Abstract
The in situ reactions of metal ions/complexes are important in understanding the mechanisms by which environmental and occupational metal particles alter lung immune responses. A better understanding of these reactions in situ will also allow for the improved specificity and controlled toxicity of novel metallocompounds to be used as inhaled diagnostics or therapeutics. Our previous work showed that inhalation of metals (e.g., chromium, vanadium, nickel) caused altered lung immune cell function and host resistance. The data also suggested that the degree of immunomodulation induced depended not only on the amount of metal deposited, but also the compound used. If specificity governs pulmonary immunomodulatory potential, it follows that physicochemical properties inherent to the metal have a role in the elicited effects. We hypothe-size that major determinants of any metal compound's potential are its redox behavior, valency (generally referred to as oxidation state and considered speciation in chemical literature), and/or solubility. In accord with the extensive work carried out with vanadium (chemical symbol V) compounds showing the importance of form used, differences in potential for a range of V agents (pentavalent [VV] insoluble vanadium pentoxide and soluble sodium metavanadate, tetravalent [VIV] vanadyl dipicolinate, and trivalent [VIII] bis(dipicolinato)vanadium) were quantified based on induced changes in local bacterial resistance after host inhalation of each agent at 100 μ g V/m3 (5 hr/d for 5 d). Differences in effect between VV forms indicated that solubility was a critical property in in situ pulmonary immunotoxicity. Among the soluble forms, oxidizing vanadate had the greatest impact on resistance; reducing VIII altered resistance to a lesser extent. Both the VIV and insoluble VV had no effect. When data was analyzed in the context of pre-infection lung V burdens, soluble V agents with different oxidation states induced varying responses, supporting the hypothesis that differences in immunomodulatory potential might be attributed to redox behavior or valency. Our findings both provide a basis for understanding why some metals could be a greater health risk than others (when encountered in equal amounts) and will assist in the design of inhalable metallopharmaceuticals by allowing researchers to preempt selection of certain metal ions or complexes for use in such products.
Keywords :
INTRODUCTION
Select metals (as free ions and complexes) present in air may account for a large number of exposure-associated respiratory diseases (EPA, Citation2004). With each breath, local cell populations are exposed to various metals that can trigger a variety of biological effects involved in disease pathogeneses. For example, modifications in immune cell structural, functional, or biochemical properties could impair local immunocompetence (Cohen et al., Citation2004). Changes in secretory activity during initiation or propagation of immune responses, alone or in conjunction with effects on phagocytosis, intracellular killing, or reactive intermediate formation could ultimately lead to increases in infectious disease. Many in vivo and in vitro studies have addressed whether, the extent, and the means by which individual metals induce these effects (see Cohen, Citation2004, Citation2006). While these studies showed that dose (i.e., amount delivered to lung) was a determinant in the extent of immunomodulation, the potential for effects also depended on the agent itself. This suggested that inherent physicochemical properties of metal compounds or metals themselves, such as redox activity, solubility, and valency, were determinative in their in situ toxicity.
Solubility of a metal alone (or a compound) depends on molecule size, ligand type, charge, and nuclearity. A review of the literature shows that most inhalation studies with metals generally investigated only soluble forms; rarely have effects of soluble and insoluble forms of a given metal been compared directly. In some cases, it seems that solubility may be most critical for effects in the lungs as insoluble materials often persist after entrainment. For example, one of our recent studies reported that solubility had a dramatic effect on the in situ immunomodulating effects of inhaled chromium compounds (Cohen et al., Citation2006).
Valency and redox behavior also likely impact on immunotoxicity of metals that can exist in several oxidation states. While the fundamental difference between a +4 and +5 state is one electron, the difference is profound and affects how the metal complexes form and their properties (McCleverty and Meyer, Citation2004). Coordinated ligands can also affect the valence state that predominates. The inherent ability of a metal to change valence (i.e., its redox behavior) generally can occur in shuttling or unidirectional processes; the latter are abundant in biology as most metals have one preferred valence. Valency also determines types of coordinating ligands, complex stereochemistry, solubility, intra-/extracellular reactivity, and means of entry into cells.
In the studies here, immunotoxic effects were examined in vivo for each of four chemically distinct vanadium (symbol V) compounds. Vanadium was selected for analyses since, among metals routinely in urban atmospheres, it has become one of the better studied for immunotoxic effects and differential effects of various V agents have been reported with respect to biological outcomes (re: insulin-enhancing properties [Crans et al., Citation2004; Thompson et al., Citation2006]). These and other studies indicated that the mode of action of V varied with the agent employed. Both animal (Crans et al., Citation2004; Buglyo et al., Citation2005; Thompson et al., Citation2006) and in vitro studies (Crans et al., Citation1990, Citation1996; Crans and Simone, Citation1991) noted differential effects from V agents with varying forms, redox states, or coordinated ligands.
Chemically, the highest oxidation state of V (i.e., pentavalent VV) is generally considered to enter into a shuttling redox process. Here, once an electron is accepted, the now tetravalent VIV can regain an electron allowing pentavalent VV to re-form. While further reduction of VIV to trivalent VIII has been inferred (Rehder, Citation1992; Baran, Citation2000; Crans et al., Citation2004), this is more likely to occur in nonmammalian cells (e.g., tunicates, sea squirts, fan worms) (Sigel and Sigel, Citation1992). It remains controversial as to whether reduction of VIV to VIII is possible under physiological conditions (Kanamori et al., Citation1997; Slebodnik et al., Citation1997).
In mammalian systems, VV ions can enter cells via transport systems used by phosphate (PO43 −) anions. Insoluble VV forms require phagocytic or pinocytic uptake and then phagosomal solubilization to yield free ions. In a cell, VV interactions with reductants (e.g., NAD(P)H, glutathione, ascorbate, catechols; Baran, Citation2000; Crans et al., Citation2004) lead to conversion to VIV. In turn, VIV interactions with oxygen (O2) or reactive oxygen species (ROS; Stern et al., Citation1992) result in oxidation back to VV. Beyond these redox-related damages, the presence of VV and VIV in a cell also affect critical enzymes and signal transduction pathways (Stern et al., Citation1993; Crans et al., Citation2004; Prophete et al., Citation2006), and leads to apoptosis/cell death (Riley et al., Citation2003; Wang et al., Citation2003; Chien et al., Citation2006). In contrast to VV, VIII entry does not appear mediated by ion channels (Yang et al., Citation2004; Zhang et al., Citation2006), but rather by simple diffusion. Recent studies also noted that VIII binds to transferrin (Nagaoka et al., Citation2002); it is thus possible VIII enters cells via iron uptake pathways. Lastly, because VIII easily complexes sulfate (Meier et al., Citation1992), it is possible VIII enters cells in a manner akin to iron polysulfates. While the exact description of how VIII enters cells is still lacking, once inside, VIII could react with O2 to be oxidized to VIV (Stern et al., Citation1992); under very rare circumstances, VIII complexes (i.e., with superoxide [Stankiewicz et al., Citation1991]) acts as an oxidant, though the metal is not reduced to VII.
