Abstract
Abundant literature exists demonstrating the immunomodulating effects of polychlorinated biphenyls (PCBs). To date, most of the research has focused on dioxin-like coplanar PCB congeners because of their high affinity for the aryl hydrocarbon receptor (AhR) and cytochrome P450-inducing capability. For this study, the impact of two structurally different PCB congeners on the immune responsiveness of bluegill sunfish (Lepomis macrochirus) was examined to evaluate the immunotoxic potential of each congener (as separate entities) and to relate effects on immune function with hepatic CYP1A induction. Fish received a single intraperitoneal injection of the: coplanar congener, PCB 126 (0.01 or 1.0 μg/g BW); noncoplanar PCB 153 (5.0 or 50.0 μg/g BW); or, the corn oil vehicle. PCB-induced effects on innate and cell-mediated immune parameters, and on hepatic CYP1A protein induction were evaluated in fish sacrificed 1, 3, 7, 14 or 21 days post-injection. In the absence of CYP1A induction, PCB 153 increased kidney phagocyte-mediated superoxide production 3 d post-injection, and at the highest dose suppressed B- and T-lymphocyte proliferation after 3 and 7 days, respectively. Treatment of fish with PCB 126 had no effect on oxyradical production, but altered B-lymphocyte proliferation after 1 day, also in the absence of CYP1A induction. Hepatic CYP1A was only induced in fish exposed to the highest PCB 126 dose; protein induction appeared at 3 d post-injection and persisted for up to 21 days. Taken together, these results demonstrate that exposure to different PCB congeners can alter immune function in the absence of CYP1A induction, suggesting that mechanisms other than the AhR pathway may play a role in PCB-induced immunotoxicity, particularly for the noncoplanar congeners.
INTRODUCTION
Polychlorinated biphenyls (PCBs) are a well-studied class of organic pollutants known for their persistence in the environment as well as for their wide realm of toxicological effects. A variety of organ systems (including the immune system) have been shown, in a diversity of species, to be sensitive targets for the toxicological effects of PCBs.
There exist 209 PCB congeners that are distinguished by the number and placement of chlorine molecules on the biphenyl rings. The persistence of a particular PCB congener in environmental and biological samples is directly proportional to the number of chlorine substitutions. The structure of a PCB congener is dependent upon the presence or absence of chlorine substitutions on the ortho position of the rings; ortho-substituted congeners take on a noncoplanar conformation, while non-ortho-substituted congeners are planar in structure (Silkworth and Grabstein, Citation1982). Coplanar PCB congeners are structurally similar to dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin) and, therefore, share properties such as binding to the aryl hydrocarbon receptor (AhR) resulting in the up-regulation of many genes, including the cytochrome P450s. Many of the toxicological effects of PCBs are attributed to their ability to bind and activate this receptor pathway (Davis and Safe, Citation1990; Van den Berg et al., Citation1998). The potential for toxicity in PCB-exposed populations is based upon toxic equivalency factors (TEFs) where individual congeners are assigned a number based upon their “dioxin-like” properties (Van den Berg et al., Citation1998). Given that noncoplanar PCB congeners have a very low affinity for the AhR, their toxic potential is generally not considered as relevant.
However, recent evidence suggests that PCB congeners assigned a low TEF (i.e., noncoplanar PCBs), may also be toxicologically significant. A number of studies have demonstrated that exposure to noncoplanar PCB congeners can adversely alter functions of the nervous, immune, and endocrine systems (Fischer et al., Citation1998; Sanchez-Alonso et al., Citation2003; Smithwick et al., Citation2003). For example, results from an in vitro investigation utilizing a rat insulinoma cell line demonstrated that treatment of cells with either a noncoplanar PCB congener or Aroclor 1254 (a mixture of noncoplanar and coplanar congeners) stimulated the release of insulin, while exposure to an equimolar concentration of a coplanar congener had no effect on cellular insulin release (Fischer et al., Citation1998). In another in vitro study, treatment of rat neuronal cells with noncoplanar PCB 153 induced apoptosis in a time- and dose-dependent manner. Apoptosis in this study was correlated with an increase in caspase-3 activity in cells treated with 100 μM PCB 153 for 90 min or greater (Sanchez-Alonso et al., Citation2003).
The immune system may be one of the most sensitive parameters for evaluating PCB-induced toxicity (Tryphonas, Citation1995). Polychlorinated biphenyls, either as Aroclors or individual congeners, have been shown in mammalian studies to disrupt all three arms of the immune response (Davis and Safe, Citation1990; Kerkvliet et al., Citation1990; Stack et al., Citation1999; Voie et al., Citation2000). However, the exact mechanism underlying PCB-induced immunotoxicity has yet to be identified. Many investigators have correlated activation of the AhR pathway in mammals with immunotoxic outcome (Silkworth and Grabstein, Citation1982; Silkworth et al., Citation1984; Lubet et al., Citation1986; Davis and Safe, Citation1990; Kerkvliet et al., Citation1990; Harper et al., Citation1993). For example, Kerkvliet et al. (Citation1990) demonstrated that mice possessing a functional Ah locus had a reduced cytotoxic T-lymphocyte (CTL) response following exposure by gavage to coplanar PCB 169 (10 mg/kg body weight [BW]); CTL activity was unaffected in mice lacking a functional AhR even at a 10-fold higher PCB dose. In contrast, other studies have demonstrated that depending upon the particular immune endpoint being evaluated, a functional AhR pathway might not be a prerequisite for PCB-induced immunotoxicity (Harper et al., Citation1993; Stack et al., Citation1999; Smithwick et al., Citation2003; Levin et al., Citation2004). For example, in the absence of AhR activation, in vitro treatment of mouse splenocytes with multi-ortho substituted noncoplanar PCB congeners inhibited lipopolysaccharide (LPS)-induced lymphocyte proliferation (Smithwick et al., Citation2003).
