Abstract
An enzyme-linked immunosorbent assay (ELISA) using whole sheep red blood cells (SRBC) has been reported as one of the methods for detecting a T-lymphocyte-dependent antibody response. However, it has not been widely used because of SRBC problems such as the weak attachment to ELISA plates, specificity and short-term stability. The objectives of this study were to address these issues and to validate the SRBC-specific antibody response assay. Male Sprague–Dawley rats were bled after 6 days of SRBC immunization. In our new procedure, glutaraldehyde was added before discarding the supernatant of inoculated SRBC suspension to attach SRBC firmly to the plate, while in the original method it was added after discarding. As a result, the attached SRBC was maintained throughout the ELISA procedures. No interference was observed in the titration curve of IgM and IgG antibodies in rats and IgM-antibody in mice when control sera were analyzed to evaluate specificity of this method. The short-term stability of SRBC was overcome by using the different lots of SRBC. They provided antibody titers, which were consistent with those measured using the same lot for immunization. In addition, cyclophosphamide, cyclosporine, prednisolone and methotrexate, well-known immunosuppressive agents, were tested to confirm the applicability of the improved ELISA method to detect the T-lymphocyte-dependent antibody response. All four compounds inhibited the IgM antibody responses dose-dependently. These results demonstrate that the improved whole SRBC-ELISA method provides reproducible and reliable results in the T-lymphocyte-dependent antibody response assay.
INTRODUCTION
The assessment of immunotoxicity on new pharmaceuticals is one of the important components of preclinical safety assessment. Since 2002, the International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use put extensive effort into the development of a harmonized approach for the testing of immunosuppression and immunoenhancement. A consensus on the ICH S8 Guideline on Immunotoxicity Testing for Pharmaceuticals was eventually achieved and has been adapted by EU in 2005, and by the FDA and MHLW in 2006. Based on this guideline document, the evaluation of a primary antibody response to a T-lymphocyte-dependent antigen (e.g., sheep red blood cells [SRBC] or keyhole limpet hemocyanin [KLH]) is recommended as one of the most sensitive immune function tests following chemical exposures (Ladics et al., Citation1995, Citation1998; Putman et al., Citation2002). The T-lymphocyte-dependent antibody response assay is a sensitive indicator of immunological integrity. When SRBC is used as the particular T-lymphocyte-dependent antigen, this response requires the coordinated interaction of various immune system cells (i.e., antigen-presenting cells, T-lymphocytes, and B-lymphocytes).
Enzyme-linked immunosorbent assay (ELISA) methods using whole SRBC (Heyman et al., Citation1984; Mori et al., Citation1989) and SRBC membranes (Van Loveren et al., Citation1991; Temple et al., Citation1993) have been reported. Because of the long-term stability of the membranes, an SRBC-ELISA using the cell membranes has been extensively used to detect T-lymphocyte-dependent antigen-induced primary antibody responses (Temple et al., Citation1995). According to the research by Van Loveren et al. (Citation1991) and Temple et al. (Citation1993, Citation1995), the method using SRBC membranes is thought to be appropriate for SRBC-ELISA. However, there are some of problems, including the complexity of the preparation process and lot-to-lot variations among membrane preparations (Van Loveren et al., Citation1991; Temple et al., Citation1993). On the other hand, there is no need to prepare the SRBC membrane in an ELISA using whole SRBC. In spite of this advantage, however, the use of the whole SRBC-ELISA for detection of antibody responses to a T-dependent antigen has been limited due to the following concerns: (1) a weak attachment of SRBC to ELISA plates; (2) inherent reactivity of mouse serum with sheep hemoglobin; and, (3) the short-term stability of intact SRBC in Alsever's solution (Van Loveren et al., Citation1991; Temple et al., Citation1995; Smith et al., Citation2003).
In the present report, an improved ELISA method for detecting antibodies binding to SRBC is described. The whole SRBC-ELISA method has been previously used only in a T-lymphocyte-dependent antibody response assay in mice (Mori et al., Citation1989). We used well-known immunosuppressive agents in order to confirm the applicability of the proposed ELISA as a T-lymphocyte-dependent antibody response assay in rats, because the rat is widely used in rodent safety assessment studies. The results show that the improved ELISA provides robust and reproducible detection of antibody responses in rats.