While the prevalence of cellular reducing agents suggests continuous shuttling between states once V enters a cell, the cycling can be broken: (1) VIV stabilization by cellular PO43 −-based ligands prevents reduction of O2/ROS (Nechay et al., Citation1986); and, (2) intact vanadate might exit the cell through as yet not positively defined mechanisms (i.e., it is likely PO43 − channels have a role [Barac-Nieto et al., Citation2002; Elmariah and Gunn, Citation2003]). These findings help explain why most intracellular V is found as VIV while VV predominates extracellularly.
Many metals, like V, have been shown to be immunotoxicants whose effects in the lungs seem to vary with the compound tested. Mechanisms have been postulated to explain how these metals act directly or indirectly to cause effects in immune cells (Cohen, Citation2004, Citation2006). Novel pathways and potential common mechanisms are also likely to exist. By examining if inherent physicochemical characteristics influence the toxicity of metal ions/complexes (here, different V compounds) on lung immune cells, the studies here document whether these properties play a role in mechanisms of respiratory disease pathogeneses. Our results should help provide clearer rationales for differing immunotoxicities of commonly-encountered ambient metals, yield information that can lead to a better appreciation for potential reactions of metals in living systems, and enable better design of new inhalable metallopharmaceuticals.
MATERIALS AND METHODS
Experimental Animals
Ten-week-old pathogen-free male F344 rats (≈ 225 g, Charles River, Wilmington, MA) were used in all exposures. On arrival, the rats were quarantined for 2 wk and then housed individually in plastic cages in temperature- (20°C) and humidity (50% RH)-controlled rooms, and provided Purina Rodent Chow and water ad libitum. Rats underwent routine clinical screening under veterinary supervision prior to initiation of exposures to each V agent (each at 100 μ g V/m3) for 5 hr/d for five consecutive days. All facilities and experimental procedures were approved by the NYU Medical Center Committee on Animal Care and Use.
Chemical Agents
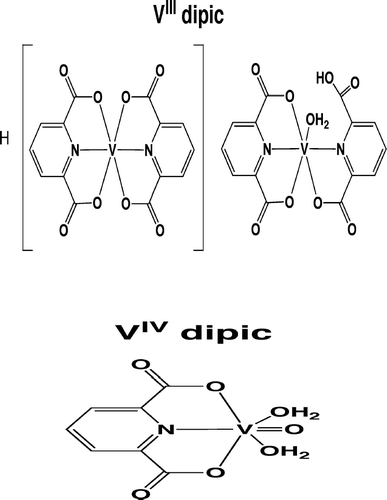
To analyze effects from soluble V agents with varying valences, sodium metavanadate (VV; NaVO3), diaqua(dipicolinato)oxovanadium(IV) ([VIVO(dipic)(OH2)2] or diaqua(2,6-pyridinedi-carboxylato)oxovanadium(IV); herein using term VIV dipic) and bis(dipicolinato)vanadium(VIII) (H[VIII(dipic)2- H2O]·3H2O or bis(2,6-pyridinedicarboxylato)vanadium(III); herein termed VIII dipic), were used for exposures. These dipic complexes of VIV and VIII were used since chloride salts of each parent ion could not be prepared at the neutral pHs needed for exposure (i.e., VCl3 precipitates at pH < 4.0). To compare effects due to solubility (here at a fixed valence state, i.e., VV), insoluble vanadium pentoxide (V2O5) was used as a counter to soluble NaVO3. Solubility values range from 0.7 g/L (in H2O at 25°C; NTP, Citation2002) for nonhydrated-V2O5 to 211 g/L for NaVO3 (for reference; given the chelation of the central metal by a ligand, solubility values for VIII dipic and VIV dipic fall between these values [i.e., ≈ 2 and 2–4 g/L, respectively]). Control rats received filtered air only. Both VV agents were purchased from Sigma (St. Louis, MO); VIII and VIV dipic complexes ( and ) were synthesized de novo. Bis(dipicolinato)-vanadium(III) was synthesized by the method of Chatterjee et al. (Citation1998) while diaqua(dipicolinato)-oxovanadium(IV) was synthesized by the method of Bersted et al. (Citation1968). Purity of each dipic agent used in exposures was 100%; methods of purity determination included UV/Vis, EPR, and IR spectroscopy, as well as elemental analyses.
Generation and Characterization of Exposure Atmospheres and Exposure System
Atmospheres of each soluble agent were generated by nebulizing a dilute solution (pH 7.2–7.4) via a Collison nebulizer (BGI, Waltham, MA) as described previously (Cohen et al., Citation1996a, Citation2006). Insoluble particle atmospheres were generated using a Wright dust feeder. As the V2O5 was not placed in water to facilitate nebulization, the overwhelming majority of the material reaching each rat's nose in that exposure group was truly V2O5 and not a complex mixture of V2O5 and variable amounts of associated vanadate (data not shown).
Each aerosol was mixed with filtered air and directly introduced into the radial, flow past design 50-port exposure system. Because of the system design, all particles reaching the nose of the rats only differ in their parent chemical composition and not in any overt way due to size, moisture content, or agglomeration. Target concentration was always 100 μ g V/m3; if a significant effect was noted, subsequent exposures used atmospheres ranging down to 0.001 μ g/m3. This range encompassed V levels used earlier and encountered in select occupational settings (mining, steel refining; OSHA PEL = 50 μ g V/m3), and was representative of V levels routinely measured in urban air (Prophete et al., Citation2006). Aerodynamic size distribution of each aerosol was confirmed via 8-stage multiple orifice impactors (MSP, St. Paul, MN) after exposure (due to flow requirement). Mass concentration was assessed during exposure by particle weight collected on 47 mm filters (Type FG, 0.2 μ m pore, Millipore, Bedford, MA). All exposures were nose-only and rats were housed in plastic restraint tubes during each exposure; earlier work has shown that rats do not undergo undue stress under these conditions. Initial air was pre-filtered and temperature-controlled; relative dilution air humidity was maintained at 50 ± 5%. Delivery of aerosol to each port was highly reproducible within and between exposure groups (a 2% CV).