Evidence is accumulating that, like mammals, fish are also sensitive to the immunomodulating effects of PCBs (Rice and Schlenk, Citation1995; Regala et al., Citation2001; Duffy et al., Citation2002, Citation2003, Citation2005; Duffy and Zelikoff, Citation2005; Maule et al., Citation2005). Fish share many immunological properties with mammals including, white blood cells and lymphoid tissue structure and function, as well as in their ability to mount a nonspecific, cell- and humoral-mediated immune response (Zelikoff, Citation1994). Due to these similarities, the use of fish for immunotoxicity testing as an alternative to mammalian species was predicted (Karol, Citation1998) and has since come to fruition (Zelikoff et al., Citation2000). Moreover, fish possess a functional AhR and are sensitive to gene upregulation (i.e., cytochrome P450 induction) by some of the same AhR agonists as mammals (including PCBs, polycyclic aromatic hydrocarbons [PAHs] and halogenated aromatic hydrocarbons [HAHs]) (Roy et al., Citation2002; Schlezinger and Stegeman, Citation2001; Carlson et al., Citation2002; Meyer et al., Citation2002). Thus, in addition to being the most relevant model for assessing PCB contamination in aquatic species, fish represent a biologically relevant and useful model for examining PCB-induced immunotoxicity in mammals, as well as for providing a better understanding of the mechanism(s) by which observed effects on the immune system might occur.
In addition to adding to the limited body of knowledge concerning the toxicological impact of noncoplanar PCB congeners, this study provides valuable new information concerning the association between PCB exposure, AhR activation (as indicated by hepatic CYP1A induction) and immunotoxicity.
MATERIALS AND METHODS
Bluegill sunfish (Lepomis macrochirus) were purchased from a New York State fish hatchery (Northeastern Aquatics, Rhinebeck, NY). Fish (between 2–4″ in length and weighing approximately 6.11 g [± 0.23 g (SE)]) were acclimated to room temperature (23–25°C) and maintained in aerated dechlorinated tap water (on a 16 hr light: 8 hr dark photoperiod) for at least 2 weeks prior to use in any of the experiments. Approximately 30 fish were maintained per 16 gallon (61 liter) tank. Fish were fed once daily (except for the day of PCB injection when they were not fed) a diet of Tetramin Richmix flake food (Tetra Sales, Blacksburg, VA). Temperature, pH and overall water quality were monitored weekly for the presence of unionized ammonia, chlorine and dissolved oxygen. Water quality criteria remained within normal ranges throughout the study.
PCB congeners used in this study were purchased from AccuStandard Inc. (New Haven, CT) and had a designated purity of 100 and 99.6% for PCB 126 (3,3′,4,4′,5-pentachlorobiphenyl) and 153 (2,2′,4,4′,5,5′-hexachlorobiphenyl), respectively. Stock solutions of PCB 126 and PCB 153 were prepared by dissolving each congener in corn oil overnight in a 60°C waterbath.
On the day of exposure, fish received a single intraperitoneal (IP) injection of either PCB 126 (0.01 or 1.0 μg/g BW) or PCB 153 (5.0 or 50.0 μg/g BW); control fish were injected with the corn-oil vehicle. Dose was calculated such that a 1.0 g fish received 5 μl of either the individual PCB congener or vehicle. These sublethal doses of PCB 126 and 153 were selected for study based upon range-finding survival studies and previous investigations with other fish species demonstrating their immunotoxic potential. Following injection, bluegill were maintained in 10 gallon (38 l) glass aquaria (3–4 fish/treatment group) until sacrificed 1, 3, 7, 14 or 21 days later. Fish were sacrificed by decapitation (Zelikoff et al., Citation1996) and kidneys and spleens aseptically removed and placed into fish physiological saline (FPS) supplemented with 1% (w/v) glucose (FPS+). The kidneys or spleen from each fish were individually homogenized, and cell counts and viability determined by hemocytometer counting and trypan blue exclusion, respectively. Cell concentrations were adjusted appropriately depending upon the assay protocol. Livers were also removed at the time of sacrifice, frozen immediately in liquid N2, and stored at −70°C until used to measure CYP1A protein induction.
To measure innate immune function, extracellular superoxide (O2.−) production by phorbol 12-myristate 13-acetate (PMA)–stimulated kidney phagocytes was measured colorimetrically by the O2.−-induced reduction of ferricytochrome C (Zelikoff et al., Citation1996). Superoxide production by bluegill kidney phagocytes was measured for up to 120 min in the presence of superoxide dismutase (SOD). Data are presented as nmoles of O2. − produced/4 × 105 cells/60 min.
As a measure of cell-mediated immune function, splenic T- and B-lymphocyte proliferation was measured in response to stimulation by either concanavalin A (ConA) or LPS, respectively. Proliferation of splenic lymphocytes was measured by reduction of 3-(4,5 dimethylthizol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) as described previously by Carlson et al. (Citation2002). Data are presented as the ratio between mitogen-stimulated/unstimulated T- or B-lymphocyte proliferation (i.e., fold-stimulation).
