Abstract
The present investigation was carried out to study the anti-arthritic activity of ethanolic extract of seeds of Moringa oleifera Lam. (MOEE) in adjuvant-induced arthritis in adult female Wistar rats. During the experimental period, body weight, paw edema volume (primary lesion) and arthritic index (secondary lesion) was observed. On the 21st day, serum from each animal was used for estimation of Rheumatoid Factor (RF) value and levels of selected cytokines (TNFα, IL-1, and IL-6). Whole blood was used for measurement of erythrocyte sedimentation rate (ESR). Liver homogenate was utilized for assessment of oxidative stress and histopathology was performed to measure degree of inflammation in synovial joint. Our results suggest that, percentage reduction in body weight was less, paw edema volume and arthritic index score was decreased significantly as compared to diseased control animals. Serum levels of RF, TNF-α, IL-1, and IL-6 also showed decreased levels as compared to those in the diseased control group. Treatment with MOEE also altered oxidative stress in relation to its anti-inflammatory activity. Histopathological observations showed mild or less infiltration of lymphocytes, angiogenesis and synovial lining thickening. From all above results and observations, it can be concluded that Moringa oleifera possesses promising antiarthritic property.
Keywords :
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized pathologically by joint edema, lymphocytic and synovial lining cells infiltration, vascular proliferation, destruction of cartilage and bone (Miossec, Citation1992). Within the RA joint, the proliferating pannus and synovial fluid are abundantly populated by inflammatory cells, and the proteolytic enzymes, prostanoids, cytokines, and oxygen-derived free radicals (Arend and Dayer, Citation1995). These free radicals released from the cells are considered the prime mediators of breakdown of cartilage and bone in this disease (Holt et al., Citation1992; McDonnell et al., Citation1999).
From the pathological point of view, synovial macrophages are believed to be important in the development and progression of tissue and joint damage in rheumatoid arthritis (Halliwell, Citation1994). These inflammatory cells release pro-inflammatory cytokines such as interleukins (ILs) and tumor necrosis factor-α (TNF-α) (Koch et al., Citation1995; Szekanecz et al., Citation2000). These mediators play a pivotal role in the initiation, evolution and persistence of chronic inflammation (Dayer and Fenner, Citation1992; Bertazzolo et al., Citation1994).
Although non-steroidal anti-inflammatory drugs (NSAIDs) are used widely to control the symptoms of both RA and osteoarthritis, the ability of the agents to preserve cartilage and bone and positively influence the underlying pathologic condition remains controversial. Due to the increasing frequency of intake of NSAIDs and their reported common side effects, there is need to focus on the scientific exploration of herbal drugs having fewer side effects. Currently, in India and other developing countries, there is a continuous search for drugs that can provide relief against chronic inflammation. As such, a search for alternative anti-arthritic therapies that do not adversely affect are of current interest. To date, no scientific study has been reported that has examined these alternative therapies in connection with the development or persistence of rheumatism. In this respect, the present investigation was undertaken to determine the efficacy of Moringa oleifera against adjuvant-induced arthritis in rats.
Moringa oleifera Lam. belongs to the family Moringaceae (genus Moringa), and is its best- known and most widely distributed species (Moron, Citation1991; Sengupta and Gupta, Citation1970). It is a small, fast-growing ornamental tree grown throughout the tropics and subtropics of Asia and Africa (Michael and Horst, Citation1998). Numerous varieties of M. oleifera have been developed to meet the tastes of local populations (Rajan, Citation1986). The medicinal values of the seeds and the different parts of the plant have long been recognized in folklore medicine (Faizi et al., Citation1995).
The whole plant possesses antimicrobial activity (Caceres et al., Citation1991) and is also used for the treatment of ascites, rheumatism, venomous bites, and for enhancing cardiac function (Nadkarni and Nadkarni, Citation1976; Chaudhri, Citation1996). The leaves exhibit strong hypotensive, diuretic, spasmolytic effects and have been seen to be useful against inflammation, helminthesis and in scurvy (Faizi et al., Citation1995; Caceres et al., Citation1992). The roots of the plant have been used as carminatives, anthelmintics, diuretics, and in the treatment of intermittent fever, epilepsy, and chronic rheumatism (Kirtikar and Basu, Citation1975). The plant's seeds have been used as purgatives, antipyretics and as anti-inflammatory agent (Varier, Citation1997).
MATERIALS AND METHODS
Procurement of Plant Material and Extraction Procedure
Seed kernels of M. oleifera were obtained from a commercial supplier of Ahmedabad and then identified and authenticated by Department of Pharmacognosy, L. M. College of Pharmacy, Ahmedabad. Voucher specimens were deposited at the Dept. of Pharmacognosy, Ahmedabad, India.
A coarse powder (500 g) of the dried seed kernels of M. oleifera was defatted using petroleum ether and then exhaustively extracted using 95% ethanol (2 L) in a Soxhlet extractor at 55°C for 6 hr. The resulting extract was then concentrated under reduced pressure to yield a syrupy mass. This final extract was stored in airtight container in a cool place and used throughout the course of the study.
Phytochemical Analysis
Preliminary phytochemical studies of the extract were performed for alkaloids, flavanoids, saponins, glycosides, phenols, steroids, tannins and terpenoids according to published standard methods (Paech and Tracey, Citation1955).