MATERIALS AND METHODS
Animals and Animal Maintenance
Male 7-week-old Crj:CD(SD)IGS rats and male 8-week-old Crj:CD-1(ICR) mice were obtained from Charles River Japan Inc. (Atsugi, Kanagawa, Japan) and acclimated over a 1-week period prior to study. Rats were individually housed in stainless steel/wire bottom cages and mice were housed in plastic cages (5 per cage) in environmentally controlled animal rooms (temperature, 22 ± 2°C; relative humidity, 55 ± 20%; 12-hr light-dark cycle). All rats received 22 g/day of Certified Rodent Diet #5002 (PMI Feeds, Inc., St. Louis, MO) throughout the study, while all mice received the chow ad libitum. Water was available to all ad libitum. The number of animals, animal procedures, and experimental designs used in this study were reviewed and approved by the Institutional Animal Care and Use Committee.
Materials
All materials were obtained from the following manufacturers: phosphate-buffered saline (PBS), Wako Pure Chemical Industries, Ltd. (Osaka, Japan); glutaraldehyde, Nacalai Tesque Co. (Kyoto, Japan); skim milk, Difco (Detroit, MI); alkaline phosphatase (ALP)-conjugated goat anti-rat IgM (μ chain-specific), Southern Biotechnology Associates, Inc. (Birmingham, AL); ALP-conjugated goat anti-mouse IgM (μ chain-specific), Bethyl Laboratories Inc. (Montgomery, TX); ALP-conjugated goat anti-rat IgG (γ chain-specific) and p-NPP Alkaline Phosphate Substrate, Chemicon International Inc. (Temecula, CA); cyclophosphamide, Shionogi & Co., Ltd. (Osaka); cyclosporin, Novartis Pharma K. K. (Tokyo, Japan); prednisolone, Sigma-Aldrich, Inc. (St. Louis, MO); and, methotrexate, Wyeth Japan (Tokyo).
Immunization with SRBC
Sheep blood diluted 1:1 in Alsever's solution was purchased from Nihon Seibutsu Zairyo Center (Tokyo, Japan) and washed with sterile saline three times by centrifugation before use. Rats were immunized with a single intravenous injection of 3 × 108 SRBC in 1 ml saline or the vehicle (negative control). Mice were immunized with a single intraperitoneal injection of 5 × 108 SRBC in 0.5 ml normal saline. Sera from naíve mice were used as a negative control. SRBC numbers were determined using a Z2 Coulter Counter (Beckman Coulter Inc., Fullerton, CA).
Blood Collection
Post-immunization with SRBC, rats were bled from the orbital sinus on Day 6 to obtain serum IgM for analysis and on Day 14 for IgG analysis. On Day 6 postimmunization, mice were bled by heart puncture to obtain serum for IgM analysis. Each sample of blood was allowed to clot for 1-3 hr at room temperature, and the serum was then separated by centrifugation at 3,000 rpm for 15 min. All sera were stored at −80°C until the ELISA studies were performed.
ELISA Using Whole SRBC
Glutaraldehyde Method for Fixing SRBC to the ELISA Plates
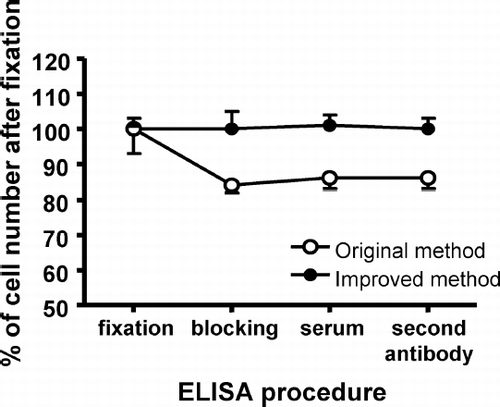
SRBC suspension (5 × 106/0.1 ml PBS) was added to each well of flat bottom 96-well ELISA microtiter plates (MAXISORP 442404, Nunc, Roskilde, Denmark) and the plates held overnight at 4°C. In the original method, after aspirating the supernatant, 100 μ l 0.3% glutaraldehyde solution was gently added to plates and the plates then held 30 min at 25°C (Rosenberg et al., Citation2002). In our improved method, without disturbing the cell layer, 20 μ l of 1.8% glutaraldehyde solution was then gently added to plates inoculated with SRBC and the plates held 30 min at 25°C. The final concentration of glutaraldehyde here was 0.3%, the same as in the original method; this low concentration does not drastically affect protein conformation and antigenicity of the proteins is retained (Stocker and Heusser, Citation1979; Johnstone and Thorpe, Citation1982).