Individual livers from each treatment group were homogenized and ultra-centrifuged at 28,000 rpm for 90 min to obtain microsomes for the determination of CYP1A protein by ELISA (Carlson et al., Citation2002) and Western blot analysis (Meyer et al., Citation2002). The protein content of each microsomal sample was first determined using a commercially available protein assay kit (BioRad Laboratories, Hercules, CA). For ELISA measurements of CYP1A protein, samples were diluted to 10 μg protein/100 μl buffer, added in triplicate to an Immunolon 96-well microtitre plate (Dynatech Laboratories, Chantilly, VA), and incubated (at 4°C) for 24 hr. Following incubation, wells were washed, blocking solution (2% [w/v] bovine serum albumin [BSA] in phosphate-buffered saline [PBS]) was added and following a 60 min incubation, monoclonal antibody (mAb) C10-7 raised against rainbow trout CYP1A1 polypeptide sequence, (Rice et al., Citation1998), currently available through Biosense, Norway was added to each well. After another 60 min incubation, wells were washed, a secondary Ab added (anti-mouse IgG alkaline phophatase conjugate [Sigma, St. Louis, MO]), and plates re-incubated for an additional 60 min. After washing, p-nitrophenyl phosphate substrate solution (Sigma) was added to each well and the plates incubated for 30 min. The reaction was halted by the addition of NaOH to each well and absorbance determined spectrophotometrically at 405 nm. Data are presented as mean absorbance-blank values for each individual plate.
Western blots were performed on 2.0 μg microsomal protein from individual liver samples (Meyer et al., Citation2002). Briefly, samples were separated by SDS-PAGE using a 10% gel and transferred to an Immun-Blot PVDF membrane (Bio-Rad). Following transfer, the membranes were blocked overnight (at 4°C) in 5% milk, washed the following day, and the same mAb solution used for ELISA (Rice et al., Citation1998) was added to each well. After 2 hr at room temperature, the membranes were rewashed, a secondary Ab (anti-mouse IgG horseradish peroxidase conjugate [Cell Signaling, Beverly, MA]) was added (1 hour) and, after a final wash, bands were visualized using LumiGLO chemiluminescent reagents (Cell Signaling).
Statistical analyses were performed between treatment groups and the corresponding time-matched control by one-way Analysis of Variance (ANOVA) followed, when appropriate, by Fisher's post-hoc testing. Significance was accepted at p < 0.05. The large variation in sample size between the exposure groups was due to the pooling of data from the vehicle-injected control groups within a single post-exposure time point. The large fluctuations observed between- and within- post-injection time points were likely due to the outbred nature of the fish, and the fact that these experiments were carried out over a 16-month period.
RESULTS
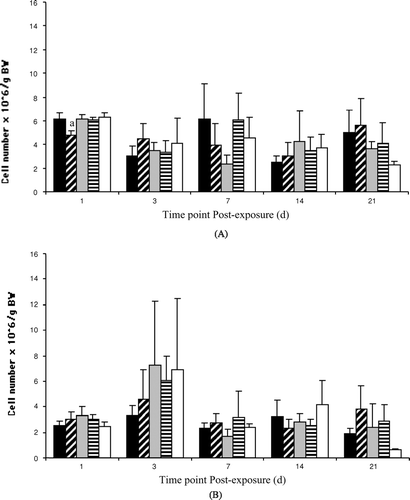
Injection of bluegill with PCB 126 or PCB 153 had no effect on host mortality, spleen cell numbers, or viability compared to the corn oil-injected controls ( and ). Kidney cell numbers were significantly lower (compared to controls) in fish exposed to 5.0 μg PCB 153/g BW, but only at 1 d post-exposure; values returned to control levels by 3 d post-exposure ().
FIG. 1 Kidney (A) and spleen (B) cell numbers from bluegill exposed to vehicle ▪, 5.0 ▪ or 50.0 □ μg PCB 153/g BW, or to 0.01 ▪ or 1.0 □ μg PCB 126/g BW at 1 (n = 7–14), 3 (n = 3–6), 7 (n = 3–9), 14 (n = 3–6) or 21 (n = 3–5) d post-injection. Each bar represents the mean ± SE. aSignificantly different (p < 0.05) from the time-matched vehicle control.
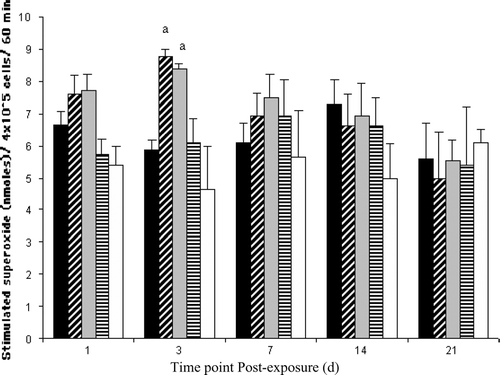
Superoxide production was affected only in bluegill exposed to the noncoplanar PCB congener 153 (). Kidney phagocyte-mediated O2.− production was significantly increased (compared to the time-matched vehicle control) at 3 d in both the low and high dose PCB 153 treatment groups. While a similar tendency was observed at 1 d post-injection, differences failed to reach statistical significance. Exposure to coplanar PCB 126 had no effect on O2.− production in fish examined for up to 21 d post-injection.
FIG. 2 Extracellular superoxide (O2. −) production by bluegill kidney phagocytes following exposure to vehicle ▪, 5.0 ▪ or 50.0 □ μg PCB 153/g BW, or to 0.01 □ or 1.0 □ μg PCB 126/g BW at 1 (n = 7–14), 3 (n = 5–8), 7 (n = 3), 14 (n = 5) or 21 (n = 3–5) d post-injection. Each bar represents the mean ± SE. aSignificantly different (p < 0.05) from the time-matched vehicle control.
