 26% and 17%, respectively. Exposure to VCZ had no effect on either the percent value or the absolute value of total T cells and T helper cells. Exposure to VCZ produced no alteration in the absolute number of NK (NKR-P1A+OX8+) cells, but a significant increase (26%) was observed at the 10 ppm VCZ level when the data were expressed as a percent value (). However, the lack of a dose-related response in these parameters or correlative changes in functional parameters raised doubts as to the changes being biologically meaningful. No change was observed for macrophages no matter whether the data were expressed as an absolute or percent value ().
Effect of vinclozolin on the number and percentage of splenocyte subpopulations in F1 generation female Sprague Dawley rats
Although exposure to VCZ seemed to produce a slight increase in the IgM AFC response, no statistically significant effect was observed when the data were expressed as either the specific activity (AFC/106 cells; ) or the total activity (AFC/spleen; ).
When the effect of VCZ on the NK cell activity was evaluated, a significant decrease in the percent cytotoxicity at all of the effector:target ratios was observed at the 150 and 750 ppm VCZ levels; a decrease was also observed at the effector:target ratios of 6.25:1 and 12.5:1 for the 10 ppm VCZ treatment group ().
DISCUSSION
The effect of VCZ on reproductive development has been studied extensively (Wong et al., Citation1995; Kelce et al., Citation1994; Gray et al., Citation1994; Monosson et al., Citation1999). Less well studied is the potential of VCZ to affect the development of immune system. Therefore, multigenerational studies have been conducted to evaluate immune responses in rats exposed to VCZ. We have examined the effects of VCZ on the immune responses in both F0 (adult exposure) and F1 generations (developmental and adult exposures) of Sprague Dawley rats exposed to VCZ-containing food at the levels of 10, 150 and 750 ppm. However, the extent to which the human population is exposed to VCZ and/or its active metabolites either directly or via the food chain is not known (Kelce and Wilson, Citation1997). The U.S. EPA's reference dose is 12 μ g/kg/day (CitationU.S. EPA, 1997); the doses selected for use in this study were based on studies conducted at the NCTR (Flynn et al., Citation2001). The lowest dose used in our study was approximately 10 times the human allowable daily intake (WHO, Citation1991).
Most experimental evidence supports that VCZ is an anti-androgen (Wong et al., Citation1995; Kelce et al., Citation1994), and the effect of VCZ is dependent on the concentrations of androgens, AR, sex hormone binding globulin and accessory proteins required for transcriptional activity (Euling et al., Citation2002). Androgens are steroids produced in the testis, ovary, and adrenal gland (Diamanti-Kandarakis et al., Citation1995). Low levels of circulating androgens appear to be associated with an increased risk of autoimmune disorders (Palaszynski et al., Citation2004). Thus, one would predict that anti-androgens might have stimulatory effects on the immune system. Indeed, in F0 females, a dose-related increase in the numbers of splenocytes, B cells, and T cells was observed following VCZ exposure. In F1 male rats, both the antibody-forming cell responses and NK cell activities were increased by VCZ. Increased NK cell activity by VCZ is also in agreement with the report that androgens inhibit NK cell activity (Hou and Zheng, Citation1988; Deschaux et al., Citation1982). Additionally, an increase in the number of NK cells was also observed in F1 males at the low concentration of VCZ. Currently, we do not understand the significance of increased responses in immune function tests designed to measure immunosuppression. However, a protective effect of testosterone is thought to be responsible for a lower susceptibility of males to autoimmune diseases than females (Liva and Voskuhl, Citation2001). Thus, it will be important to determine if VCZ-mediated immunostimulation may increase the potential for developing self-reactivity.
Exposure to VCZ produced an increase in the antibody-forming cell responses in F1 male rats. However, no effect on the antibody-forming cell responses was produced in either female F0 or F1 rats. The reason for this sexually dimorphic effect is currently unknown, but it is possible that the effect of antiandrogenic effect of VCZ in females is small because the levels of androgens in females are low.