Studies of Host Resistance/In Situ Bacterial (Listerial) Clearance After V Agent Exposure
To determine effects on in situ antibacterial responses, resistance to pneumonia-inducing Gram-positive Listeria monocytogenes (strain L242/73 Type 4b) was assessed. Listeria was grown 16 hr in trypticase soy (TS) broth at 37°C, its concentration spectrophotometrically determined at 540 nm, and an aliquot then diluted with saline to the needed concentration for intratracheal instillation (110 μ l/rat) under light halothane anesthesia. Extrapolation to predict Listeria concentration is known to be within 90% of predicted values (Cohen et al., Citation2002, Citation2006).
One day after their final exposure, 24 V rats and 15 air controls were infected with 4 × 106 bacteria/rat (< LD10 in this strain at this age). Six naive rats were also infected; three were analyzed immediately to establish baseline bacterial burdens and the rest 72 hr later to monitor virulence. A separate set of 10 V-exposed rats was not infected, but instead used for determining lung V burden at time of infection (i.e., Day 0 rats; n = 5) or for determining immune cell population profiles in the lungs prior to infection. The five rats in the latter set underwent standard lavaging procedures (Cohen et al., Citation2002) to obtain the cells used in post-cytospin differential analyses. After infection, all rats were monitored for any signs of morbidity. Within each infected set, cohorts of 6, 8, and 10 V-exposed rats (along with 5 controls) were weighed and then euthanized by Nembutol (100 mg/kg, IP) overdose at 24, 48, and 72 hr postinfection, respectively—a period encompassing the innate response to Listeria in the lung. Each lung was isolated en bloc; the trachea and extrapulmonary bronchi were removed and the tissue weighed and processed for estimation of listerial burden by homogenization and plating of serial dilutions (triplicate) on TS agar/0.6% yeast extract plates for 24 hr at 37°C. The remaining homogenate volume was then measured and the material placed at 4°C for later use in determining lung V burden at sacrifice.
Total Listeria and Listeria/g lung were calculated for each rat. The percentage change in total Listeria and in Listeria/g lung from those of each parameter seen in control rats at each timepoint were used as indices of any induced modulation of resistance. Because of potential differences in deposition/clearance of each agent, these percentages were further analyzed in the context of the total amount of V present in the lungs at sacrifice and/or pre-infection.
Assessment of Lung Metal Burden
NIEHS Center Analytical Core procedures used earlier (Cohen et al., Citation2006) were applied to determine the amount of V (for each agent) in lung samples isolated at Day 0 and each timepoint. Each final isolate was analyzed using inductively coupled plasma optical emission spectroscopy (ICPOES-Model Optima 4300-DV; Perkin Elmer, Norwalk, CT) operating at 290.880 nm. The minimal detectable V was 1 ppb; extraction efficiency, regardless of matrix, was > 99%. All materials were reagent grade and standards National Institute of Standards and Technology-traceable. All standards dilutions were made up in ultrapure H2O. Standard curves consisted of 6-point calibration with a standard blank to assure accurate baselines.
Data Analysis
Effects from each V agent on each test endpoint were analyzed by two-way ANOVA (analysis of variance); individual factors were exposure group (air or V agent) and assessment time. All data were tested to assure assumptions of normality and homogeneity of variance were met, and transformations applied as needed. Data were also screened for outliers using Dixon and Grubb's analyses (Taylor, Citation1990). Significant time or group effects, or effects associated with interaction between the two were sub-tested using t-tests corrected for multiple comparisons. Outcomes were considered significant at p < 0.05.
RESULTS
Lung V Burdens as Function of Test Agent
Rats in each V treatment group were expected to be exposed to ≈ 100 μ g V/m3 during each 5 hr regimen. Analyses of filter samples collected during exposures indicated that rats exposed to V2O5 received slightly (but, not significantly) more V over the 5 exposure days than any V-exposed counterpart (). Average particle sizes delivered to each rat were consistent.
TABLE 1 Exposure parameters from the exposure studies
Twenty-four hrs after the final exposure (i.e., Day 0), rats in each group were either infected or sacrificed for analyses of lung V burdens. Data shown in indicates that rats exposed to V2O5 had the highest total lung V content among all treatment groups on Day 0. Their V levels were 7.0-, 3.8-, and 3.6-fold greater than those in NaVO3-, VIV dipic-, and VIII dipic-exposed rats, respectively. Initial levels of V in VIII- and VIV-treated rats were comparable, and both were 95 and 84% greater, respectively, than that in rats exposed to soluble NaVO3. When the lungs of infected rats in each treatment group were analyzed after 3 d of infection, there were again significant differences in total V content. Levels of V in the lungs of rats in the insoluble V2O5 group were the greatest (e.g., 7.6-, 5.2-, and 5.2-fold greater than in lungs of NaVO3-, VIV dipic-, and VIII dipic-exposed rats, respectively). Levels of V in rats that received soluble NaVO3 were even significantly lower (≈ 32%) than that of rats that were exposed to either dipic complex. Analyses of Day 0 V burdens in the context of lung weight revealed the same trends and relative differences noted above among all the treatment groups (). Levels of V/g lung in rats that received V2O5 were 6.8-, 2.8-, and 3.6-fold greater than those in rats exposed to NaVO3, VIV dipic, and VIII dipic, respectively. When V levels at Day 3 of infection were similarly analyzed, relative differences among the groups were similar to those obtained using the absolute burdens.
FIG. 2 Lung V burdens at Day 0 (i.e., pre-infection) and Day 3 of infection with Listeria. Each bar represents the average burden ([A] ng V; [B] ng V/g lung) (± SE) in the lungs of n = 5 (Day 0; solid bar) or n = 8–10 (Day 3; hatched bar) rats/treatment with V2O5 (insoluble V[V]), NaVO3 (soluble V[V]), VIII dipic, or VIV dipic. In Day 0 sets; *value significantly (p < 0.05) different from that in rats in all other groups; †value significantly (p < 0.05) different from that in soluble VIII dipic and VIV dipic-treated rats. In Day 3 sets: *value significantly (p < 0.05) different from that in rats in all other groups; # value significantly (p < 0.05) different from that in soluble VIII dipic and VIV dipic-treated rats.
