Abstract
A T-cell dependent antibody response (TDAR) model to evaluate compounds for potential immunotoxicity in dogs has not been reported. The objective of these studies was to develop and validate a dog TDAR model using the T-cell dependent antigen, keyhole limpet hemocyanin (KLH). Studies were conducted to determine the appropriate dose of KLH, immunization route and kinetics of the antibody response to KLH in the dog. To validate the sensitivity of this method, we investigated the TDAR to KLH in the dog with a known immunosuppressive drug, cyclosporine (Neoral). The results of this study demonstrate that a robust primary IgM and IgG response to KLH can be generated in dogs and the IgG response was sensitive to cyclosporine treatment.
INTRODUCTION
The dog is commonly used for safety assessment of drugs in development. If standard toxicology studies indicate immunotoxicity, additional in-depth testing may be required. The in vivo primary T-cell dependent antibody response (TDAR) has been recommended to assess the acquired immune response in species used in preclinical toxicology studies. The measurement of primary responses (IgM and IgG) to T-cell dependent antigens such as keyhole limpet hemocyanin (KLH) has been described in mice (Moynihan et al., Citation2000), rats (Moraska and Fleshner, Citation2001; Smith et al., Citation2003; Gore et al., Citation2004; Roman et al., Citation2004), dogs (Mates and Hinton, Citation1976; Weissman et al., Citation1992; Jones et al., Citation1993), rabbits (Mates and Hinton, Citation1976), pigs (Bruininx et al., Citation2000), non-human primates (Martin et al., Citation2004), and humans (Korver et al., Citation1984; Snyder et al., Citation1990). Various routes of administration have been described: intraperitoneal (mice and rats), intramuscular (pigs and non-human primates), intrapulmonary (dogs and rabbits), and subcutaneous (rats and humans). Peak antibody responses to KLH in most species occurred between 5–10 days for IgM responses and 10–14 days for IgG responses. Weissman et al. (Citation1992) demonstrated that with intrapulmonary immunization of KLH in dogs, KLH-specific IgG, IgM and IgA peaked around day 7 and remained significantly elevated above baseline through the end of study (day 20) for the three Ig classes measured.
Reports of evaluation of TDAR in the presence of potential immunomodulators have been described for rats (Koller and Exon Citation1983; Conner et al., 2001; Smith et al., Citation2003; Gore et al., Citation2004; Roman et al., Citation2004) and humans (Amlot et al., Citation1986); however, studies evaluating the effect of immunosuppressive compounds on the TDAR to KLH in canines have not been reported. Hence, the objectives of these studies were to evaluate different doses of KLH, routes of immunization and kinetics of the IgM and IgG response to KLH. In addition, the immunosuppressive agent, cyclosporine (Neoral) was used to validate the sensitivity of the dog TDAR model.
METHODS
Animals
Male and female beagle dogs (approximately 7–12 months old) were obtained from Marshall Farms (North Rose, NY). Dogs were maintained in a room at 70°F (±5°) with approximately 10% humidity and 12 hours on/12 hours off light exposure. Dogs were fed 5L66 (Purina Mills), once per day. The animals were randomized into treatment groups. Studies were conducted in accordance with the current guidelines for animal welfare (Guide for the Care and Use of Laboratory Animals). The procedures used in the study were reviewed and approved by the Institutional Animal Care and Use committee.
Immunization and Dosing Procedures
Keyhole limpet hemocyanin (KLH, Pierce catalogue number 77600) was used for immunization. The KLH was rehydrated with sterile water to a concentration of 10 mg/ml. Vaccination volume varied depending upon desired concentration. The route used was either subcutaneous (SC) or intramuscular (IM) in the initial study, with all subsequent studies using the IM route. Approximately 5–10 ml of blood was collected in serum separator tubes for antibody testing and serum chemistry or in EDTA tubes for hematology and cyclosporine trough levels. Hematology evaluation included: WBC, RBC, HCT, HGB, MCH, PLT, WBC differential and reticulocyte count. Serum chemistry evaluation included: glucose, BUN, creatinine, total protein, albumin, calculated globulin, A/G ratio, calcium, sodium, potassium, chloride, total cholesterol, ALT, AST, gamma GT, ALP, and total bilirubin. Cyclosporine levels were measured using the Abbott TDx Fluorescence Polarization Immunoassay at the School of Veterinary Medicine, North Carolina State University. Neoral (Novartis; 100 mg/ml) was diluted in sterile water to the proper concentration at each time of use. Dogs were dosed with either Neoral or sterile water by oral gavage based upon weight.