There were differential responses to VCZ exposure in the NK cell activity in female F0 and F1 rats. Exposure to VCZ in F1 female rats both developmentally and postnatally decreased the NK activity in all the treatment groups while the effect in F0 female rats was minimal. This might be due to an enhanced sensitivity of the developing fetus to the anti-androgenic effects of VCZ because there were reduced levels of competing endogenous androgens in the fetus compared to the adult (Kelce and Wilson, Citation1997). Thus, the sequential events occurred in F1 female rats could be as follows: VCZ exposure led to decreases in responses to androgen (Monosson et al., Citation1999; Kubota et al., Citation2003), which would cause an increase in circulating estrogen (Wolf et al., Citation2004). Estrogen at high concentrations would inhibit NK cell activity (Luster et al., Citation1984; Nilsson and Carlsten, Citation1994; Olsen and Kovac, Citation1996). Additionally, the developing fetus is deficient with respect to certain feedback mechanisms that regulate the endocrine system in the adult (Nilsson, Citation2000). These mechanisms may also be responsible for the decreases observed in the number and percentage of cytotoxic T cells in F1 female rats.
At the concentrations used in our study, exposure to VCZ did not affect the terminal body weights in either F0 or F1 generation rats. Effects on the weights of spleen and thymus were also minimal, which suggested that there was minimal systemic toxicity. However, it should be noted that the level of VCZ that produced more immunomodulation in F1 male rats was at the middle concentration, e.g. 150 ppm. There is evidence that at high concentrations in the absence of dihydrotestosterone (DHT), M2 targets AR to the nucleus and acts as an agonist (Kelce and Wilson, Citation1997). Thus, the attenuated potentiating effect of VCZ on the immune responses at the high concentration when compared to the middle concentration might be related to its androgenic effect.
Although an increase in the NK cytotoxicity was observed at the effector:target ratios of 6.25:1 and 12.5:1 in the 150 ppm VCZ-treated F0 female rats, there was no effect at higher effector:target ratios of 25:1, 50:1, 100:1 and 200:1. Additionally, exposure to VCZ had no effect on NK cell activity at 10 and 750 ppm of VCZ in this group of animals. Furthermore, exposure to VCZ did not affect the number and percentage of splenic NK cells in F0 female rats at the 150 ppm VCZ concentration. Taken together, the lack of effects at the higher effector:target ratios, lack of a dose response and comparable changes in NK cell numbers at 150 ppm VCZ in F0 female rats suggest that the observed changes in NK cytotoxicity at the effector:target ratios of 6.25:1 and 12.5:1 may not be biologically meaningful.
In summary, the results from the present study indicate that dietary exposure to VCZ can alter certain immune parameters in the rat. Differential immunological changes were observed in F0 female, F1 male and female rats. Increases in the number of total splenocytes and differential subsets were observed in F0 females. Increases in the antibody forming cell responses and NK cell activity were observed in F1 males. On the other hand, decreases in the NK cell activity and the number of cytotoxic T cells were observed in F1 females. However, it should be noted that the F1 rats were exposed continuously from GD7 to PND64. VCZ may have differential effects on the immune system when the exposure occurs in utero during lactation, childhood, puberty, or adult. In future studies, VCZ modulation of the immune system at different stages of the development will be evaluated to determine which period is more sensitive to VCZ exposure. Additionally, host resistance models should be included into an in-depth assessment of VCZ immunomodulatory properties over several generations to evaluate the biological implications of these immunological changes.
Supported by NTP Contract No. ES 55094. The authors would like to thank Shirley S. Griffey for her technical assistance. The part of the study conducted at the NCTR (animals, diet, compound, animal care, etc.) was funded by the NCTR/FDA-NIEHS/NTP Interagency Agreement 224-93-0001.