Drugs and Chemicals
All organic solvents were obtained from the S.D. Fine Chemicals Private Limited (Mumbai, India) and all were of analytical grade. Rheumatoid Factor was assessed with a Turbilatex kit obtained from Spinreact Co. (Girona, Spain), ELISA kits for cytokine measurements were obtained from Biosource (Camarillok, CA). Complete Freund's Adjuvant (CFA) containing 10 mg/ml of heat-killed Mycobacterium tuberculosis, hydrogen peroxide, trichloroacetic acid (TCA), thiobarbituric acid (TBA), Tris buffer, 5, 5′-dithiobis-2-nitrobenzoic acid (DTNB), epinephrine and dexamethasone were all obtained from Sigma-Aldrich (St. Louis, MO).
Animals
Wistar albino rats procured from Zydus Cadila Limited (Ahmedabad, India) were housed at ambient temperature (22 ± 1°C), relative humidity (55 ± 5%) and 12-hr light-dark cycle. Animals had free access to standard pellet diet (certified Amrut brand rodent feed, Pranav Agro Industries, Pune, India) and filtered tap water given ad libitum. Freshly prepared solutions of drugs or chemicals were used throughout the study. All the experiments complied with the University guidelines for animal experimentation. Throughout the entire study period, the rats were monitored for growth, health status and food intake capacity to be certain that they were healthy.
Complete Freund's Adjuvant-Induced Arthritis in the Rats
Adult female Wistar rats, weighing 150–180 g were divided into five different groups. Each group consists of six animals. Group-I: Non-arthritic control (distilled water); Group-II: Diseased control; Group-III: dexamethasone (5 mg/kg body weight [BW]/day per os, as reference standard); Group-IV: MO1 (100 mg/kg/day per os, of ethanolic extract of M. oleifera); and, Group-V: MO2 (200 mg/kg/day per os, of ethanolic extract of M. oleifera). These daily doses were selected based upon preliminary studies screening for overall anti-arthritic activity using three different doses (MOEE at 100, 200 and 400 mg/kg per os); promising dose dependent activity was obtained at both the 100 and 200 mg/kg levels (i.e., the highest dose had little more effect than the 200 mg/kg dose).
Experimental arthritis was induced according to the modified method of Pearson and Wood (Citation1963). Briefly, 0.1 ml of Complete Freund's Adjuvant (CFA) containing 10 mg/ml of heat-killed M. tuberculosis was injected once intradermally in the right hind paw of each animal (in Groups II-V); this time of adjuvant injection was referred to hereon as Day 0. Treatments with water, MOEE, or dexamethasone were started on Day 0 as well and continued daily for 21 days thereafter.
Assessment of Arthritis
During the experimental period, changes in body weight paw volume, and arthritic index score were recorded. Degree of inflammation (in terms of edema formation was measured plethysmographically) (Winter et al., Citation1962) as well as extent of primary lesions were measured on Days 1, 3, 5, 9, and 21 of the exposures to the various treatments. The secondary lesions were assessed on Day 21 in terms of arthritic index.
Measurement of Primary and Secondary Lesions
Primary lesions refer to the edema formation in the injected hind paw that peaks 3-5 days after injection of the CFA. The edema volume of the injected paw was measured plethysmographically on Day 5.
Secondary lesions are immunologically mediated changes characterized by inflammation of the non-injected sites. Accordingly, secondary lesions were evaluated by calculating the percent inhibition of paw volume of the non-injected left hind paw on Day 21 of the drug/vehicle treatment regimen and using arthritic index as the sum of scores for each animal according to the method of Schorlemmer et al. (Citation1999). Redness and nodules in ear: none = 0, visible = 1, swelling of connective tissue of nose: none = 0, visible = 1, nodules in tail: none = 0, visible = 1, forepaw inflammation: none = 0, visible = 1, hindpaw inflammation: none = 0, slight = 1, moderate = 2 and marked = 3. The average of the treated animals was compared with the control group.
Measurement of the Inflammatory Mediators in Serum
At the end of Day 21, blood was collected (under light ether anaesthesia) from the retro-orbital veins of each animal and each sample was divided into two portions. The first aliquot was placed in a non-heparinized tube for serum separation and it was then kept at −20°C for the quantitative determination of the levels of Rheumatoid Factor (RF) and the cytokines TNF-α, IL-1, and IL-6 by ELISA (following manufacturer instructions). The second portion of the blood was placed in a heparinized tube and used for estimation of erythrocyte sedimentation rate (ESR) by the method of Westerngren (Al-Fadhli and Al-Awadhi, Citation2005).
Assessment of Pro-oxidant and Antioxidant Status
After the measurement of serum parameters, all the animals were sacrificed by cervical dislocation and their livers isolated. Each liver was homogenized in Tris HCl buffer (0.01 M, pH. 7.4) using a REMI homogeniser (REMI Motor, Bombay, India) to generate a 10% homogenate (Hogberg et al., Citation1974). The method of Devasagayam (Citation1986) was used to measure the presence of lipid peroxides in the homogenate and is based upon the release of malondialdehyde. For the estimation of antioxidant enzyme activities in the samples, superoxide dismutase (SOD) was assayed according to the method of Misra and Fridovich (Citation1972), with the degree of inhibition of the auto-oxidation of epinephrine by SOD being used as the index of activity. Catalase activity was assayed by the method of Sinha (Citation1972). The method of Moron et al. (Citation1979) was used for estimations of total reduced glutathione (GSH). Protein was estimated according to the method of Lowry et al. (Citation1951). All the parameters were estimated spectrophotometrically (UV 1601, Shimadzu (Asia Pacific) Pte Ltd., Sydney, Australia) at their respective specified wavelengths.