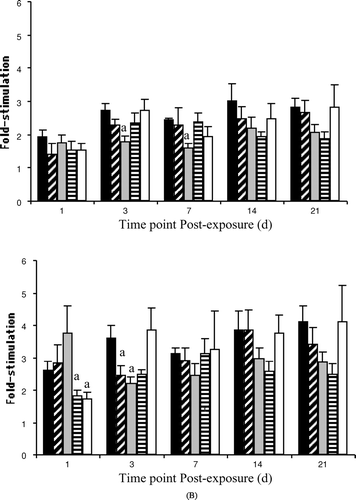
Proliferation by splenic lymphocytes was altered in bluegill exposed to either PCB congener ( and ). Concanavalin A-stimulated T-lymphocyte proliferation by fish injected with 50.0 μg PCB 153/g BW was significantly suppressed (compared to the time-matched vehicle control) at both 3 and 7 days post-exposure (). Mitogen-stimulated B-lymphocyte proliferation was suppressed in fish exposed to both PCB 153 doses, but only at 3 d post-injection (). While PCB 126 failed to alter T-lymphocyte proliferation at any dose or post-injection time point, exposure of bluegill to the same PCB congener and dose significantly suppressed (compared to the time-matched vehicle control) the proliferative response of B-cells 1 day following injection; effects of PCB 126 on B-lymphocyte activity were no longer obvious by 3 d post-exposure.
FIG. 3 ConA (A)- and LPS (B)-stimulated lymphocyte proliferation following exposure of bluegill to vehicle ▪, 5.0 ▪ or 50.0 □ μg PCB 153/g BW, or to 0.01 □ or 1.0 □ μg PCB 126/g BW at 1 (n = 3–13), 3 (n = 4–6), 7 (n = 3–5), 14 (n = 5–8) or 21 (n = 3–7) d post-injection. Each bar represents the mean ± SE. aSignificantly different (p < 0.05) from the time-matched vehicle control.
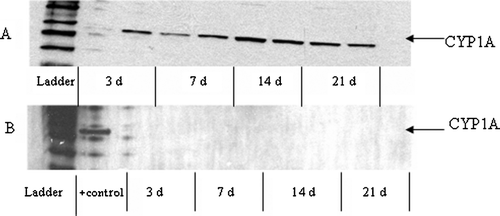
Hepatic CYP1A protein levels, measured by ELISA () and Western Blot analysis ( and ), were significantly induced in bluegill injected with the highest dose of PCB 126 (compared to controls); CYP1A protein levels in PCB 126-injected fish were significantly increased at 3 d, peaked at 14 d, and elevated levels persisted for up to 21 d post-injection ( and ). In contrast, exposure of bluegill to the noncoplanar congener (PCB 153) had no effect on CYP1A induction at either PCB dose or at any time point examined post-exposure ( and ).
FIG. 4 Hepatic CYP1A protein induction measured by ELISA in bluegill exposed to vehicle, PCB153 or PCB 126 at 1 ▪, 3 ▪, 7 □, 14 □ or 21 □ d post-injection. Each bar represents the mean (n = 3–5 individual liver samples/group) ± SE. aSignificantly different (p < 0.05) from all other time-matched treatment groups including control. bSignificantly different (p < 0.05) from the 14 d PCB 126 group exposed to 1.0 μg/g BW.
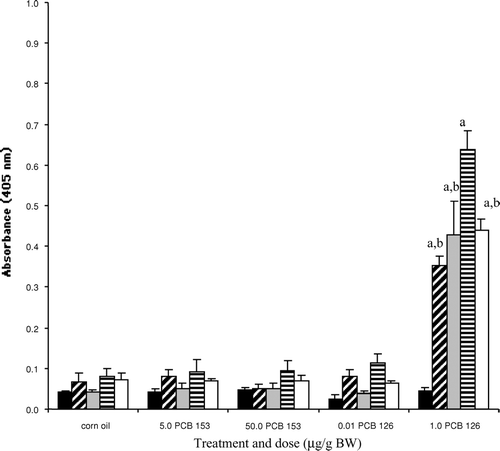
FIG. 5 Hepatic CYP1A protein induction measured by Western Blot in bluegill exposed to 1.0 μg PCB 126/g BW (A) or 50.0 μg PCB 153/g BW (B). Individual liver samples were performed in duplicate per post-injection time point. In , a liver sample taken from bluegill injected with 1.0 μg PCB 126/g BW and sacrificed at 14 d served as the “positive control.”
DISCUSSION
Although the effects of PCBs on the immune system have been well studied in mammals, and to a lesser extent in fish, the exact mechanism(s) by which PCBs alter the immune response has yet to be clearly defined. That 209 PCB congeners exist, each with their own chemical and biological activity, adds to the difficulty in establishing an exact mechanism by which PCBs affect host immunocompetence. Because of their ability to bind and activate the AhR, coplanar congeners have been the focus of most immunotoxicological studies. Results from these investigations suggested that, like dioxin, coplanar PCB congeners exert their immunotoxic effects primarily through activation of the AhR pathway. That the plaque forming cell (PFC) response to a T-dependent antigen was only depressed by exposure to Aroclor 1254 in Ah+ mice, illustrate this conclusion (Lubet et al., Citation1986). Similarly, AhR responsive mice administered 100 mg/kg BW of coplanar PCB congener 77 (3,3′,4,4′-tetrachlorobiphenyl) exhibited a suppressed PFC response (compared to controls) that correlated with an increase in hepatic cytochrome P450 protein levels (Silkworth and Grabstein, Citation1982). Exposure of the same Ah+ mice to up to 100 mg/kg of the noncoplanar 2,2′,5,5′-tetrachlorbiphenyl had no effect on the PFC response or hepatic cytochrome P450 protein levels.