![FIG. 2 Lung V burdens at Day 0 (i.e., pre-infection) and Day 3 of infection with Listeria. Each bar represents the average burden ([A] ng V; [B] ng V/g lung) (± SE) in the lungs of n = 5 (Day 0; solid bar) or n = 8–10 (Day 3; hatched bar) rats/treatment with V2O5 (insoluble V[V]), NaVO3 (soluble V[V]), VIII dipic, or VIV dipic. In Day 0 sets; *value significantly (p < 0.05) different from that in rats in all other groups; †value significantly (p < 0.05) different from that in soluble VIII dipic and VIV dipic-treated rats. In Day 3 sets: *value significantly (p < 0.05) different from that in rats in all other groups; # value significantly (p < 0.05) different from that in soluble VIII dipic and VIV dipic-treated rats.](/cms/asset/b7b77bf8-c534-4f87-83fd-43e4d9afdf99/iimt_a_211869_uf0002_b.gif)
To determine if there were compound-related differences in retention of each V agent, their respective changes in lung V burdens over the three-day infection period were compared. Data evaluated in the context of absolute V content indicated that retention of V when delivered as soluble or insoluble VV was significantly greater (≈ 52–56% of Day 0 levels) than with either dipic agent (). Exposure to soluble VIII dipic yielded the least retention (≈ 39%) of the metal after the 3-day period, whereas this was ≈ 42% with the soluble VIII dipic. If analyses were performed in the context of V/g lung, the retention values for V from soluble or insoluble VV-exposed rats were all now 20-22%, while that from VIII- and VIV dipic-exposed hosts dropped to 15 and 12%, respectively. It remains uncertain to what extent the presence of Listeria impacted on clearance of each agent (as opposed to what would occur had uninfected rats been monitored on Day 3). Thus, as in our studies using various chromium (Cr) agents (Cohen et al., Citation2006) it appears that analyses of V burden in the context of lung mass for later use in relating to bacterial burdens is inappropriate during the infection-to-resolution process.
FIG. 3 Average retention of V in the lungs of Listeria-infected rats in each V treatment group. Each bar represents the average retention (%; ± SE) of Day 0 burden in the lungs of n = 8–10 Day 3 rats per treatment with V2O5 (insoluble V[V]), NaVO3 (soluble V[V]), VIII dipic, or VIV dipic. Data analyzed in terms of ng V (solid bar) or of ng V/g lung (hatched bar). †Value significantly (p < 0.05) different from that in rats in the soluble VIII and VIV groups.
![FIG. 3 Average retention of V in the lungs of Listeria-infected rats in each V treatment group. Each bar represents the average retention (%; ± SE) of Day 0 burden in the lungs of n = 8–10 Day 3 rats per treatment with V2O5 (insoluble V[V]), NaVO3 (soluble V[V]), VIII dipic, or VIV dipic. Data analyzed in terms of ng V (solid bar) or of ng V/g lung (hatched bar). †Value significantly (p < 0.05) different from that in rats in the soluble VIII and VIV groups.](/cms/asset/0f1b5ca4-90de-43bb-892d-b5a5fc4f958f/iimt_a_211869_uf0003_b.gif)
Lung Listeria Burdens as Function of Test Agent
Following the 5 d exposures and infection with Listeria, rats in each group were assessed for bacterial burdens at 24, 48, and 72 hr post-infection. All data were then compared to burdens in the lungs of air-exposed infected rats to determine whether a particular V compound induced significant immunomodulation at any of the timepoints. While exposures to V2O5, NaVO3, or VIV dipic led to no significant effects on Listeria burdens during the first 48 hr post-infection (, , ), VIII dipic rats had significant increases in pathogen burden at 48 hr (). Only on Day 3 did it become apparent that of the four agents analyzed, soluble NaVO3 and VIII dipic caused significant reductions in pathogen clearance with NaVO3 having the most impact. Corresponding patterns were apparent when data were analyzed in the context of lung weight at time of sacrifice. In no cases were significant differences in morbidity over the 72 hr period nor in body weight due to pre-infection regimen or as related to the differences in lung Listeria burdens detected between the air- or V-treated rats. When evaluated at an exposure dose of 10 μ g V/m3, neither NaVO3 nor VIII dipic retained their effect. Analyses of effects produced by exposure to dipic alone (as sodium dipic) indicated no effect on host resistance.
FIG. 4 Listeria (bacterial) burdens in the lung of rats at each day postinfection. (A) V2O5; (B) NaVO3; (C) VIV dipic; (D) VIII dipic regimens. Values shown with ◊ represent the mean (± SE) of n = 6, 8, and 10 rats at 24, 48, and 72 hr post-infection/V regimen, respectively; values indicated with λ represent mean (± SE) of n = 5 air control rats at same timepoints. Statistical significance (p value) of result (V vs. air)—when present—is indicated.
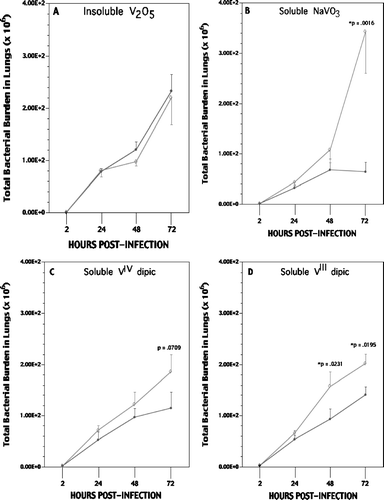
Rats that received soluble NaVO3 displayed a 435% greater total lung burden of Listeria compared to time-matched control counterparts (); these levels were significantly greater than those in all other V treatment groups as well. Inhaled soluble VIII dipic led to a significant ≈ 43% increase in Listeria burden compared to the air controls; rats exposed to soluble reducing VIV dipic or insoluble V2O5 had burdens similar to (or even less than) those in the controls. When data were re-analyzed to take into account changes in lung size that reflect increased mass due to bacteria, edema, immune cell migration, etc., the same pattern of results were obtained.
TABLE 2 Relative difference in Listeria burden in lungs of rats at day 3 post-infection
Estimation of Relative Immunomodulatory Potential of Each Test Agent
Analyses of the percentage change in Listeria burden in the context of total V present in the lungs yielded a weak negative correlation between Day 3 lung V burden and relative change in Listeria burden. That is, rats with the highest V burdens seemed to have the lowest percentage changes in Listeria levels compared to controls. For this and other reasons outlined in our earlier Cr paper (Cohen et al., Citation2006), we determined that use of Day 3 V burden as a factor in assessing each agent's relative immunomodulatory potential was unreliable.
Using Day 0 V burdens as a predictor of ultimate changes in host resistance, clear potential immunomodulation patterns were apparent (). When the percentage change in Listeria burdens from control levels (as absolute number or as per gram lung tissue) were estimated, the data demonstrated that soluble vanadate had the greatest effect on resistance as measured at a per ng V burden pre-infection. For both estimate types, relative changes in Listeria burdens were significantly greater than that from exposures to any other V agent. The results also indicated that immunomodulatory potentials for the dipic compounds did not differ though their valences did. Thus, while these data show that—among the soluble forms—complexes in different oxidation states induce varying responses in situ, indications are that differences in their potential to cause immunomodulation may be more dependent on redox behavior than anticipated.