Histopathological Studies
Histopathological studies of synovial joint obtained from rats were carried out on Day 21. Dissected joints were washed with normal saline and then kept in 10% formal saline. The joint then kept in Bouin's fixative for 18–24 hr and were then washed twice with distilled water and placed in 70% alcohol. A pinch of lithium carbonate was added to remove excessive stain. The joints were washed and placed in 70% alcohol again. After that, they were transferred to 90% alcohol overnight. Joint samples were then transferred into 100% alcohol for 3 hr, then into xylene until they become transparent. At this point, the samples were fixed in paraffin to performed sectioning. Several sections (3 μ m thickness) were taken from each joint and sections with uniform shape and size were selected for histology. Selected sections were fixed on clear glass slides and stained using Heamotoxylin and Eosin (H & E) stain.
Statistical Analysis
Results were reported as mean ± SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA). If the overall F value was found statistically significant (p < 0.05), further comparisons among groups were made according to post-hoc Tukey's test. All statistical analyses were performed using Graph Pad software (San Diego, CA). Though data was considered statistical significant at p < 0.05, when data was found to be very (i.e., p < 0.01) or highly (i.e., p < 0.001) significant, this is indicated in the Results.
RESULTS
Phytochemical Study
The preliminary phytochemical screening of the ethanolic extract of seeds of M. oleifera showed the presence of alkaloids, flavanoids, glycosides, tannins and terpenoids ().
TABLE 1 List of major phytochemical constituents of the seeds of Moringa oleifera
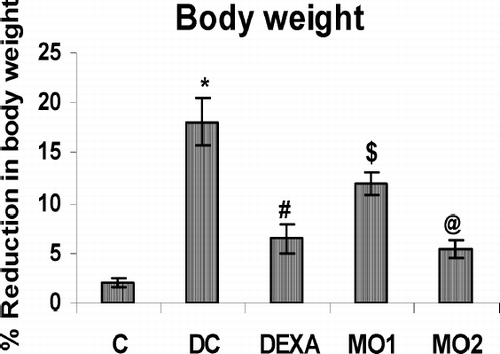
Effect of MOEE on Body Weight
The results shown in illustrate the body weight changes during the arthritic and drug-treated conditions. The percentage reduction in body weight over the entire 21-day treatment period was seen to be significantly and highly significantly less in the MO1 and MO2 treatment groups, respectively, as compared to those in diseased control animals.
FIG. 1 Effect of MOEE on body weight over the total treatment period. Value is highly significantly different from non-arthritic control (* p < 0.001). Value different from diseased controls (at differing levels of significance; @ p < 0.001, # p < 0.01, $ p < 0.05). Values shown are the mean ± SEM from nonarthritic, disease control, and treatment regimen rats (n = 6 rats/group).
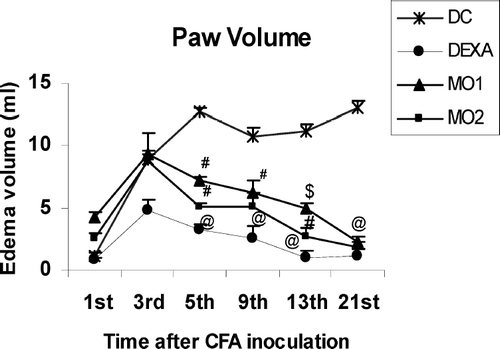
Effect of MOEE on Paw Edema Volume (Primary Lesions)
indicates that, in diseased control animals, paw edema was induced by CFA inoculation and the process was seen to be biphasic. An acute phase was evidenced on Day 5 followed by a delayed sustained chronic phase that reached a plateau starting from Day 11 onwards to Day 21. Significant, very significant, and highly significant decreases in the primary lesion were evident on Day 5 in rats in the MO1-, MO2-, and dexamethasone-treated groups, respectively, as compared to the diseased controls. Paw edema volume of each animal was measured on Days 1, 3, 5, 9, and 21 of the exposures to the various treatments.
Effect of MOEE on Secondary Lesions
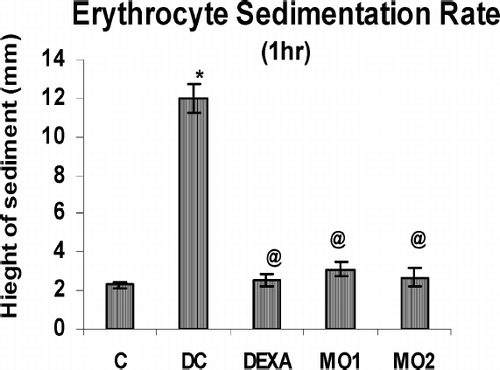
With regard to the secondary lesions, these could only be partially evaluated as no edema formation occurred in the contralateral left hind paw of control animals. However, on the 21st day, significant, very significant, and highly significant decrease in edema volume were observed in the injected paw of MO1-, MO2-, and dexamethasone-treated rats, respectively (). The mean arthritic index scores of the MOEE-treated groups were also significantly different from the disease control group, but comparable to that of dexamethasone-treated rats (). As seen in , the erythrocyte sedimentation rates (ESR) on this day were found to be very significantly higher in arthritic rats than healthy controls. However, treatments with MOEE were seen to decrease these rates back to normal levels.
FIG. 3 Effect of MOEE on arthritic index on Day 21 of the treatment regimens. Value different from diseased controls (at differing levels of significance; @ p < 0.001, # p < 0.01, $ p < 0.05). Values shown are the mean ± SEM from disease control and treatment regimen rats (n = 6 rats/group).