However, evidence is now emerging which demonstrates that AhR activation may not necessarily be required for PCB-induced toxicity, including immunotoxicity. For example, mice injected with highly chlorinated noncoplanar PCB congeners (nona-chlorobiphenyl isomers and decachlorobiphenyl) demonstrated a suppressed PFC response to sheep red blood cells (SRBCs) in the absence of 7-ethoxyresorufin O-deethylase (EROD) activity; reduced PFC numbers were observed in Ah+ and Ah− mice exposed to both noncoplanar congeners, suggesting an AhR-independent mechanism of immunotoxicity (Harper et al., Citation1993). Disparate effects between coplanar and noncoplanar PCB congeners on phagocytosis, a measure of innate immune function, have also been reported. For example, a study by Levin et al. (Citation2004) demonstrated that exposure to only noncoplanar congeners altered the phagocytic ability of bottlenose dolphins and beluga whale peripheral blood leukocytes.
The relationship between PCB-induced immunotoxicity and hepatic CYP1A induction was examined in the current study using two PCB congeners having different affinities for the AhR. Polychlorinated biphenyl 126 has the distinction of being the most potent PCB congener with an assigned TEF of 0.1 for humans and other mammals, and 0.005 for fish (Van den Berg et al., Citation1998). In contrast, PCB 153 is considered relatively inert and not taken into consideration when evaluating toxic potential using the TEF method. However, exposure to noncoplanar PCB congeners have been shown in this and mammalian investigations to impact innate immune function by altering oxyradical production (Voie and Fonnum, Citation1998; Voie et al., Citation1998, Citation2000). In those investigations, ortho-substituted PCB congeners, specifically those with a 2,4,6-substitution pattern on one biphenyl ring, were shown to stimulate respiratory burst activity in human granulocytes; PCB-induced free radical production was suggested to be due to increases in intracellular calcium levels (Voie and Fonnum, Citation1998; Voie et al., Citation1998, Citation2000).
In contrast to the lack of effects of PCB 126 on extracellular oxyradical production observed in this study, investigations using other fish species have demonstrated that i.p. administration of the same congener decreased intracellular O2. − production (Rice and Schlenk, Citation1995; Regala et al., Citation2001; Duffy et al., Citation2005). Duffy et al. (Citation2003) demonstrated that injection of PCB 126, at the same doses used for this study (0.01 and 1.0 μg/g BW), could both increase and decrease PMA-stimulated intracellular O2. − production in Japanese medaka depending upon host age and time point examined post-exposure. Differences observed between this study examining extracellular free radical production and other studies investigating the effects of PCB 126 on intracellular oxyradical formation could be attributed to a reduced sensitivity of bluegill (compared to other fish species) for the toxic effects of PCB 126, or that extracellular O2. − production is simply not affected by PCBs. In support of the latter, extracellular O2. − production by kidney leukocytes from dab was shown to be unaltered following a 7-day exposure to PCBs in the sediment (Hutchinson et al., Citation2003).
In this study, exposure of bluegill to PCB 153 suppressed the proliferative response of both B- and T-lymphocytes. Although PCB-induced effects on this particular immune endpoint have not been well-studied in fish, results observed herein are similar to those observed in mammalian studies following exposure to noncoplanar PCB congeners (Stack et al., Citation1999; Nakata et al., Citation2002; Smithwick et al., Citation2003; Lyche et al., Citation2004). In one such study, in vitro exposure to various Aroclors (1–50 μg/ml), PCB 153 (25 μg/ml), or a tertiary mixture of PCB congeners (25 μg/ml) containing equal concentrations of the noncoplanar PCB 153, the mono-ortho coplanar PCB 118, and the coplanar PCB 126, significantly depressed LPS-stimulated B-lymphocyte proliferation by mouse splenocytes (Stack et al., Citation1999). However, in the same study, in vitro treatment of mouse splenocytes with either PCB 126 alone or the mono-ortho coplanar PCB 118 had no effect on LPS-stimulated lymphocyte proliferation at doses ranging from 0.01–25 μg/ml. Disparate effects of PCB congeners on lymphocyte proliferation have also been observed following in vivo PCB exposure. Goat kids, exposed prenatally and during lactation to PCB 153, demonstrated decreased phytohemagglutinin and ConA-stimulated proliferation of peripheral blood lymphocytes; similar effects were not observed in offspring exposed in utero to coplanar PCB 126 (Lyche et al., Citation2004).
Injection of bluegill with PCB 126 in this study had no effects on T-lymphocyte proliferation. This finding is similar to that observed following in vitro exposure of Dall's porpoises' peripheral blood mononuclear cells to concentrations of PCB 126 up to 30 nM (Nakata et al., Citation2002). To the contrary, treatment of bluegill splenocytes with PCB 126 inhibited B-cell proliferation 1 d following exposure. This same congener has been shown to inhibit IgM secretion and heavy chain mRNA expression in an LPS-stimulated murine B-cell lymphoma cell line at doses as low as 3 nM (Suh et al., Citation2003). This time-dependent response to PCB 126 is in contrast to that seen for the noncoplanar congener in that effects on B-lymphocyte proliferation in this study were not observed until 3 d post-injection, suggesting that the two PCB congeners are acting via different mechanisms to induce their suppressive effects on humoral immunity. Based upon the apparent sensitivity of B-lymphocytes to treatment with PCB congeners, B-cells may represent a particularly sensitive target for the effects of PCBs. The sensitivity of B-cells to other AhR agonists has been demonstrated in studies using dioxin, where IgM heavy chain gene expression and protein secretion were inhibited in a murine B-cell lymphoma cell line (Sulentic et al., Citation2000).