Measurement of KLH-Specific IgG and IgM Antibodies
KLH-Specific IgM and IgG were measured by ELISA using 96-well plates that were coated with KLH (same source and lot used for immunization) at 2 μ g/mL in carbonate-bicarbonate buffer. Plates were blocked with 1% casein and 2-fold serial dilutions of test serum were added to the plate. After a 60-min incubation period, plates were washed and goat anti-dog IgM or IgG-horseradish peroxidase (Kirkegaard and Perry Laboratories, Gaithersburg, MD) was added to the plates. Following a 60-min incubation period and wash, 2,2′-azino-bis-3-ethyl benzthiazoline-6-sulfonic acid (ABTS) was added. The OD of the colored product was measured after 10 min at 405 nm. Each ELISA plate contained negative and positive control sera. The endpoint titer was calculated using the average OD of the negative control sample (8 wells) plus 3 SD that was subtracted from the OD values of all test sample wells. Interpolation of the serial dilutions of the test samples calculated the endpoint titer as the dilution at which the line crossed the value of the negative OD + 3 SD. Data was expressed as log2 of the endpoint titers.
RESULTS
Study 1
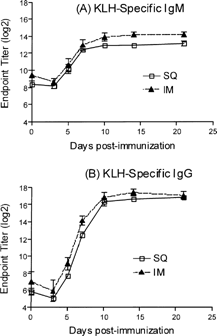
This study compared KLH immunization by the SC and IM routes and the kinetics of the IgG and IgM responses. Four dogs per group (2 per sex) were immunized with 50 mg of KLH either IM or SC routes. Blood was collected pre-immunization and 3, 5, 7, 10, 14, and 21 days post-immunization. Serum was tested for KLH-specific IgM and IgG antibodies. The antibody response with IM administration was slightly higher, although not significantly greater than that obtained with the SC route (). Peak responses occurred at day 10 for both IgG and IgM. KLH administration by the IM route was selected for subsequent experiments. Baseline titers of IgM were higher than baseline titers for IgG. This phenomenon was observed in all studies.
FIG. 1. Comparing routes of KLH immunization. Groups of dogs were immunized with KLH (50 mg/dog) by the subcutaneous (SC) and intramuscular routes (IM) and the KLH-specific IgM (A) and IgG (B) antibody titers were determined 3, 5, 7, 10, 14, and 21 days post-immunization. Values represent the mean ± SEM. n = 4 dogs/group.
Study 2
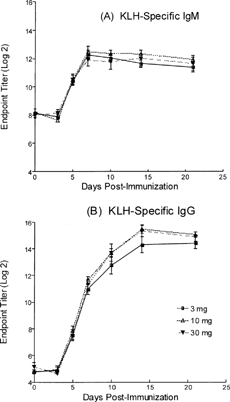
This study evaluated different KLH doses and the kinetics of the antibody response at each dose. Eight dogs per dose group (4 per sex) received 3, 10, or 30 mg of KLH (IM). Blood was collected pre-immunization and 3, 5, 7, 10, 14, and 21 days post-immunization. Serum was tested for KLH-specific IgM and IgG. Regardless of the dose, the peak IgM responses were approximately day 7 while the peak IgG response was around day 14 (). Both IgG and IgM titers remained elevated throughout the duration of the study. A dose of 3 mg/dog and collection time points of 7 (IgM) and 12 (IgG) days post-KLH immunization were selected for subsequent experiments.
FIG. 2. Evaluating different KLH doses and the kinetics of the antibody response. Groups of dogs were administered 3, 10, or 30 mg of KLH per dog by the intramuscular route (quadriceps) and the KLH-specific IgM (A) and IgG (B) antibody titers were determine 3, 5, 7, 10, 14, and 21 days post-immunization. Values represent the mean ± SEM. n = 8 dogs/group.