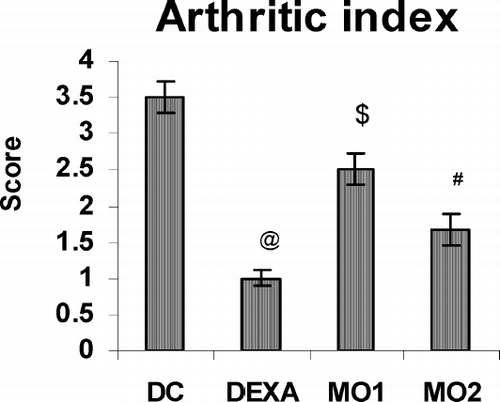
FIG. 4 Effect of MOEE on ESR using blood obtained on Day 21 of the treatment regimens. Value is highly significantly different from non-arthritic control (*p < 0.001); value highly different from diseased control (@ p < 0.001). Values shown are the mean ± SEM from nonarthritic, disease control, and treatment regimen rats (n = 6 rats/group).
Effect of MOEE on Serum Parameters
The data in reflects the highly significantly increased (p < 0.001) levels of rheumatoid factor (RF) during the arthritic condition; however, these Day 21 levels were significantly reduced to near normal by MO1 and MO2 treatments. As shown in , which reports the serum levels of TNF-α, IL-1, and IL-6 in all animals. Very significantly-elevated levels of TNF-α found on Day 21 in arthritic rats (vs. non-arthritic controls) were found to be significantly restored to near normal levels by the dexamethasone and MO2 treatments. This testifies the protective effect of MOEE against increased level of TNF-α during the course of arthritis.
FIG. 5 Effect of MOEE on RF levels in serum obtained on Day 21 of the treatment regimens. Value is highly significantly different from non-arthritic control (*p < 0.001). Value different from diseased controls (at differing levels of significance; @ p < 0.001, # p < 0.01, $ p < 0.05). Values shown are the mean ± SEM from nonarthritic, disease control, and treatment regimen rats (n = 6 rats/group).
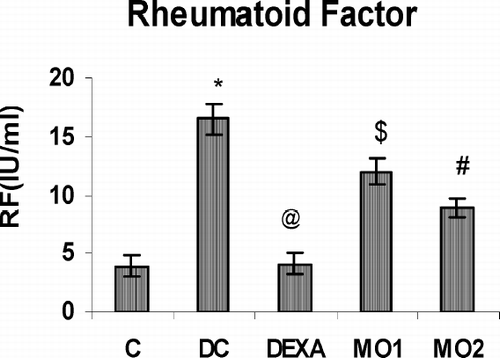
TABLE 2 Effect of MOEE on levels of inflammatory mediators in serum obtained on Day 21 of the treatment regimens
A basal systemic IL-1 concentration of (56.7 ± 7.08) was observed in the non-arthritic rats. Although the systemic IL-1 levels in diseased controls was significantly increased (151.3 ± 5.9), MO2 treatment was found to significantly inhibit the increase in IL-1 levels, and it was also appeared that dexamethasone treatment led to significantly decreased IL-1 concentrations.
Levels of IL-6 in diseased control rats were increased very significantly as compared to those in non-arthritic rats. However, a significant protective effect against this increase was observed by treatment of the rats with MO2. There was no effect from MO1 treatment on IL-1 and IL-6 levels.
Effect of MOEE on Lipid Peroxidation (MDA Levels)
The data presented in shows that significantly decreased levels of malondialdehyde (MDA) were found in diseased control rats (vs. nonarthritic controls) on Day 21. However, compared to these diseased controls, treatment with MO1 and MO2 led to highly significantly increased levels of MDA; these values were comparable to those in the dexamethasone-treated animals and approached those in the nondiseased controls.
TABLE 3 Effect of MOEE on oxidative stress in arthritic rats on Day 21 of the treatment regimens
Effect of MOEE upon Antioxidant Enzymes SOD and CAT and upon GSH
The data in also indicates that the activities of superoxide dismutase (SOD) and catalase (CAT) were highly and very significantly increased, respectively, in rats with adjuvant-induced arthritis as compared to those in nonarthritic controls. There was no effect of MO1 on antioxidant enzyme levels; however treatment with MO2 very significantly reduced the levels of both enzymes significantly. In fact, this treatment brought about values nearly that of the nonarthritic control rats. On the other hand, there were no significant changes observed in total reduced glutathione levels (GSH) with any of the treatments.
Histopathology Results
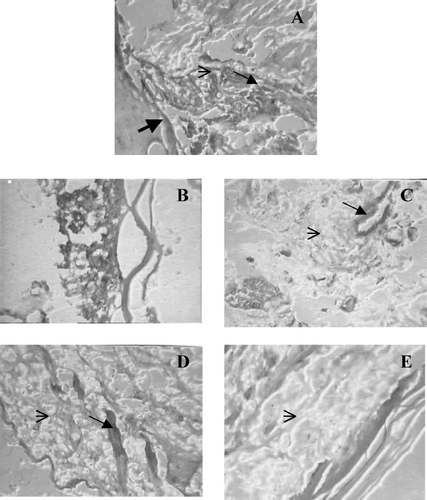
Histological observations suggest that, sections from diseased control animals clearly show the pathological changes like bone destruction, cartilage erosion, severe angiogenesis and infiltration of lymphocytic cells. However, the synovial sections from MOEE- and dexamethasone-treated rats clearly showed some degree of protection against these above-noted changes ().