Stability of SRBC Attachment to the ELISA Plates
After glutaraldehyde fixation of the SRBC to each well (5 × 106/0.1 ml PBS), the number of the cells was assayed. Briefly, 100 μ l 5 N NaOH was added to each well to lyse the SRBC and the optical density (OD) value at 405 nm in each was measured using a microplate reader (Wallac 1420 ARVOTMSX, EG&G WALLAC, Turku, Finland). The number of cells was then calculated by extraplolation from a standard curve prepared using wells containing fixed numbers of SRBC (Mori et al., Citation1989)
ELISA for IgM and IgG anti-SRBC Antibodies in Rats and Mice:
Prior to the subsequent steps in the ELISA protocol, plates were washed 4 times with 200 μ l of PBS. Non-specific binding sites were blocked with 1% skim milk in PBS for 1.5 hr. Four-fold serial dilutions (starting at a 1:50 dilution) of sera from rats or a 1:16 dilution of sera from mice were then added in duplicate to dedicated wells and the plate was incubated for 1.5 hr at 37°C. The secondary antibody, ALP-conjugated anti-rat IgM, anti-rat IgG, or anti-mouse IgM, was then added and the plate incubated a further 1 hr at 37°C. Alkaline phosphatase substrate was then added to each well and the plate incubated 1 hr at 25°C before the extent of color formation in each well was determined at 405 nm using a microtiter plate reader. As a control for possible plate-to-plate and/or assay-to-assay variability, a standard serum sample, anti-SRBC IgM or IgG from 15 immunized rats, or anti-SRBC IgM from 15 immunized mice, was included in each ELISA plate.
The method of Temple et al. (Citation1995) was used to determine the titer of IgM in each test sample. All of the absorbance (OD405) values from each of the dilutions of the standard serum sample were plotted against the log-scale values of each reciprocal of the dilutions. The resulting standard curve was then used to extrapolate the reciprocal of the test sample dilution at which an arbitrary selected OD was achieved (for example, an OD = 1.0 was used in ). The anti-SRBC IgM titer in each sample was then calculated as the reciprocal of this serum dilution.
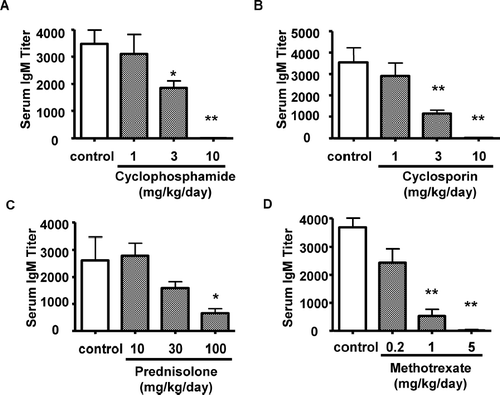
FIG. 4 Effect of immunosuppressive agents on IgM antibody response to SRBC. Rats were treated orally with cyclophosphamide, cyclosporine, prednisolone, or methotrexate for 14 consecutive days. Serum samples were collected at Day 15. The vertical axis shows the serum IgM titer required to yield a value of OD = 1.0. Each bar represents the mean ± SE of 10 animals. Asterisks indicate the value is significantly different from SRBC-immunized vehicle control group (*p < 0.05; ** p < 0.01).
Effect of Immunosuppressive Drugs on Primary IgM Antibody Response to SRBC
The effects of cyclophosphamide, cyclosporin, prednisolone and methotrexate on SRBC antibody response were assessed following single immunization with SRBC. Based on the existing information about immunosuppressive activities of these compounds, 14-day repeated dose toxicity studies were conducted. Male rats (10/group) were dosed once daily with cyclophosphamide (1, 3 and 10 mg/kg/day), cyclosporin (1, 3 and 10 mg/kg/day), prednisolone (10, 30 and 100 mg/kg/day), methotrexate (0.2, 1 and 5 mg/kg/day) or respective vehicle by oral gavage for 14 consecutive days. On Day 9, all rats were immunized with SRBC (3 × 108 cells/rat). Blood was then collected from the orbital sinus of each rat on Day 15 (corresponding to Day 6 post-immunization).
Statistical Analysis
Data from T-lymphocyte-dependent antibody response assays were analyzed using a one-way analysis of variance (ANOVA) and post-hoc Dunnett's test to determine differences between the experimental and control groups. Statistical significance was concluded at p < 0.05; values found to have a p < 0.01 were considered very significant.