FIG. 5 Relative difference in Listeria burden in the lungs of rats at Day 3 postinfection as a function of Day 0 lung V burdens. Each bar represents the mean from n = 10 Day 3 rats/indicated treatment with 100 μ g V/m3 as V2O5 (insoluble V[V]), NaVO3 (soluble V[V]), VIII dipic, or VIV dipic; ± SE) average percentage differences in Listeria levels (LM; solid bar) or of total Listeria/g lung (LM/g; hatched bar) compared to respective values in air controls, in the context of ng V in lungs at Day 0. †Value significantly (p < 0.05) different from that in rats in soluble NaVO3 group.
![FIG. 5 Relative difference in Listeria burden in the lungs of rats at Day 3 postinfection as a function of Day 0 lung V burdens. Each bar represents the mean from n = 10 Day 3 rats/indicated treatment with 100 μ g V/m3 as V2O5 (insoluble V[V]), NaVO3 (soluble V[V]), VIII dipic, or VIV dipic; ± SE) average percentage differences in Listeria levels (LM; solid bar) or of total Listeria/g lung (LM/g; hatched bar) compared to respective values in air controls, in the context of ng V in lungs at Day 0. †Value significantly (p < 0.05) different from that in rats in soluble NaVO3 group.](/cms/asset/030959bf-6f99-4a51-b5c8-9cc55c9d4fde/iimt_a_211869_uf0005_b.gif)
Lung immune cell population profiles on Day 0 were also examined to determine whether there was any pre-infection predisposition for an alteration in resistance to Listeria among the rats in each regimen (). The results indicated that only inhalation of the soluble VIII dipic significantly reduced (i.e., 5.9–8.4%) the percentage of AM while concurrently increasing (i.e., 87–200%) that of lymphocytes as compared to rats in the other exposure groups. In all treatment groups, the percentages of neutrophils never exceeded 1%.
TABLE 3 Percentages of immune cell types in BAL fluid of day 0 rats
DISCUSSION
We hypothesized that physicochemical properties are major determinants of pulmonary immunomodulating potentials of any metal, including vanadium (V). The results of this study analyzing several V compounds showed that the properties of solubility, redox behavior, and valency likely govern these potentials in situ. This conclusion is based on studies that employed Listeria monocytogenes in a resistance model suited for evaluation of lung cell-mediated components and their dysregulation by inhaled chemicals (reviewed in Cohen et al., Citation2006).
Differences in how V particles are handled in the lung are best reflected by clearance or retention patterns (see Rhoads and Sanders, Citation1985; Dill et al., Citation2004). Insoluble V2O5 particles localize in AM phagosomes after ingestion and undergo slow dissolution to vanadate ions. As such, rapid diffusion through the lung epithelia is limited and clearance would rely on mucociliary transport of uningested particles and V2O5-bearing AM. In comparison, soluble VV and VIV agents—if not complexed with nascent lining fluid constituents—are more readily deposited in epithelia and AM, with the limiting step being entry via membrane PO43 − transport systems. The results here clearly reflect differences in postentrainment processing; as rats that received V2O5 had the greatest lung V burdens and those exposed to NaVO3 the least. Soluble VIII dipic and VIV dipic exposures led to comparable retentions and both were closer to that of NaVO3 than V2O5.
Solubility as a factor in immunotoxicity is also reflected in the differing effects of VV agents on AM release of cytokines critical to resistance to Listeria. Our past studies (Cohen et al., Citation1993, Citation1996b) showed that while IL-1 production by rodent macrophages was reduced below control levels by vanadate or V2O5, significant effects were noted with vanadate at nM levels but nearly 100 μ M V2O5 was needed. Similarly, V2O5 effects on TNFα production started at a μ M level, but vanadate impacted in the fM range. Though such reductions could not be quantified in situ after vanadate was inhaled (Cohen et al., Citation1997), the effect could still be implied as levels of both cytokines fell below basal values. Pierce et al. (Citation1996) noted that ≈ 100% more V2O5 than NaVO3 was required to induce MIP-2 and KC chemotactin production in the lungs of rats. These differences ultimately were mirrored in the time periods to onset of lung inflammation (i.e., increased neutrophil levels) in the rats. Though no major effects from the V agents on immune cell profiles were noted in the current study, the short exposure combined with relatively low V level (compared to that in Cohen et al. [Citation1996b] or Pierce et al. [Citation1996]) might be a major factor why this predisposing factor for altered resistance was not observed.
Solubility was also important across several in vitro studies of effects of VV agents on phagocytic function. Consistently, higher levels of V2O5 (compared to vanadate) were needed to induce significantly reduced activity (Graham et al., Citation1975; Labedzka et al., Citation1989). A comparison of results of two in vivo studies also showed that exposure of rats to more V2O5 over a longer timeframe was needed to attain reductions in AM phagocytic activity on par with that from vanadate (Cohen et al., Citation1996a vs. NTP, Citation2002). These solubility-related effects were likely critical to how rats here responded to Listeria. If the AM only displayed a reduced phagocytic function, increased amounts of the instilled Listeria would stay extracellular and need to be ingested and killed by any still-functional AM. If the AM had normal phagocytic activity but compromised killing function, they would be less able to resist any ingested bacteria and would quickly attain high burdens; on cell death, progeny bacteria would be released to infect neighboring cells. Here, V2O5-exposed rat AM are likely to contain V and so their phagocytic and killing activities would be depressed less than AM in NaVO3-exposed rats. Thus, while the situation in V2O5-exposed rats would be more in line with the first scenario above, that in the NaVO3-exposed rats would be worse than even the second scenario, i.e., there would be a delayed, but substantive, release of Listeria into a lung that had few uncompromised AM remaining to process the progeny.
To examine whether differing solubility-related effects here among the V compounds were not simply due to differences in how they were generated and administered, comparative analyses of data obtained here with that from studies with other metal agents (i.e., chromium, lead, and zinc; Cohen et al., Citation2006 and unpublished data) of varied solubility was performed. The analyses showed that not all soluble nor insoluble agents induced equivalent immunomodulating effects. We thus conclude that the means of particle generation was not a factor dictating the observed differences in effects from each V compound. This finding is in agreement with that of Rau (Citation2005), whose review of common aerosol delivery device (including dry powder deliverers and nebulizers) drug-delivery efficiencies showed that each provided equivalent lung deposition and that treatment by these aerosol devices can be considered “clinically equivalent.”