FIG. 6 Histopathological effects from the various treatments. On Day 21 synovial joint sections of treated and untreated CFA arthritis animals stained with hematoxylin-eosin. A. Shows typical pathological changes in arthritic joint with bone destruction, cartilage erosion, severe angiogenesis and infiltration of lymphocytic cells. B. Normal synovial joint. C. Shows less angiogenesis and mild lymphocytic infiltration by dexamethasone treatment. D. MO1 shows less lymphocytic infiltration but does not protected joint against angiogenesis. E. MO2 shows significant protection against lymphocytic infiltration, bone destruction and cartilage erosion. ![]()
DISCUSSION AND CONCLUSIONS
Adjuvant-induced arthritis in rats is a well-established experimental model for the study of the pathophysiology of various types of human arthritis, especially rheumatoid arthritis (RA) (Pearson and Wood, Citation1963; Owen, Citation1980; Billingham, Citation1983). Adjuvant-induced arthritis is known to produce both primary and secondary lesions, with a majority of the pathological changes that develop being similar to those observed in RA. Considering these advantages we utilized the Complete Freund's Adjuvant (CFA)-induced RA model in rats (Schorlemmer et al., Citation1999) to assess the potential effects of extract of M. oleifera upon inflammatory parameters associated with the development of the disease state.
Changes in body weight have been found to occur in parallel to those in the incidence and severity of RA (Walz et al., Citation1971). Results from the present study shows that the adverse physical changes like paw edema volume, arthritic index, and loss of body weight in arthritic animals were reversed to a considerable extent by oral administration of MOEE. Changes in erythrocyte sedimentation rate (ESR) are also a useful measure of inflammation during RA and are directly proportional to the levels of disease severity (Sarbon et al., Citation2005). Animals from the MOEE treatment groups showed normal ESR rates (i.e., similar to that of the nonarthritic controls). Lastly, rheumatoid factor (RF) is useful as a measure of the amount of IgM present in the serum of diseased hosts. It has been reported that RF can be synthesized by B-cells and plasma cells that have infiltrated into the synovium of RA patients (Kim et al., Citation1999; Ernest et al., Citation2001). The data here showed that treatment with MOEE significantly lessened serum RF levels as compared to those in diseased control rats.
These apparent anti-inflammatory outcomes attained with the M. oleifera treatments can likely be attributed to the presence of flavanoids and antioxidants present in its extracts. MOEE have been purported to be a rich source of antioxidants, including flavanoids and polyphenolic compounds (Sanchez et al., Citation2006). Flavonoids have been shown to be responsible for preventing osteoporosis by causing an increase in bone mineral density (Nijveldt et al., Citation2001).
In this model of immunologically mediated chronic synovial inflammation and arthritis, macrophages and fibroblast play a pivotal role. Specifically, after activation, they are each capable of synthesizing several of the inflammatory mediators such as PGE2, cytokines (TNF-α, IL-1, and IL-6), and granulocyte-monocyte colony stimulating factor (GMCSF) that are abundantly expressed during the development of RA. In turn, these products induce production of a variety of enzymes that initiate cartilage and bone destruction (Hopkins, Citation1990).
Pro-inflammatory TNF-α has been implicated in the pathological mechanisms of synovial tissue proliferation, joint destruction, and programmed cell death in RA (Kennedy et al., Citation1979). Elevated TNF-α levels have been found in the sera and joints of adjuvant-induced arthritis for up to 50 days after adjuvant injection (Szekanecz et al., Citation2000). It was also reported that the expression of TNF-α and tissue enzymes (like matrix metalloproteinases) were observed to be increased in the subchondral bone region of knee joint samples obtained from human osteoarthritis or RA patients (Kaneko et al., Citation2001). Anti-TNF monoclonal antibody treatment in adjuvant-induced arthritis improved clinical score and was paralleled by an inhibition of leukocyte accumulation in the joints (Issekutiz et al., Citation1994). Similarly, IL-1 stimulates synoviocytes and monocytes, and promotes bone and cartilage degradation via inflammatory cascade; thus, IL-1 is involved in the pathogenesis of RA synovitis (Evans et al., Citation2005). Recently, many strategies have been aimed at blocking the activity of IL-1 with a view toward achieving a therapeutic effect against autoimmune arthritis. IL-6 is the most abundantly expressed cytokine in rheumatoid synovium. IL-6 can be derived from Type B synovial lining cells and fibroblasts, and its release is likely in response to insults that lead to joint damage (Madhok et al., Citation1993; Bertazolo et al., Citation1994). A highly significant correlation between local IL-6 level and the severity of chronic arthritis in rats has been shown (Brauer et al., Citation1994). Thus, IL-6 is a good marker of arthritis, including the type induced by Freund's adjuvant (Leisten et al., Citation1990). Anti-IL-6 therapy has been proposed to be a promising approach to treatment of chronic inflammation (Brauer et al., Citation1994).
The results here tend to indicate that the MOEE treatments act upon the formation of each of these three critical cytokines during the mitigation of the development of RA in the rats due to adjuvant treatment. MO2 was significantly (p < 0.05) reduced IL-6 levels in serum as compared to arthritic rats. Indeed, certain immunosuppressive agents such as cyclosporine-A succeeded in inhibiting chronic arthritis associated with normalization of IL-6 level (Theisen et al., Citation1992)
Inflammation and tissue injury-related oxidative stress have been implicated in the pathogenesis of RA (Ostrakhovitch and Afanas'ev, Citation2001). Free radicals are produced in large quantities at the site of inflammation and/or tissue injury (Gotia et al., Citation2001). Lipid peroxides that are generated at the site of inflammation (tissue injury) diffuse into the blood and can be estimated in isolated serum or plasma; in turn, these levels can be used to reflect the severity of the original (or ongoing) tissue damage (Gutteridge, Citation1995). In the present study, we observed a suppression of lipid peroxidation in the liver of diseased control rats may be due to the decline in the level of hepatic cytochrome P-450 (Carthrone et al., Citation1976). This cytochrome is involved in NADPH-dependent lipid peroxidation and is responsible for the decreased rate of oxidation of lipids in the liver. Another possible explanation is increased removal of lipid peroxides from liver into the blood of arthritic animals resulting in its elevation in serum (Geetha et al., Citation1998). This is evident from the fact that the serum of arthritic rats has reduced ability to inhibit the lysis of rabbit polymorphonuclear leucocytes after Triton X-100 induction and further they even labilise cell membranes (Robak, Citation1978).