Study 3
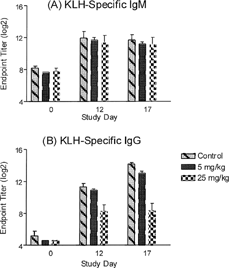
This study evaluated the TDAR in dogs receiving cyclosporine. Four dogs per dose group (2 per sex) received water, 5 or 25 mg/kg cyclosporine orally once a day for 17 days. Blood was drawn pre-dosing and on days 12 and 17. Dogs were immunized with KLH on day 5. Serum was tested for KLH-specific IgM and IgG titers. Hematology and chemistry responses were performed on blood drawn pre-dose and on days 12 and 17. The KLH-specific IgM and IgG titers of dogs in the 5 and 25 mg/kg dose groups were compared to vehicle controls (). The 25 mg/kg dose group had significantly lower IgG titers than the control group, while the 5 mg/kg dose group was similar to the control group. The KLH-specific IgM titers in both dose groups were similar to controls. Hematology, chemistry, and clinical parameters were all within normal ranges at the end of the study.
FIG. 3. Evaluating the effects of cyclosporine dosed once per day (SID dosing) on the TDAR to KLH. Groups of dogs were treated with water (vehicle) or cyclosporine (5 and 25 mg/kg) once daily for 17 days. All dogs were immunized KLH (3 mg/dog) by the intramuscular route on day 5 and the KLH-specific IgM (A) and IgG (B) antibody titers were determined on study day 12 and 17 (7 and 12 days post-immunization). Values represent the mean ± SEM. n = 4 dogs/group.
Study 4
Since decreases in KLH-specific IgM were not observed in Study 3, Study 4 was performed to evaluate cyclosporine trough levels over a 4-day period with the doses used in Study 3. Three dogs were dosed with cyclosporine at 5 or 25 mg/kg once a day for 4 days. Blood was collected pre-dose on days 2 and 4 to measure for cyclosporine trough levels. Monitoring cyclosporine trough levels is commonly used in veterinary medicine to determine the appropriate doses of cyclosporine in dogs receiving organ transplants. Mathews et al. (Citation2000) used cyclosporine for kidney transplantation in dogs to achieve 500–600 ng/ml of cyclosporine. M.G. Papich (North Caroline State University, Personal Communication) indicated the cyclosprorine levels of 300–600 ng/ml are considered anti-inflammatory and 600–1200 ng/ml are currently considered immunosuppressive, however these levels are still being defined. Trough levels of the 25 mg/kg dose group were much higher than the 5 mg/kg dose group. Trough levels for the 5 mg/kg were considered to be below the levels needed to produce immunosuppression, while all animals receiving 25 mg/kg had trough levels that would be considered to by immunosuppressive after 4 days of dosing ().
TABLE 1 Cyclosporine trough levels at doses of 5 and 25 mg/kg SID
Study 5
Given the results from Study 4, the TDAR was evaluated in dogs receiving cyclosporine twice a day at higher doses. Four dogs per group (2 per sex) were dosed with either water or cyclosporine (20 or 40 mg/kg) twice a day (BID) for 17 days. Dogs were immunized with KLH on day 5. Blood was drawn just prior to the first daily cyclosporine dose on days 5, 12, and 17 for KLH antibody titers and cyclosporine trough levels. Hematology and chemistry responses were performed on blood drawn pre-dose and on days 12 and 17. The KLH-specific IgM titers for animals in the 20 and 40 mg/kg dose group were similar to controls, whereas KLH-specific IgG titers for both dose groups were lower than controls (). The KLH-specific IgG response at 20 mg/kg was similar to that observed with the 40 mg/kg dose. Hematology and clinical parameters were all within normal ranges at the end of the study. Cyclosporine trough levels for the 40 mg/kg dose group was higher than that observed for the 20 mg/kg dose group (). Trough levels for the 25 mg/kg once a day (SID) dose group on day 4 () was similar to that observed for the 20 mg/kg BID dose group () on days 12 and 17. Cyclosporine trough levels for all dogs in the 20 and 40 mg/kg dose groups by days 12 and 17 were at levels that would be efficacious for dog transplant studies.
FIG. 4. Evaluating the effects of higher doses of cyclosporine dosed twice per day (BID dosing) on the TDAR to KLH. Groups of dogs were treated with water (vehicle) or cyclosporine (20 and 40 mg/kg) twice daily for 17 days. All dogs were immunized KLH (3 mg/dog) by the intramuscular route on day 5 and the KLH-specific IgM (A) and IgG (B) antibody titers were determined on study day 12 and 17 (7 and 12 days post-immunization). Values represent the mean ± SEM. n = 4 dogs/group.