Observations here about the extent and direction of any immunomodulation induced by the three soluble V agents confirmed expectations that redox behavior also governed potency. Among the valence forms examined, VV is the strongest oxidant under physiological conditions (depending on parent ion, E°(VV/VIV) = +1.02 to +1.31 V; Rehder, Citation1992). Extrapolating what we have learned about the possible chemistry of V from isolated systems, we can infer that on entry into cells of a live organism, VV will oxidize/complex with glutathione [GSH] and NAD(P)H (Nechay et al., Citation1986; Baran, Citation2000; Crans et al., Citation2004). NAD(P)H levels may also be affected by activation of vanadate-dependent membrane-associated NAD(P)H oxidation (Liochev and Fridovich, Citation1990; Minasi and Willsky, Citation1991). This ongoing loss of nicotinamide equivalents in an AM would be expected to impair its overall capacity to form adequate amounts of ROS for use in intraphagosomal killing, due to potential effects on NADPH oxidase activity (Cohen et al., Citation1989). Similarly, the loss of GSH would affect GSH redox cycle use for protection against peroxidative damage arising during intracellular killing or any increased presence of reactive species derived from VV/VIV reactions with cellular O2/ROS (Cohen et al., Citation1988). Because of the critical need to repair peroxidative damage (to maintain viability), VV-induced increases in GSH oxidation would force the AM to consume most remaining available NAD(P)H equivalents for GSSG reduction rather than for ROS formation. Based on these scenarios, it could be assumed that the presence of oxidizing VV in AM of NaVO3-exposed rats may have decreased killing of ingested Listeria, in part, via both direct (self-perpetuating ROS← →VV/VIV reactions) and indirect diminution of levels of ROS needed for killing.
Unlike VV, VIII is a poor oxidant (E°(VIII/VII) = −0.24 to −0.27 V) and will act as a reductant with cellular O2 or ROS. It would then be expected that airway interactions with O2 should result in VIII conversion to VIV and ultimately VV. With levels of V delivered in the VIII dipic and NaVO3 atmospheres not differing significantly, since VIII exposure caused a one-fold higher pre-infection V burden but a far smaller impact on resistance than VV (and only marginally greater than VIV), we infer that most of the inhaled VIII was not converted to VV in the airways.
While this conversion was expected based on its chemical properties, a recent study by Buglyo et al. (Citation2005) showed that in animals orally administered VIII dipic, the compound was more stable than expected. In the absence of significant conversion to the more potent VV form, it would seem possible one way VIII dipic caused a significant decline in resistance to Listeria was via direct reductive effects on ROS. It is also possible VIII indirectly perturbed AM function. With the highest affinity for transferrin (Tf) among the three valences tested, any in situ VIII complexation with Tf (Nagaoka et al., Citation2002) could have altered delivery of iron (Fe) to AM and thereby lower its capacity for ROS generation (Mateos et al., Citation1998; Ward et al., Citation2002). Whether effects here with VIII dipic (and proposed mechanisms of effect) are representative of all soluble VIII agents will require comparison of immunotoxic potentials of the dipic form vs. other VIII complexes.
That VIV dipic appeared to be retained on Day 0 more than its soluble VV counterpart, yet had much less immunomodulating effect in situ, was an interesting finding related to differences in valency and redox potential. The ability of VIV (as vanadyl unit chelated to dipic ligand) to complex with PO43 − ions is many-fold that of VV (Nechay et al., Citation1986) and reflects a difference in each compound's valency. Rat lung lining fluid contains ≈ 1 mM PO43 − (Cowley et al., Citation1997), and the total V in the 90 μ l lining fluid (Hatch, Citation1992) of the VIV dipic-exposed rats is only ≈ 50 μ M on Day 0. Thus, unlike for VV, it is likely that most VIV entrained here may not have been able to enter AM or epithelia (and induce toxicity) but instead remained extracellular. Analyses of Day 0 rat lavage V levels are underway to confirm if this was the case.
An additional important factor to consider is that vanadate was not chelated. Previous studies have suggested that V salts were more toxic than their chelated counterparts (Willsky et al., Citation2001; Crans et al., Citation2004; Thompson et al., Citation2006). Since the [VO2dipic]− system is unstable in a pH neutral reducing environment (Crans et al., Citation2000) like the lung and likely to dissociate (Jakusch et al., Citation2003), and VV ions are converted intracellularly to VIV, similar effects were expected to arise from the vanadate and VIV dipic. Instead, the lack of any substantive effect on host resistance suggests that VIV (in dipic form) is really not a potent direct toxicant. That VIV had any effect at all in the rats may be attributed to its ability to bind Tf and possibly affect AM Fe homeostasis. Whether the weak effects with VIV dipic were representative of all soluble VIV agents will require comparison of immunotoxic potentials of the chelated dipic form vs. that of a free salt (i.e., vanadyl sulfate) or other VIV complexes.
In summary, these studies in rats exposed to V agents differing in solubilility (at a fixed valence) or valency and redox behavior (soluble forms only) show that each physicochemical property can govern immunomodulatory potentials in situ. It was clear that at approximately equivalent levels of exposure, inhalation of soluble pentavalent V (i.e., VV as NaVO3) was more detrimental than its insoluble V2O5 counterpart. Overall, soluble NaVO3 was the most potent modulant of the four agents. As the modulatory potentials of the lower oxidation state (VIII and VIV) complexes did not significantly differ from one another, nor did either agent likely directly initiate oxidation processes, these studies suggest that the redox behaviors of low valency compounds are not likely to be very important in determining immunotoxicities of individual V agents in the lung.
In contrast, solubility is a critical factor in the pulmonary immunotoxicity of inhaled VV compounds. Ongoing studies of other metal agents of varied solubility, valency, and redox behavior will yield further information about the role of physicochemical properties in observed toxic outcomes after inhalation. Once completed, these lines of investigation should provide a better basis for understanding why certain metals could be a greater health risk than others even if encountered in equal amounts. This improved understanding, in turn, will help researchers in the design of inhalable diagnostic and therapeutic metallopharmaceuticals by preempting the selection of certain metal ions/complexes for potential use in these products.
ACKNOWLEDGMENT
This study was supported primarily by funds from NIGMS/NIH Grants GM065458 and GM40525 as well as NCI/NIH Grant 1U19CA105010. The Authors are grateful to the services and assistance provided, in part, by Center Programs in the NYU Department of Environmental Medicine supported by NIEHS (Grant ES00260) and the USEPA/PM Center Grant R82735101.