RESULTS
Stability of SRBC Attached to an ELISA Plate
Stability of the SRBC monolayer was determined by measuring the number of SRBC attached to the plate at each step of the ELISA procedure. As shown in , when the cells were fixed to the ELISA plate by the original method—where the supernatant of SRBC suspension in each well of the microtiter plate was discarded prior to fixation with 0.3% glutaraldehyde solution (Rosenberg et al., Citation2002)—the number of SRBC attached to the plate after blocking with 1% skim milk was decreased by ≈ 15% compared to that after fixation by 0.3% glutaraldehyde solution. In our postfixation procedure, 20 μ l 1.8% glutaraldehyde solution (i.e., to yield the same final concentration [i.e., 0.3%] as in the original method) was gently added into the wells of the plate before discarding the buffer from the SRBC suspension. This addition of glutaraldehyde was performed carefully to prevent disturbance of the cell layer. As a result of this improvement in the cell fixation procedure with glutaraldehyde, the attachment of SRBC to the ELISA plates was maintained throughout all of the ELISA procedures ().
ELISA for IgM and IgG anti-SRBC Antibodies in Rats and Mice
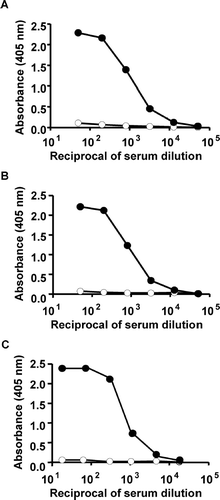
Typical examples of titration curves of IgM and IgG antibodies in rat sera and IgM antibody in mouse sera from SRBC-immunized and negative control animals are shown in . For titration of IgM anti-SRBC response, a pool of sera from rats immunized with SRBC was pre-diluted at 1:50. The maximum extinction in pooled sera taken at Day 6 from rats that were immunized (on Day 0) with SRBC was 2.29 at a dilution of 1:50, and was decreased to 0.03 in a 1:51,200 dilutions of the sera. Negative control sera yielded very low OD values, i.e., maximum of 0.11 at a serum dilution of 1:50 (). In titration curves of IgG anti-SRBC antibodies in pooled sera taken at Day 14 from SRBC-immunized rats, the maximum extinction was 2.22; these extinctions decreased to 0.01 at a serum dilution of 1:51,200. Negative control sera yielded very low OD values, i.e., a maximum of 0.07 at a serum dilution of 1:50 (). In titration curves of IgM anti-SRBC antibodies in pooled positive sera taken at Day 6 from mice immunized with SRBC, the maximum extinction was 2.37; these extinctions decreased to 0.01 at a serum dilution of 1:16,384. Negative control sera yielded very low OD values of a maximal 0.06 at a serum dilution of 1:16 ().
FIG. 2 Titration curve of anti-SRBC antibodies. IgM (A) and IgG (B) in rats and IgM (C) in mice. Negative control sera were showed open symbols and positive control sera were showed closed symbols. For positive control, pooled anti-sera for IgM and IgG were collected at Days 6 and 14 after immunization (rats: 3× 108 cells, intravenously. and mice: 5 × 108 cells, intraperitoneally). IgM and IgG antibody levels were determined using the whole SRBC-ELISA.
The above data demonstrated that the improved fixation technique and the use of whole SRBC as an antigen in ELISA are adequate for the determination of anti-SRBC antibodies of IgM and IgG in rats and IgM in mice.
SRBC Lot Difference in ELISA
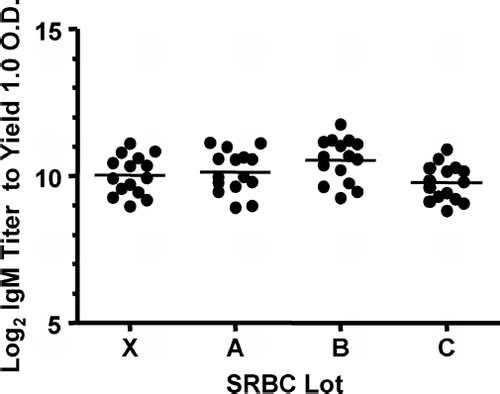
The differences in the SRBC lots used in the ELISA runs were also evaluated (). Fifteen rats were immunized with 3 × 108 SRBC of Lot X, the anti-SRBC-containing sera were then collected six days later. Individual serum IgM titers of these sera were then measured by ELISA using whole SRBC from Lots X, A, B, or C, respectively. When individual serum from rats immunized with Lot X SRBC was measured in the ELISA using SRBC from Lot X, the average log2 titer of the anti-SRBC-specific IgM was 10.0 ± 0.7. The average log2IgM titer of these sera measured by ELISA using different SRBC from Lots A, B and C were 10.1 ± 0.7, 10.5 ± 0.7 and 9.8 ± 0.6, respectively. Thus, there were no meaningful differences in the SRBC lots.