Superoxide radicals play an important role as a chemical mediator on the inflammatory response of rheumatoid arthritis (Kaneda, Citation1982). Here, as expected, an increased level of superoxide dismutase (SOD) was observed in the diseased control rats. In the study here, administration of MOEE caused a significant decrease in the elevated SOD activity seen in diseased animals. The main function of catalase is to detoxify hydrogen peroxide (H2O2). Similarly, although catalase is significantly increased in RA, treatment with MOEE assured that catalase levels began to trend toward those seen in the nonarthritic control rats. The GSH level in the diseased control group or in any of the treatment groups was not affected. This might be due to the fact that the reduced GSH was being continuously maintained at an adequate rate by the activities of the glutathione redox cycle (Mortensen, Citation1953).
All of these findings likely reflect the overall decrease in the original formation of inflammatory reactive oxygen species (ROS) (and as a result, decreased tissue damage) that resulted from the MOEE treatment. With less ROS generated, there is a lowered need for SOD and catalase, and decreased consumption of GSH (and redox cycle activity) due to the lessened presence of peroxidative damage to cell membranes. Except for the changes in GSH, each of these findings were observed in the various treatment groups receiving either dexamethasone or moreover, the higher dose of MOEE.
The possible implication from this study is that therapeutic dosing with MOEE might also be able to have a positive effect upon already-established arthritis in rats (or other hosts). As noted earlier, many recently strategies have focused on blocking the activity of TNFα, IL-1, and IL-6 with a view toward achieving a therapeutic effect against autoimmune arthritis. Our findings suggest that MOEE at a dose of 200 mg/kg suppresses the formation/release of key proinflammatory cytokines and inhibit leukocyte infiltration into the potential target sites for the disease (confirmed here by histopathological observation).
Moreover, the qualitative phytochemical investigations of seed extracts of M. oleifera here and by other investigators have shown the presence of an array of active chemical constituents including alkaloids, flavanoids, glycosides, tannins and terpenoids. Most of the active principles reported have specific pharmacologic activity (). In our laboratory, we have also succeeded in isolating benzylisothiocyanate from the extract; investigations are ongoing to verify this and to even better characterize the composition of the extract.
From the results reported here, it can be concluded that the MOEE has significant anti-arthritic activity and also alters oxidative stress in treated rats. Further studies are needed to elucidate in detail precise mechanisms for these outcomes and the role of each major constituent responsible for such activities.
REFERENCES
- Al Fadhli S. M., Al-Awadhi A. M. Comparison of erythrocyte sedimentation rate measurement by the automated SEDI system and conventional Westergren Method using the Bland and Altman statistical method. Med. Princ. Pract. 2005; 14: 241–244
- Arend W. P., Dayer J. M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor- ∝ in rheumatoid arthritis. Arthritis Rheum. 1995; 38: 151–160
- Bertazzolo N., Punzi L., Stefani M., Cesaro G., Pianon M., Finco B., Todesco S. Interrelationships between interleukin IL-1, IL-6 and IL-8 in SF of various arthropathies. Agents Action 1994; 41: 90–92
- Billingham M. E. Models of arthritis and the search for antiarthritic drugs. Pharm. Ther. 1983; 21: 389–428
- Blake D. R., Hall N. D., Terby D. A., Halliwell B, Gutteridge J. M. Protection against superoxide and hydrogen peroxide in synovial fluid from rheumatoid patients. Clin. Sci. 1981; 61: 483–488
- Brauer R., Kette H., Henzgen S., Thoss K. Influence of cyclosporine-A on cytokine levels in synovial fluid and serum of rats with antigen induced arthritis. Agents Action 1994; 41: 96–98
- Caceres A., Cebreva O., Morales O., Miollined P., Mendia P. Pharmacological properties of M. oleifera 1. Preliminary screening for antimicrobial activity. J. Ethnopharmacol. 1991; 33: 213–216
- Caceres A., Saravia A., Rizzo S., Zabala L., De-Leon E., Nave F. Pharmacological properties of M. oleifera 2. Screening for antispasmodic, anti-inflammatory and diuretic activity. J Ethnopharmacol. 1992; 36: 233–237
- Carthrone M. A., Palmer E. D., Green J. Adjuvant induced arthritis and drug metabolizing enzymes. Biochem. Pharmacol. 1976; 25: 2683–2688
- Herbal Drug Industry: A Practical Approach to Industrial Pharmacognosy, R. D. Chaudhri. Eastern Publisher, New Delhi 1996; 58–62
- Colpaert F. C. Evidence that adjuvant arthritis in the rat is associated with chronic pain. Pain. 1987; 28: 201–222