REFERENCES
- Barac-Nieto M., Alfred M., Spitzer A. Basolateral phosphate transport in renal proximal tubule-like OK cells. Exp. Biol. Med. 2002; 227: 626–631
- Baran E. J. Oxovanadium(IV) and oxovanadium(V) complexes relevant to biological systems. J. Inorg. Biochem. 2000; 80: 1–10
- Bersted B. H., Belford R. L., Paul I. C. Crystal and molecular structure of orthorhombic vanadyl(IV)pyridine-2,6-dicarboxylate tetrahydrate. Inorg. Chem. 1968; 7: 1557–1562
- Buglyo P., Crans D. C., Nagy E. M., Lindo R. L., Yang L., Smee J. J., Jin W., Chi L. H., Godzala M. E., Willsky G. R. Aqueous chemistry of the vanadium(III) (V(III)) and the V(III)-dipicolinate systems and a comparison of the effect of three oxidation states of vanadium compounds on diabetic hyperglycemia in rats. Inorg. Chem. 2005; 44: 5416–5427
- Chatterjee M., Maji M., Ghosh S., Mak T. C. Studies of V(III) complexes with selected alpha-N-heterocyclic carboxylato NO donor ligands: Structure of a new seven-coordinated pentagonal bipyramidal complex containing picolinato ligands. J. Chem. Soc.-Dalton Trans. 1998; 21: 3641–3645
- Chien P. S., Mak O. T., Huang H. J. Induction of COX-2 protein expression by vanadate in A549 human lung carcinoma cell line through EGF receptor and p38 MAPK-mediated pathway. Biochem. Biophys. Res. Commun. 2006; 339: 562–568
- Cohen M. D. Pulmonary immunotoxicology of select metals: Aluminum, arsenic, cadmium, chromium, manganese, nickel, vanadium, and zinc. J. Immunotoxicol. 2004; 1: 39–70
- Cohen M. D. Pulmonary Immunotoxicology. Chapter 9. Toxicology of the Lung, 4th Edition, D. Gardner. Taylor and Francis/CRC Press, Boca Raton, FL 2006; 351–420
- Cohen M. D., Becker S., Devlin R., Schlesinger R. B., Zelikoff J. T. Effects of vanadium upon polyI:C-induced responses in rat lung and alveolar macrophages. J. Toxicol. Environ. Health 1997; 51: 591–608
- Cohen M. D., Chen C. M., Wei C. I. Decreased resistance to Listeria monocytogenes in mice following vanadate exposure: Effects upon the function of macrophages. Int. J. Immunopharmacol. 1989; 11: 285–292
- Cohen M. D., Parsons E., Schlesinger R. B., Zelikoff J. T. Immunotoxicity of in vitro vanadium exposures: Effects on IL-1, TNFα, and prostaglandin E2 production by WEHI-3 macrophages. Int. J. Immunopharmacol. 1993; 15: 437–446
- Cohen M. D., Prophete C., Maureen Sisco M., Lung-Chi Chen L. C., Zelikoff J. T., Smee J. J., Holder A. A., Crans D. C. Pulmonary immunotoxic potentials of metals are governed by select physicochemical properties: Chromium agents. J. Immunotoxicol. 2006; 3: 69–81
- Cohen M. D., Sen A. C., Wei C. I. Effects of ammonium metavanadate treatment upon macrophage glutathione redox cycle activity, superoxide production, and intracellular Glutathione status. J. Leukocyte Biol. 1988; 44: 122–129
- Cohen M. D., Sisco M., Baker K., Li Y., Lawrence D., van Loveren H., Zelikoff J. T., Schlesinger R. B. Effect of inhaled ozone on pulmonary immune cells critical to antibacterial responses in situ. Inhal. Toxicol. 2002; 14: 599–619
- Cohen M. D., Yang Z., Qu Q., Schlesinger R. B., Zelikoff J. T. Vanadium affects macrophage IFN-γ binding and -inducible responses. Toxicol. Appl. Pharmacol. 1996b; 138: 110–120
- Cohen M. D., Yang Z., Zelikoff J., Schlesinger R. B. Pulmonary immunotoxicity of inhaled ammonium metavanadate in Fisher 344 rats. Fundam. Appl. Toxicol. 1996a; 33: 254–263
- Cowley E. A., Govindaraju K., Lloyd D. K., Eidelman D. H. Airway surface fluid composition in the rat determined by capillary electrophoresis. Am. J. Physiol. 1997; 273: L895–899
- Crans D. C., Simone C. M. Nonreductive interaction of vanadate with an enzyme containing a thiol group in the active site: Glycerol-3-phosphate dehydrogenase. Biochemistry 1991; 30: 6734–6741
- Crans D. C., Keramidas A. D., Drouza C. Organic vanadium compounds—Transition state analogy with organic phosphorus compounds. Phosphorous Sulfur, Silicon, Related Elem. 1996; 110: 245–248
- Crans D. C., Smee J. J., Gaidamauskas E., Yang L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem. Rev. 2004; 104: 849–902
- Crans D. C., Willging E. M., Butler S. R. Vanadate tetramer as the inhibiting species in enzyme reactions in vitro. J. Amer. Chem. Soc. 1990; 112: 427–432
- Crans D. C., Yang L., Jakusch T., Kiss T. Aqueous chemistry of ammonium dipicolinatooxovanadium(V): The first organic vanadium(V) insulin-mimetic compound. Inorg. Chem. 2000; 39: 4409–4416
- Dill J. A., Lee K. M., Mellinger K. H., Bates D. J., Burka L. T., Roycroft J. H. Lung deposition and clearance of inhaled vanadium pentoxide in chronically exposed F344 rats and B6C3F1 mice. Toxicol. Sci. 2004; 77: 6–18
- Elmariah S., Gunn R. B. Kinetic evidence that the Na-PO4 co-transporter is the molecular mechanism for Na/Li exchange in human red blood cells. Am. J. Physiol. Cell. Physiol. 2003; 285: C446–C456
- EPA. United States Environmental Protection Agency. Air Quality Criteria for PM, 2004. United States Environmental Protection Agency, Washington, DC 2004, EPA/600/P-99/002aF, bF
- Graham J. A., Gardner D. E., Waters M. D., Coffin D. L. Effect of trace metals on phagocytosis by alveolar macrophages. Infect. Immun. 1975; 11: 1278–1283
- Hatch G. Comparative biochemistry of airway lining fluid. Comparative Biology of the Normal Lung, Vol. 1, R. A. Parent. CRC Press, Boca Raton, FL 1992; 617–632