As seen in other fish and mammalian studies examining the effects of PCB 126 on P450 induction (van der Weiden et al., Citation1994; Rice and Schlenk, Citation1995; Rice and Roszell, Citation1998; Duffy et al., Citation2003; Chubb et al., Citation2004), exposure to the same coplanar congener caused a dose- and time-related induction of hepatic CYP1A protein in bluegill. However, at these sublethal, environmentally relevant doses, exposure to PCB 153 failed to induce hepatic CYP1A in bluegill examined as early as 1 day and as long as 21 days post-exposure. Studies utilizing other fish models have also demonstrated an inability of PCB 153 to increase hepatic CYP1A protein levels, mRNA and/or EROD activity (Kleinow et al., Citation1990; van der Weiden et al., Citation1994). For example, levels of hepatic CYP1A and EROD activity were unaltered in mirror carp 14 days following IP injection of PCB 153 (0.36–45.25 μg/kg) (van der Weiden et al., Citation1994). Treatment with PCB 153 also failed to induce CYP1A protein or mRNA levels in exposed rainbow trout (Kleinow et al., Citation1990). Studies by Suh et al. (Citation2003) using a murine B-cell lymphoma cell line have also demonstrated that PCB 153 can antagonize the AhR by reducing AhR agonist-induced CYP1A induction. On the other hand, exposure to PCB 153 has been reported to induce hepatic CYP1A protein (Foster et al., Citation1998) and increase EROD activity (DaCosta and Curtis, Citation1995) in rainbow trout. In these studies, protein levels and activity were increased in rainbow trout 4 weeks following a single IP injection of 50 or 250 μg PCB 153/g BW (Foster et al., Citation1998). Similarly, EROD activity was increased in the same species fed a diet of 20 μg PCB 153/g for 4 or 12 weeks (DaCosta and Curtis, Citation1995). A longer exposure duration, examination of CYP1A protein levels and/or activity at a later post-exposure time point, or species sensitivity to noncoplanar PCB congeners (specifically, PCB 153), may have contributed to the divergent results.
In the current study, both PCB congeners produced immune alterations in bluegill sunfish in the absence of hepatic CYP1A induction. This suggests that PCB-induced effects on respiratory burst activity and lymphocyte proliferation may have occurred via an AhR-independent mechanism. Taken together with results from the aforementioned long-term PCB 153 exposure studies in rainbow trout, it is possible that the immunotoxic effects of noncoplanar PCB 153 (and, possibly other noncoplanar congeners) may occur via both an AhR-independent and -dependent mechanism depending upon the exposure duration and/or examination time point post-exposure.
In conclusion, the results from this study are significant in that they provide compelling evidence that noncoplanar PCB congeners are, indeed, toxicologically important, and that PCB-induced effects on the immune response may occur via mechanisms independent of the AhR pathway. These findings highlight the importance of examining both coplanar and noncoplanar PCB congeners when evaluating toxic potential so as not to underestimate risk to exposed populations. Finally, given: the similarities between the fish and mammalian immune response; that the AhR pathway in fish and mammals is activated by many of the same agonists; and, the importance of developing alternative, non-mammalian species for assessing toxicological risk, fish may prove to be a valuable asset for assessing PCB-induced immunotoxicity.
ACKNOWLEDGMENTS
We would like to thank Dr. C.D. Rice (Clemson University) for providing monoclonal anti-trout CYP1A1 antibody C10-7 and American Aquatic Testing (Allentown, PA) for purchasing some of the fish used in these experiments. This work was supported, in part, by a Hudson River Graduate Fellowship and U.S. Army Contract no. DAMD 17-99-9011 and the NYU NIEHS Center Grant (ES00260).
The views, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as official Department of the Army position, policy, or decision, unless so designated by other official documentation. Research was conducted in compliance with the Animal Welfare Act, and other Federal statues and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals (NRC 1996) in facilities that are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International.