REFERENCES
- Bleavins M. R., de la, Iglesia F. A., McCay J. A., White K. L., Jr., Munson A. E.. 1995. Immunotoxicologic studies with CI-959, a novel benzothiophene cell activation inhibitor. Toxicology. 98: 111–123. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Burns-Naas L. A., Mast R. W., Klykken P. C., McCay J. A., White K. L., Jr., Mann P. C., Naas D. J.. 1998. Toxicology and humoral immunity assessment of decamethylcyclopentasiloxane (D5) following a 1-month whole body inhalation exposure in Fischer 344 rats. Toxicol. Sci.. 43: 28–38. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Delclos K. B., Newbold R. R.. A comprehensive protocol for the evaluation of reproductive, neurological, behavioral, immunological, and carcinogenic effects of potential endocrine disrupting chemicals, Presented at Estrogens in the Environment IV. Washington, D.C. July 21, 1997
- Deschaux P., Paucod J. C., Ardail D.. 1982. Interaction between thymosin, testosterone and estradiol on natural killer cell activity in mice. Tohoku J. Exp. Med.. 136: 367–372. [PUBMED], [INFOTRIEVE]
- Diamanti-Kandarakis E., Tolis G., Duleba A. J.. 1995. Androgens and therapeutic aspects of antiandrogens in women. J. Soc. Gynecol. Investig.. 2: 577–592. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Doerge D. R., Churchwell M. I., Delclos K. B.. 2000. On-line sample preparation using restricted-access media in the analysis of the soy isoflavones, genistein and daidzein, in rat serum using liquid chromatography electrospray mass spectrometry. Rapid. Commun. Mass. Spectrom.. 14: 673–678. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Euling S. Y., Gennings C., Wilson E. M., Kemppainen J. A., Kelce W. R., Kimmel C. A.. 2002. Response-surface modeling of the effect of 5alpha-dihydrotestosterone and androgen receptor levels on the response to the androgen antagonist vinclozolin. Toxicol. Sci.. 69: 332–343. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Flynn K. M., Delclos K. B., Newbold R. R., Ferguson S. A.. 2001. Behavioral responses of rats exposed to long-term dietary vinclozolin. J. Agric. Food Chem.. 49: 1658–1665. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Gray L. E., Jr., Ostby J. S., Kelce W. R.. 1994. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxicol. Appl. Pharmacol.. 129: 46–52. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Guo T. L., White K. L., Jr., Brown R. D., Delclos K. B., Newbold R. R., Weis C., Germolec D. R., McCay J. A.. 2002. Genistein modulates splenic natural killer cell activity, antibody-forming cell response, and phenotypic marker expression in F0 and F1 generations of Sprague-Dawley rats. Toxicol. Appl. Pharmacol.. 181: 219–227. [PUBMED], [INFOTRIEVE]
- Holladay S. D., Blaylock B. L., Comment C. E., Heindel J. J., Fox W. M., Korach K. S., Luster M. I.. 1993. Selective prothymocyte targeting by prenatal diethylstilbe sterol exposure. Cell Immunol.. 152: 131–142. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Hou J., Zheng W. F.. 1988. Effect of sex hormones on NK and ADCC activity of mice. Int. J. Immunopharmacol.. 10: 15–22. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Jerne N. K., Nordin A. A.. 1963. Plaque formation in agar by single antibody-producing cells. Science. 140: 405–406. [PUBMED], [INFOTRIEVE]