- Jakusch T., Jin W., Yang L., Kiss T., Crans D. C. Vanadium(IV/V) speciation of pyridine-2,6-dicarboxylic acid and 4-hydroxy-pyridine-2,6-dicarboxylic acid complexes: Potentiometry, EPR spectroscopy and comparison across oxidation states. J. Inorg. Biochem. 2003; 95: 1–13
- Kanamori K., Kinebuchi Y., Michibata H. Reduction of vanadium(IV) to vanadium(III) by cysteine methyl ester in water in the presence of amino polycarboxylates. Chem. Lett. 1997; 5: 423–424
- Labedzka M., Gulyas H., Schmidt N., Gercken G. Toxicity of metallic ions and oxides to rabbit alveolar macrophages. Environ. Res. 1989; 48: 255–274
- Liochev S. I., Fridovich I. Vanadate-stimulated oxidation of NAD(P)H in the presence of biological membranes and other sources of O2−. Arch. Biochem. Biophys. 1990; 279: 1–7
- Mateos F., Brock J. H., Perez-Arellano J. L. Iron metabolism in the lower respiratory tract. Thorax 1998; 53: 594–600
- Comprehensive Coordination Chemistry II—From Biology and Nanotechnology, Volume 4 Transition Metal Groups 3–6, J. A. McCleverty, T. J. Meyer. Elsevier Pergamon, San Diego, CA 2004
- Meier R., Boddin M., Mitzenheim S., Kanamori K. Solution properties of vanadium(III) with regard to biological systems. Metal Ions in Biological Systems, Vol. 31. Vanadium and Its Role in Life, H. Sigel, A. Sigel. Marcel Dekker, New York 1992; 45–88
- Minasi L. A., Willsky G. R. Characterization of vanadate-dependent NADH oxidation stimulated by Saccharomyces cerevisiae plasma membranes. J. Bacteriol. 1991; 173: 834–841
- Nagaoka M. H., Yamazaki T., Maitani T. Binding patterns of vanadium ions with different valence states to human serum transferrin studied by HPLC/high-resolution ICP-MS. Biochem. Biophys. Res. Commun. 2002; 296: 1207–1214
- Nechay B. R., Nanninga L. B., Nechay P. S. Vanadyl (IV) and vanadate (V) binding to selected endogenous phosphate, carboxyl, and amino ligands; Calculations of cellular vanadium species distribution. Arch. Biochem. Biophys. 1986; 251: 128–138
- NTP. National Toxicology Program. NTP toxicology and carcinogenesis studies of vanadium pentoxide (CAS No. 1314-62-1) in F344/N rats and B6C3F1 mice (inhalation). National Toxicology Program Technical Report Series 2002; 507: 1–343
- Pierce L. M., Alessandrini F., Godleski J. J., Paulauskis J. D. Vanadium-induced chemokine mRNA expression and pulmonary inflammation. Toxicol. Appl. Pharmacol. 1996; 138: 1–11
- Prophete C., Maciejczyk P., Salnikow K., Gould T., Larson T., Koenig J., Jaques P., Sioutas C., Lippmann M., Cohen M. Effects of PM-associated metals on alveolar macrophage phosphorylated ERK-1/-2 and iNOS expression during ongoing alteration in iron homeostasis. J. Toxicol. Environ. Health 2006; 69: 935–951
- Rau J. L. The inhalation of drugs: Advantages and problems. Resp. Care 2005; 50: 367–382
- Rehder D. Inorganic considerations on the function of vanadium in biological systems. Metal Ions in Biological Systems, Vol. 31. Vanadium and Its Role in Life, H. Sigel, A. Sigel. Marcel Dekker, New York 1992; 1–44
- Rhoads K., Sanders C. L. Lung clearance, translocation, and acute toxicity of arsenic, beryllium, cadmium, cobalt, lead, selenium, vanadium, and ytterbium oxides following deposition in rat lung. Environ. Res. 1985; 36: 359–378
- Riley M. R., Boesewetter D. E., Kim A. M., Sirvent F. P. Effects of metals Cu, Fe, Ni, V, and Zn on rat lung epithelial cells. Toxicology 2003; 190: 171–184
- Metal Ions in Biological Systems, Vol. 31. Vanadium and Its Role in Life, H. Sigel, A. Sigel. Marcel Dekker, New York 1992
- Slebodnick C., Hamstra B. J., Pecoraro V. L. Modeling the biological chemistry of vanadium: Structural and reactivity studies elucidating biological function. Struct. Bond. 1997; 89: 51–108
- Stankiewicz P. J., Stern A., Davison A. J. Oxidation of NADH by vanadium: Kinetics, effects of ligands and role of H2O2 or .O2−. Arch. Biochem. Biophys. 1991; 287: 8–17
- Stern A., Davison A. J., Wu Q., Moon J. Effects of ligands on reduction of oxygen by vanadium(IV) and vanadium(III). Arch. Biochem. Biophys. 1992; 299: 125–128
- Stern A., Yin X., Tsang S. S., Davison A., Moon J. Vanadium as a modulator of cellular regulatory cascades and oncogene expression. Biochem. Cell Biol. 1993; 71: 103–112
- Statistical Techniques for Data Analysis, J. K. Taylor. Lewis Publishers, Chelsea, MI 1990
- Thompson K. H., Barta C. A., Orvig C. Metal complexes of maltol and close analogues in medicinal inorganic chemistry. Chem. Soc. Rev. 2006; 35: 545–556
- Wang L., Medan D., Mercer R., Overmiller D., Leornard S., Castranova V., Shi X., Ding M., Huang C., Rojanasakul Y. Vanadium-induced apoptosis and pulmonary inflammation in mice: Role of reactive oxygen species. J. Cell. Physiol. 2003; 195: 99–107
- Ward R. J., Wilmet S., Legssyer R., Crichton R. R. The influence of iron homoeostasis on macrophage function. Biochem. Soc. Trans. 2002; 30: 762–765
- Willsky G. R., Goldfine A. B., Kostyniak P. J., McNeill J. H., Yang L., Khan A. R., Crans D. C. Effect of vanadium (IV) compounds in the treatment of diabetes: In vivo in vitro studies with vanadyl sulfate and bis(maltolato)oxovandium(IV). J. Inorg. Biochem. 2001; 85: 33–42
- Yang X., Yand X., Yuan L., Wang K., Crans D. The permeability and cytotoxicity of insulin-mimetic vanadium compounds. Pharm. Res. 2004; 21: 1026–1033
- Zhang Y., Yang X., Wang K., Crans D. The permeability and cytotoxicity of insulin-mimetic vanadium (III, IV, V)-dipicolinate complexes. J. Inorg. Biochem. 2006; 100: 80–87