REFERENCES
- Carlson E. A., Li Y., Zelikoff J. T. Exposure of Japanese medaka (Oryzias latipes) to benzo[a]pyrene suppresses immune function and host resistance against bacterial challenge. Aquat. Toxicol. 2002; 56: 289–301, [PUBMED], [INFOTRIEVE], [CSA]
- Chubb L. S., Andersen M. E., Broccardo C. J., Legare M. E., Billings R. E., Dean C. E., Hanneman W. H. Regional induction of CYP1A1 in rat liver following treatment with mixtures of PCB 126 and PCB 153. Toxicol. Pathol. 2004; 32: 467–473, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Da Costa E. G., Curtis L. R. Bioaccumulation of dietary 2,2′,4,4′,5,5′-hexachlorobiphenyl and induction of hepatic arylhydrocarbon hydroxylase in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 1995; 14: 1711–1717, [CSA]
- Davis D., Safe S. Immunosuppressive activities of polychlorinated biphenyls in C57BL/6N mice: Structure-activity relationships as Ah receptor agonists and partial antagonists. Toxicology 1990; 63: 97–111, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Duffy J. E., Carlson E., Li Y., Prophete C., Zelikoff J. T. Impact of poylchlorinated biphenyls (PCBs) on the immune function of fish: Age as a variable in determining adverse outcome. Mar. Environ. Res. 2002; 54: 559–563, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Duffy J. E., Carlson E. A., Li Y., Prophete C., Zelikoff J. T. Age-related differences in the sensitivity of the immune response to a coplanar PCB. Ecotoxicology 2003; 12: 251–259, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Duffy J. E., Li Y., Zelikoff J. T. PCB-induced hepatic CYP1A induction is associated with innate immune dysfunction in a feral teleost fish. Bull. Envrion. Contam. Toxicol. 2005; 74: 107–113, [CSA], [CROSSREF]
- Duffy J. E., Zelikoff J. T. Approaches and models for the assessment of chemical-induced immunotoxicity in fish. Investigative Immunotoxicology, H. Tryphonas, M. Fournier, B. R. Blakley, J. E. G. Smits, P. Brousseau. CRC Press, Boca Raton, Florida 2005; 49–61
- Fischer L. J., Seegal R. F., Ganey P. E., Pessah I. N., Kodavanti P. R. S. Symposium overview: Toxicity of non-coplanar PCBs. Toxicol. Sci. 1998; 41: 49–61, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Foster E. P., Vrolijk N. H., Chen T. T., Curtis L. R. Interaction with 2,2′,4,4′,5,5′-hexachlorobiphenyl with hepatic cytochrome P-4501A in rainbow trout (Oncorhynchus mykiss). J. Toxicol. Environ. Health A 1998; 53: 313–325, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Harper N., Howie L., Connor K., Dickerson R., Safe S. Immunosuppressive effects of highly chlorinated biphenyls and diphenyl ethers on T-cell dependent and independent antigens in mice. Toxicology 1993; 85: 123–135, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hutchinson T. H., Field M. D. R., Manning M. J. Evaluation of non-specific immune functions in dab, Limanda limanda L., following short-term exposure to sediments contaminated with polyaromatic hydrocarbons and/or polychlorinated biphenyls. Mar. Environ. Res. 2003; 55: 193–202, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Karol M. H. Target organs and systems: Methodologies to assess immune system function. Environ. Health Perspect. 1998; 106(Supp. 2)533–540, [PUBMED], [INFOTRIEVE], [CSA]
- Kerkvliet N. I., Baecher-Steppan L., Smith B. B., Youngerberg J. A., Henderson M. C., Buhler D. R. Role of the Ah locus in suppression of cytotoxic T-lymphocyte activity by halogenated aromatic hydrocarbons (PCBs and TCDD): Structure-activity relationships and effects in C57Bl/6 mice congenic at the Ah locus. Fundam. Appl. Toxicol. 1990; 14: 532–541, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kleinow K. M., Haasch M. L., Williams D. E., Lech J. J. A comparison of hepatic P450 induction in rat and rainbow trout (Oncorhynchus mykiss): Delineation of the site of resistance of fish to phenobarbital-type inducers. Comp. Biochem. Physiol. 1990; 96C: 259–270, [CSA]
- Levin M., Morsey B., Mori C., De Guise S. Specific non-coplanar PCB-mediated modulation of bottlenose dolphin and beluga whale phagocytosis upon in vitro exposure. J. Toxicol. Environ. Health A 2004; 67: 1517–1535, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lubet R. A., Lemaire B. N., Avery D., Kouri R. E. Induction of immunotoxicity in mice by polyhalogenated biphenyls. Arch. Toxicol. 1986; 59: 71–77, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lyche J. L., Larsen H. J. S., Skaare J. U., Tverdal A., Dahl E., Johansen G. M., Ropstad E. Effects of perinatal exposure to low doses of PCB 153 and PCB 126 on lymphocyte proliferation and hematology in goat kids. J. Toxicol. Environ. Health A 2004; 67: 889–904, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Maule A. G., Jorgensen E. H., Vijayan M. M., Killie J. A. Aroclor 1254 exposure reduces disease resistance and innate immune responses in fasted arctic charr. Environ. Toxicol. Chem. 2005; 24: 117–124, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Meyer J. N., Nacci D. E., Di Giulio R. T. Cytochrom P4501A (CYP1A) in killifish (Fundulus heteroclitus): Heritability of altered expression and relationship to survival in contaminated sediments. Toxicol. Sci. 2002; 68: 69–81, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Nakata H., Sakakibara A., Kanoh M., Kudo S., Wntanabe H., Nagai N., Miyazaki N., Asano Y., Tanabe S. Evaluation of mitogen-induced responses in marine mammal and human lymphocytes by in-vitro exposure of butyltins and non-ortho coplanar PCBs. Environ. Pollut. 2002; 120: 245–253, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Regala R. P., Rice C. D., Schwedler T. E., Dorociak I. R. The effects of tributyltin (TBT) and 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) mixtures on antibody responses and phagocyte oxidative burst activity in channel catfish, Ictalurus punctatus. Arch. Environ. Contam. Toxicol. 2001; 40: 386–391, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rice C. D., Schlenk D. Immune function and cytochrome P4501A activity after acute exposure to 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) in channel catfish. Aquat. Anim. Health 1995; 7: 195–204, [CSA], [CROSSREF]