- Dahot M. U., Memon A. R. Nutritive significance of oil extracted from Moringa oleifera seeds. J. Pharm. Univ. Karachi. 1985; 3: 75–80
- Das B. R., Kurup P. A., Narasimha Rao P. L. Antibiotic principle from Moringa Pterosperma Part IX. Inhibition of transaminase by isocyanates. Ind. J. Med. Res. 1958; 46: 75–77
- Dayer J., Fenner H. The role of cytokines and their inhibitors in arthritis. Bailliere's Clin. Rheumatol. 1992; 6: 485–516
- Dayrit F. M., Alcantara A. D., Villasenor I. M. The antibiotic compound and its deactivation in aqueous solution. Phil. J. Sci. 1990; 119: 23–26
- Devasagayam T. P. Lipid peroxidation in rat uterus. Biochim. Biophys. Acta. 1986; 876: 507–514
- Ermel R. W., Kenny T. P., Chen P. P., Robbins D. L. Molecular analysis of rheumatoid factors derived from rheumatoid synovium suggests an antigen-driven response in inflamed joints. Arthritis Rheum. 1993; 36: 380–388
- Ernest H. S., Choy M. D., Gabriel S. P. Cytokine pathway and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001; 344: 907–916
- Evans C. H., Robbins P. D., Ghivizzani S. C., Wasko M. C., Tomaino M. M., Kang R. Gene transfer to human joints: Progress toward a gene 485 therapy of arthritis. Proc. Natl. Acad. Sci. USA 2005; 102: 8698–8703
- Faizi S., Siddiqui B. S., Saleem R., Siddiqui S., Aftab K., Gilani A. H. Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry (Oxford) 1995; 38: 957–963
- Firestein G. S., Alvaro-Gracia J. M., Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J. Immunol. 1990; 144: 3347–3353
- Geetha T., Varlakshmi P., Mary R. L. Effect of triterpenes from Crataeva nurval a stem bark on lipid peroxidation in adjuvant induced arthritis in rats. Pharmacol Res. 1998; 37(3)191–195
- Gotia S., Popovici I., Hermeziu B. Antioxidant enzymes levels in children with juvenile rheumatoid arthritis. Rev. Med. Chai. Soc. Med. Nat. Les. 2001; 105: 499–503
- Guevara A. P., Vargas C., Sakurai H., Fujiwara Y., Hashimoto K., Maoka T., Kozuka M., Ito Y., Tokuda H., Nishino H. An antitumor promoter from Moringa oleifera Lam. Mutat. Res. 1999; 440: 181–188
- Gutteridge J. M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995; 41: 1819–1828
- Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence?. Lancet 1994; 344: 721–724
- Hogberg J., Larson R. E., Kristoferon A., Orrenius S. NADPH-dependent reductase solubilised from microsomes of peroxidation and its activity. Biochem. Biophys. Res. Commun. 1974; 56: 836–842
- Holt I., Cooper R. G., Denton J. Cytokine inter-relationships and their association with disease activity in arthritis. Br. J. Rheumatol. 1992; 31: 725–733
- Hopkins S. J. Cytokines and eicosanoids in rheumatic diseases. Ann. Rheum. Dis. 1990; 49: 207–210
- Issekutiz A. C., Meager A., Otterness I., Issekutiz T. B. The role of tumor necrosis factor-alpha and IL-1 in polymorphonuclear leukocyte and T-lymphocyte recruitment to joint inflammation in adjuvant arthritis. Clin. Exp. Immunol. 1994; 97: 26–32
- Kaneda H. A. Study on the lipid peroxide and its scavenging enzymes in rheumatoid arthritis. Nippon Seikeigeka Gakkai Zasshi 1982; 56: 387–397
- Kaneko M., Tomita T., Nakase T., Ohsawa Y., Seki H., Takeuchi E., Takano K., Shi K., Takahi K., Kominami E., Uchiyama Y., Yoshikawa H., Ochi T. Expression of proteinases and inflammatory cytokines in subchondrial bone regions in the destructive joint in rheumatoid arthritis. Rheumatology 2001; 40: 247–255
- Kennedy A. C., Allam B. F., Rooney P. J., Watson M. E., Fairney A., Buchanan K. D., Hillyard C. J. Hypocalcaemia in rheumatoid arthritis: Investigation of its causes and implication. Ann. Rheum. Dis. 1979; 38: 401–412
- Kim H. J., Krenn V., Steinhouser G., Berek C. Plasma cell development in synovial germinal centers patients with rheumatoid and reactive arthritis. J. Immunol. 1999; 162: 3053–3062
- Kirtikar K. R., Basu B. D. Indian Medicinal Plants, 2nd Edition, Vol. 1, D. Dun, B. Singh, M. P. Singh. New Cannaught Place—Dehradun, M/s Bishen Singh Mahendra Pal Singh 1975; 676–683
- Koch A. E., Kunkel S. L., Strieter R. M. Cytokines in rheumatoid arthritis. J. Invest. Med. 1995; 43: 28–38
- Laandrault N., Pouchert P., Ravel P., Gase F., Cros G., Teissedro P. L. Antioxidant activities phenolic level of French wines from different varieties and vintages. J. Agr. Food Chem. 2001; 49: 3341–3343
- Leisten J. C., Gaarde W. A., Scholz W. Interleukins-6 levels were correlate with footpad swelling in adjuvant-induced arthritic Lewis rats treated with cyclosporine A or indomethacin. Clin. Immunol. Immunopathol. 1990; 56: 108–115
- Lowry O. H., Rosenbrought N. J., Farr A. L., Randall R. J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 1951; 193: 265–275
- Madhok R., Crilly A., Watson J., Capell H. Serum interleukin-6 levels in rheumatoid arthritis: Correlations with clinical and laboratory indices of disease activity. Ann. Rheum. Dis. 1993; 52: 232–234