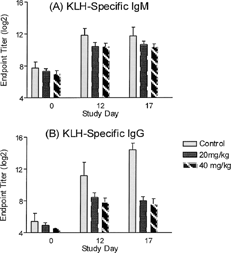
TABLE 2 Cyclosporine trough levels at doses of 20 and 40 mg/kg BID
DISCUSSION AND CONCLUSIONS
The kinetics of the canine IgM and IgG responses to KLH are similar to those described for typical primary and secondary responses to T-dependent antigens in most species. A study by Weissman et al. (Citation1992) examined primary and secondary antibody responses to KLH in canines exposed to KLH immunization via the pulmonary route; however, there is no published data of antibody responses to KLH when immunized by the IM or SC routes. In the Weissman study, kinetics of KLH-specific IgM antibodies in serum were similar to those observed in these studies, however, IgG peaked earlier than observed in this study. These differences may be due to differences in the route for KLH immunization.
It was observed that baseline (pre-immunization) KLH-specific IgM titers were considerably higher than IgG baseline titers in our studies. Previous human studies with KLH demonstrated the presence of IgM antibodies to KLH prior to immunization (Korver et al., Citation1984). Such antibodies have been referred to as natural antibodies (Boyden, Citation1966). Work by Guilbert et al. (Citation1982) resulted in the formulation of hypothesis that natural antibodies to numerous antigens may be found in normal human serum. Such antibodies are assumed to have been generated by shared epitopes, primarily polysaccharides. The presence of natural antibodies in canines has not previously been described; however, our data suggest that such antibodies exist.
The assessment of TDAR in canines exposed to immunosuppressive compounds has not been previously described, however studies in rats (Gore et al., Citation2004; Roman et al., Citation2004) evaluated the effects of immunosuppressive compounds on the KLH specific antibody responses. Differences in the magnitude of IgG and IgM specific responses were observed, with the magnitude of the IgG suppression being considerably greater that the IgM. Gore et al. (Citation2004) hypothesized that the differences may be related to the effect of the immunosuppressant on cytokine production and class switching or due to the fact that the dosing regimen may have a greater impact on the IgG since it occurs later than the IgM. Fuleihan et al. (Citation1994) described the inhibition of CD40 ligand expression in vitro in stimulated T-lymphocytes from cyclosporine treated patients. Patients with defects in the gene encoding CD40L, are unable to synthesize immunoglobulins other than IgM and IgD (Geha et al., Citation1979), suggesting that interactions between CD40 on B-cells and its ligand on activated T-cells are critical for immunoglobulin class switching. Thus, if cyclosporine acts in a similar manner in canines, this may explain the observed lack of inhibition in the KLH-specific IgM response in our studies.
In conclusion, these studies determined the optimal KLH dose, route of immunization and blood collection time points that were then used to validate the sensitivity of the canine model for TDAR assessment to cyclosporine. Cyclosporine inhibited the KLH-specific IgG response but not the IgM response. The lack of effect on IgM may be due to the mode of action of cyclosporine.
The authors thank Mark Papich, D.V.M. and staff at North Carolina State University for cyclosporine trough level testing, staff at Pfizer Clinical Pathology for blood hematology and chemistry, and Pfizer Toxicology Staff for animal dosing and blood collection.