- Rice C. D., Schlenk D., Ainsworth J., Goksoyr A. Cross-reactivity of monoclonal antibodies against peptide 277-294 of rainbow trout CYP1A1 with hepatic CYP1A among fish. Mar. Environ. Res. 1998; 46: 87–91, [CSA], [CROSSREF]
- Rice C. D., Roszell L. E. Tributyltin modulated 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) – induced hepatic CYP1A activity in channel catfish, Ictalurus punctatus. J. Toxicol. Environ. Health A 1998; 55: 197–212, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Roy N. K., Courtenay S., Maxwell G., Yuan Z., Chambers R. C., Wirgin I. Cytochrome P4501A1 is induced by PCB 77 and benzo[a]pyrene treatment but not by exposure to the Hudson River environment in Atlantic tomcod (Microgadus tomcod) post-yolk sac larvae. Biomarkers 2002; 7: 162–173, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sanchez-Alonso J. A., LopezAparicio P., Recio M. N., Perez-Albarsanz M. A. Apoptosis-mediated neurotoxic potential of a planar (PCB 77) and a nonplanar (PCB 153) polychlorinated biphenyl congener in neuronal cell cultures. Toxicol. Lett. 2003; 144: 337–349, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Schlezinger J. J., Stegeman J. J. Induction and suppression of cytochrome P450 1A by 3,3′,4,4′,5-pentachlorobiphenyl and its relationship to oxidative stress in the marine fish scup (Stenotomus chrysops). Aquat. Toxicol. 2001; 52: 101–115, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Silkworth J. B., Antrim L., Kaminsky L. S. Correlations between polychlorinated biphenyl immunotoxicity, the aromatic hydrocarbon locus, and liver microsomal enzyme induction in C57BL/6 and DBA/2 mice. Toxicol. Appl. Pharmacol. 1984; 75: 156–165, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Silkworth J. B., Grabstein E. M. Polychlorinated biphenyl immunotoxicity: Dependence on isomer planarity and the Ah gene complex. Toxicol. Appl. Pharmacol. 1982; 65: 109–115, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Smithwick L. A., Smith A., Quensen J. F., III, Stack A., London L., Morris P. Inhibition of LPS-induced splenocyte proliferation by ortho-substituted polychlorinated biphenyl congeners. Toxicology 2003; 188: 319–333, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Stack A. S., Altman-Hamamdzic S., Morris P. J., London S. D., London L. Polychlorinated biphenyl mixtures (Aroclors) inhibit LPS-induced murine splenocyte proliferation in vitro. Toxicology 1999; 139: 137–154, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Suh J., Kang J. S., Yang K. H., Kaminski N. E. Antagonism of aryl hydrocarbon receptor-dependent induction of CYP1A1 and inhibition of IgM expression by di-ortho-substituted polychlorinated biphenyls. Toxicol. Appl. Pharmacol. 2003; 187: 11–21, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sulentic C. E. W., Holsapple M. P., Kaminski N. E. Putative link between transcriptional regulation of IgM expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin and the aryl hydrocarbon receptor/dioxin-responsive enhancer signaling pathway. J. Pharmacol. Exp. Ther. 2000; 295: 705–716, [PUBMED], [INFOTRIEVE], [CSA]
- Tryphonas H. Immunotoxicity of PCBs (Aroclors) in relation to great lakes. Environ. Health Perspect. 1995; 103(Supp. 9)35–46, [PUBMED], [INFOTRIEVE], [CSA]
- Van den Berg M., Birnbaum L., Bosveld A. T. C., Brunstrom B., Cook P., Feeley M., Giesy J. P., Hanberg A., Hasegawa R., Kennedy S. W., Kubiak T., Larsen J. C., Rolaf van Leeuwen F. X., Liem A. K., Djien Nolt C., Peterson R. E., Poellinger L., Safe S., Schrenk D., Tillitt D., Tysklind M., Younes M., Waern F., Zacharewski T. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans, and wildlife. Environ. Health Perspect. 1998; 106: 775–792, [PUBMED], [INFOTRIEVE], [CSA]
- van der Weiden M. E. J., de Vries L. P., Fase K., Celander M., Seinen W., van den Berg M. Relative potencies of polychlorinated dibenzo-p-dioxins (PCDDS), dibenzofurans (PCDFS) and biphenyls (PCBs), for cytochrome P450 1A induction in the mirror carp (Cyprinus carpio). Aquat. Toxicol. 1994; 29: 163–182, [CSA], [CROSSREF]
- Voie O. A., Fonnum F. Ortho-substituted polychlorinated biphenyls (PCB) elevates intracellular [Ca2 +]i in human granulocytes. Environ. Toxicol. Pharmacol. 1998; 5: 105–112, [CSA], [CROSSREF]
- Voie O. A., Wiik P., Fonnum F. Ortho-substituted polychlorinated biphenyls activate respiratory burst measured as luminol-amplified chemoluminescence in human granulocytes. Toxicol. Appl. Pharmacol. 1998; 150: 369–375, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Voie O. A., Tysklind M., Andersson P. L., Fonnum F. Activation of respiratory burst in human granulocytes by polychlorinated biphenyls: A structure-activity study. Toxicol. Appl. Pharmacol. 2000; 167: 118–124, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zelikoff J. T. Fish immunotoxicology. Immunotoxicology and Immunopharmacology 2nd ed., J. H. Dean, M. I. Luster, A. E. Munson, I. Kimber. Raven Press Ltd., New York 1994; 71–95
- Zelikoff J. T., Raymond A., Carlson E., Li Y., Beaman J. R., Anderson M. Biomarkers of immunotoxicity in fish: From the lab to the ocean. Toxicol. Lett. 2000; 112–113: 325–331, [CSA], [CROSSREF]
- Zelikoff J. T., Wang W., Islam N., Twerdok L. E., Curry M., Beaman J., Flescher E. Assays of reactive oxygen intermediates and antioxidant enzymes: Potential biomarkers for predicting the effects of environmental pollution. Techniques in Aquatic Toxicology, G. K. Ostrander. Lewis Publishers, New York 1996; 287–306