- Memon G. M., Khatri L. M. Isolation and spectroscopic studies of mono-palmitic, di-oleic triglyceride from seeds of Moringa oleifera Lam. Pak. J. Sci. Ind. Res. 1987; 30: 393–395
- Michael L., Horst K. Synthesis of active principles from the leaves of Moringa oleifera using S-pent-4-enyl thioglycosides. Carbohydr. Res. 1998; 312: 33–44
- Miossec P. Cytokine abnormalities in inflammatory arthritis. Bailliere's Clin. Rheumatol. 1992; 6: 373–391
- Misra H. P., Fridovich I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. Int. Biol. Chem. 1972; 247: 3170–3175
- McDonnell J., Hoerrner L. A., Lark M. W. Recombinant human interleukin-1 beta induced increase in levels of proteoglycans, stromelysin, and leukocytes in rabbit synovial fluid. Arthritis Rheum. 1999; 35: 799–805
- Moron M. S., Defierre J. W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979; 582: 67–78
- Mortensen R. A. The effect of diet on the glutathione content of erythrocytes. J. Biol. Chem. 1953; 204: 239–243
- Morton J. F. The Horseradish tree, Moringa pterosperma (Moringaceae)—a boon to arid lands?. Econ. Bot. 1991; 45: 318–333
- Indian Materia Medica, Vol. 1, K. M. Nadkarni, A. K. Nadkarni. Popular Prakashan, Mumbai 1976; 811–816
- Nijveldt R. J., Nood E. V., Hoorn D. E., Boelens P. G., Norren K. V., Paul A. M., Leeuwen V. Flavonoids: A review of probable mechanism of action and potential applications. Am. J. Clin. Nutr. 2001; 74: 418–425
- Njoku O. U., Adikwu M. U. Investigation on some physicochemical antioxidant and toxicological properties of Moringa oleifera seed oil. Acta. Pharma. Zagr. 1997; 47: 287–290
- Ostrakhovitch E. A., Afanas'ev I. B. Oxidative stress in rheumatoid arthritis leucocytes; Suppression by rutin and other antioxidant and chelators. Biochem Pharmacol. 2001; 62: 743–746
- Owen R. T. Adjuvant induced polyarthritis—an overview. Methods and findings. Exp. Clin. Pharmacol. 1980; 2: 199–204
- Modern Methods of Plant Analysis, Vol. IV, D. Paech, M. V. Tracey. Springer-Verlag, Berlin 1955; 373–374
- Pearson C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc. Soc. Exp. Biol. Med. 1956; 91: 95–101
- Pearson C. M., Wood F. Studies of arthritis and other lesions induced in rats by the injection of mycobacterium adjuvant. Am. J. Pathol. 1963; 42: 93–95
- Rajan B. K. Apiculture and farm forestry in semi-acid tracts of Karnataka. My Forest 1986; 22: 41–49
- Robak J. Adjuvant-induced and carrageenan-induced inflammation and lipid peroxidation in rat liver, spleen and lungs. Biochem. Pharmacol. 1978; 27: 531–533
- Roos D., Weening R. S. Defects in the oxidative killing of microorganisms by phagocytic leukocytes. Oxygen Free Radical and Tissue Damage Ciba Symp. Experta Medica, Amsterdam 1979; 225–262, 65, New Series
- Sanchez M. D. I., Lopez C. J., Rios V. High-performance liquid chromatography method to measure ∝ - and ϒ -tocopherol in leaves and fresh beans from Moringa oleifera. J. Chromatogr A. 2006; 1105: 111–114
- Sarban S., Kocyigit A., Yazar M., Isikan U. E. Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin. Biochem. 2005; 8: 981–986
- Schorlemmer H. U., Kurrle R., Schleyerbach R., Bartlett R. R. Disease modifying activity of malononinitrilamides, derivates of leflunomide's active metabolite, on models of rheumatoid arthritis. Inflamm. Res. 1999; 48: 113–114
- Sengupta A., Gupta M. P. Studies on seed fat composition of Moringacea family. Fette Seifen Anstrich. 1970; 72: 6–10
- Sinha A. K. Colorimetric assay of catalase. Anal. Biochem. 1972; 47: 389–394
- Stossel T. P. Phagocytosis. N. Engl. J. Med. 1974; 290: 717–723
- Szekanecz Z., Halloran M. M., Volin M. M., Woods J. M., Strieter R. M., Haines G. K., Kunkel S. J., Burdick M. D., Koch A. E. Temporal expression of inflammatory cytokines and chemokines in rat adjuvant induced arthritis. Arthritis Rheum. 2000; 43: 1266–1277
- Theisen-Popp P., Pape H., Muller P. R. Interleukin-6 (IL-6) in adjuvant arthritis of rats and its pharmacological modulation. J. Immunopharmacol. 1992; 14: 565–571
- Varier V. P. Indian Medicinal Plants Compendium of 500 Species, P. K. Warrier V. P. Nambiar, C. Ramankutty. Orient Longman Ltd., Madras 1997; Vol. 4: 58–62
- Villasenor I. M. Bioactive metabolites from Moringa oleifera Lam. Kimika 1994; 110: 47–52
- Villasenor I. M., Dayrit F., Lim-Sylianco C. Y. Studies on M. oleifera seeds, Part II. Thermal degradation of roasted seeds. Phil. J. Sci. 1990; 119: 33–39
- Villasenor I. M., Lim-Sylianco C. Y., Dayrit F. Mutagens from roasted seeds of Moringa oleifera. Mutat. Res. 1989; 224: 209–212
- Walz D. T., Dimartino J. J., Misner A. Adjuvant-induced arthritis in rats, drug effect on physiologic, biochemical and immunologic parameters. J. Pharm. Exp. Ther. 1971; 178: 223–231
- Winter C. A., Risley E. A., Nuss W. Carrageenan induced edema in hind paw of rats as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962; 111: 544–547