- Kelce W. R., Monosson E., Gamcsik M. P., Laws S. C., Gray L. E., Jr.. 1994. Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol. Appl. Pharmacol.. 126: 276–285. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Kelce W. R., Wilson E. M.. 1997. Environmental antiandrogens: developmental effects, molecular mechanisms, and clinical implications. J. Mol. Med.. 75: 198–207. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Kubota K., Ohsako S., Kurosawa S., Takeda K., Qing W., Sakaue M., Kawakami T., Ishimura R., Tohyama C.. 2003. Effects of vinclozolin administration on sperm production and testosterone biosynthetic pathway in adult male rat. J. Reprod. Dev.. 49: 403–412. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Liva S. M., Voskuhl R. R.. 2001. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol.. 167: 2060–2067. [PUBMED], [INFOTRIEVE]
- Luster M. I., Faith R. E., McLachlan J. A.. 1978. Alterations of the antibody response following in utero exposure to diethylstilbestrol. Bull. Environ. Contam. Toxicol.. 20: 433–437. [PUBMED], [INFOTRIEVE]
- Luster M. I., Faith R. E., McLachlan J. A., Clark G. C.. 1979. Effect of in utero exposure to diethylstilbestrol on the immune response in mice. Toxicol. Appl. Pharmacol.. 47: 279–285. [PUBMED], [INFOTRIEVE]
- Luster M. I., Hayes H. T., Korach K., Tucker A. N., Dean J. H., Greenlee W. F., Boorman G. A.. 1984. Estrogen immunosuppression is regulated through estrogenic responses in the thymus. J. Immunol.. 133: 110–116. [PUBMED], [INFOTRIEVE]
- Monosson E., Kelce W. R., Lambright C., Ostby J., Gray L. E., Jr.. 1999. Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat. Toxicol. Ind. Health. 15: 65–79. [CROSSREF], [PUBMED], [INFOTRIEVE]
- National Research Council. Hormonally active agents in the environment. Washington, DC, National Academy Press. 1999
- Nilsson N., Carlsten H.. 1994. Estrogen induces suppression of natural killer cell cytotoxicity and augmentation of polyclonal B cell activation. Cell. Immunol.. 158: 131–139. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Nilsson R.. 2000. Endocrine modulators in the food chain and environment. Toxicol. Pathol.. 28: 420–431. [PUBMED], [INFOTRIEVE]
- Olsen N. J., Kovacs W. J.. 1996. Gonadal steroids and immunity. Endocr. Rev.. 17: 369–384. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Palaszynski K. M., Loo K. K., Ashouri J. F., Liu H. B., Voskuhl R. R.. 2004. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J. Neuroimmunol.. 146: 144–152. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Ronis M. J., Badger T. M.. 1995. Toxic interactions between fungicides that inhibit ergosterol biosynthesis and phosphorothioate insecticides in the male rat and bobwhite quail (Colinus virginianus). Toxicol. Appl. Pharmacol.. 130: 221–228. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Shelat S. G., Aird F., Redei E.. 1997. Exposure to dehydroepiandrosterone in utero affects T-cell function in males only. Neuroimmunomodulation. 4: 154–162. [PUBMED], [INFOTRIEVE]
- U.S. EPA, 1997. Vinclozolin; pesticide tolerance. Final rule. U.S. Environmental Protection Agency. Fed. Reg.. 62: 38464–38474
- U.S. EPA, 2001. Vinclozolin; notice of use cancellations. U.S. Environmental Protection Agency. Fed. Reg.. 66: 44134–44136
- Wolf C. J, LeBlanc G. A., Gray L. E., Jr.. 2004. Interactive effects of vinclozolin and testosterone propionate on pregnancy and sexual differentiation of the male and female SD rat. Toxicol. Sci.. 78: 135–143. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Wong C., Kelce W. R., Sar M., Wilson E. M.. 1995. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J. Biol. Chem.. 270: 19998–20003. [PUBMED], [INFOTRIEVE]
- Woolhiser M. R., McCay J. A.. 1999. Delayed assessment of immunlogical function allowing for transportation of lymphoid tissues to distinct laboratory sites. Toxicology Methods. 9: 165–171
- World Health Organization, ICPS—International Programme on Chemical Safety: Summary of toxicological evaluations performed by the joint FAO/WHO meeting on pesticide residues, JMPR. 1991, (UNEP, ILO, WHO).