REFERENCES
- Amlot P. L., Hayes A. E., Gray D., Gordon-Smith E. C., Humphrey J. H. Human immune responses in vivo to protein (KLH) and polysaccharide (DNP-Ficoll) neoantigens: Normal subjects compared with bone marrow transplant patients on cyclosporine. Clin. Exp. Immunol. 1986; 64: 125–135, [PUBMED], [INFOTRIEVE], [CSA]
- Boyden S. V. Natural antibodies and the immune response. Adv. Immunol. 1966; 5: 1–28, [PUBMED], [INFOTRIEVE], [CSA]
- Bruininx E. M., Swinkels J. W., Parentier H. K., Jetten C. W., Gentry J. L., Schrama J. W. Effects of an additional iron injection on growth and humoral immunity of weanling pigs. Livestock Prod. Sci. 2000; 67: 31–39, [CSA], [CROSSREF]
- Connor T. J., Dympna B., Kelly J. P. Methylenedioxymethamphetamine (MDMA; ‘Ecstasy’) suppresses antigen specific IgG2a and IFNγ production. Immunol. Lett. 2001; 78: 67–71, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Fuleihan R., Ramesh N., Horner A., Ahern D., Belshaw P., Alberg D. G., Stamenkovic I., Harmon W., Geha R. Cyclosporine A inhibits CD40 ligand expression in T-lymphocytes. J. Clin. Invest. 1994; 93: 1315–1320, [PUBMED], [INFOTRIEVE], [CSA]
- Geha R., Hyslop N., Alami S, Farah F., Schneeberger E., Rosen F. Hyper-immunoglobulin M immunodeficiency (Dysgammaglobulinemia). Presence of immuno-globulin M-secreting plasmacytoid cells in peripheral blood and failure of immunoglobulin G switch in B-cell differentiation. J. Clin. Invest. 1979; 64: 2385–2391, [CSA]
- Gore E. R., Gower J., Kurali E., Sui J. L., Bynum J., Ennulat D., Herzyk D. Primary antibody response to keyhole limpet hemocyanin in rat as a model for immunotoxicity evaluation. Toxicology 2004; 197: 23–35, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Guilbert B., Dighiero G., Avarameas S. Naturally-occurring antibodies against nine common antigens in human serum. J. Immunol. 1982; 26: 2779–2787, [CSA]
- Jones S. E., Davila D. R., Haley P. J., Bice D. E. The effects of age on immune responses in the antigen-instilled dog lung. Antibody responses in the lung and lymphoid tissues flowing primary and secondary antigen instillation. Mech. Age. Develop. 1993; 68: 191–207, [CSA], [CROSSREF]
- Koller L. D., Exon J. H. Induction of humoral immunity to protein antigen without adjuvant in rats exposed to immunosuppressive chemicals. J. Toxicol. Environ. Health 1983; 12: 173–181, [PUBMED], [INFOTRIEVE], [CSA]
- Korver K., Zeijlemaker W. P., Schellekens P. T., Vossen J. M. Measurement of primary in vivo IgM- and IgG-antibody response to KLH in humans: Implications of pre-immune IgM binding in antigen-specific ELISA. J. Immunol. Meth. 1984; 74: 241–251, [CSA], [CROSSREF]
- Martin P. L., Cornacoff J., Prabhakar U., Lohr T., Treacy G., Sutherland J. E., Hersey S., Martin E. Preclinical safety and immune modulating effects of therapeutic monoclonal antibodies to interleukin-6 and tumor necrosis factor-α in Cynomolgus macaques. J. Immunotoxicol 2004; 1: 131–139, [CSA], [CROSSREF]
- Mates A., Hinton A. Immune response in dog: Antibody formation in dogs and rabbits to human serum proteins and keyhole limpet haemocyanin. Microbios 1976; 17: 175–187, [PUBMED], [INFOTRIEVE], [CSA]
- Mathews K. A., Holmberg D. L., Miller C. W. Kidney transplantation in dogs with naturally occurring end-stage renal disease. J. Am. Animal Hosp. Assoc. 2000; 36: 294–301, [CSA]
- Moraska A., Fleshner M. Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001; 281: R484–R489, [PUBMED], [INFOTRIEVE], [CSA]
- Moynihan J. A., Karp J. D., Cohen N., Ader R. Immune deviation following stress odor exposure: Role of endogenous opioids. J Neuroimmunol. 2000; 102: 145–153, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Roman D., Ulrich P., Paul G., Court M., Vit P., Kehern J., Mahl A. Determination of the effect of calcineurin inhibitors on the rat's immune system after KLH immunization. Toxicol. Lett. 2004; 149: 133–140, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Smith W. H., Winstead C. J., Stank K. K., Halstead B. W., Wierda D. A predictive F344 rat immunotoxicology model: Cellular parameters combined with humoral response to NP-Cγ G and KLH. Toxicology 2003; 194: 129–145, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Snyder B. K., Roghmann K. J., Sigal L. H. Effect of stress and other biopsychosocial factors on primary antibody response. J. Adolescent Health Care 1990; 11: 472–479, [CSA], [CROSSREF]
- Weissman D. N., Bice D. E., Muggenburg B. A., Haley P. J., Shopp G. M., Schuyler M. R. Primary immunization in the canine lung: Soluble antigen induces a localized response. Am. Rev. Respir. Dis. 1992; 145: 6–12, [PUBMED], [INFOTRIEVE], [CSA]