FIG. 3 Potential effect of lot differences in SRBC used for ELISA on IgM antibody response to SRBC. The sera (n = 15/group) were collected at Day 6 after immunization with 3× 108 SRBC of Lot X. IgM antibody levels were determined by whole SRBC-ELISA of Lots X, A, B, and C, respectively. The bar indicates average in each group.
Applicability of the Proposed ELISA Method to Typical Immunosuppressive Agents
Treatment with cyclophosphamide, cyclosporin, prednisolone, or methotrexate produced dose-related decreases in SRBC-specific IgM antibodies in sera. Exposure of male rats to 3 and 10 mg/kg/day of cyclophosphamide for 14 days elicited a 47 and 100% decrease, respectively, in SRBC-specific serum IgM levels (). The serum levels of anti-SRBC IgM in rats treated with 3 and 10 mg/kg/day of cyclosporin were 68 and 99 % lower (), with 30 and 100 mg/kg/day of prednisolone were 39 and 75% lower (), with 1 and 5 mg/kg/day of methotrexate were 86 and 99% lower than that of the vehicle control rats ().
DISCUSSION
Attachment of the antigen to the plates is very critical in ELISAs, so the optimal method to obtain firm attachment of SRBC to the plates was investigated first. Mori et al. (Citation1989) reported that SRBC, which has a surface negative charge, is attached directly to the bottom of the plate, i.e., aminoplate. However, attachment of the SRBC to the aminoplate without glutaraldehyde treatment was unstable in this study (data not shown). Rosenberg et al. (Citation2002) reported that glutaraldehyde treatment of cells attached to plates gave a more stable monolayer of the antigen cells. When the cells were fixed to the ELISA plate by the method of Rosenberg et al. (Citation2002), the number of SRBC attached to the plate after blocking was decreased by approximately 15% compared to that after glutaraldehyde treatment. The weak attachment of SRBC by this method was probably due to disturbance of the cell layer by addition of the glutaraldehyde solution. To alleviate this problem and to fix and attach SRBC to the plate, a small amount of high-concentrated glutaraldehyde solution was added after SRBC inoculation. As a result of this simple but effective improvement in the cell fixation procedure, SRBC attachment to ELISA plates was maintained during the washing and incubation procedures of an ELISA.
The use of the whole SRBC-ELISA method has been limited due to a concern about the inherent reactivity of mouse serum with sheep hemoglobin (Temple et al., Citation1995). In this present study, no interference on titration curves for IgM and IgG antibodies was observed when sera from negative control rats were analyzed by the whole SRBC-ELISA method. As a result, no interference on titration curves for the IgM antibody was observed in naive sera from mice. These results suggest that the whole SRBC-ELISA method can be applicable to T-dependent antibody response assay both in rats and mice.
The short-term stability of SRBC could be one of the problems in the whole SRBC-ELISA, because SRBC can be used only for several weeks after bleeding (Temple et al., Citation1995). Plates older than several weeks after coating with SRBC resulted in lower SRBC-specific OD values and increases in variance (Heyman et al., Citation1984). Plates freshly coated with SRBC should be used in the whole SRBC-ELISA. With respect to SRBC lot differences in the ELISA assay used in the present study, antibody titers measured using the same lot used for immunization were nearly identical to those measured using different lots of SRBC. This experiment demonstrated that the use of different lots overcome the problem for the short-term stability of SRBC in whole SRBC-ELISA.
Cyclophosphamide, cyclosporin, prednisolone and methotrexate have been commonly used as typical immunosuppressive agents. It has been reported that many immunosuppressive agents, including cyclophosphamide, were detected by the ELISA method of SRBC membrane (Van Loveren, et al., Citation1991; Temple, et al., Citation1993; Ladics, et al., Citation1995; Ladics, et al., Citation1998). To determine whether the suppressive effects of immunosuppressive agents on the IgM antibody response to SRBC could be detected by the presently proposed ELISA method, we tested cyclophosphamide, cyclosporin, prednisolone and methotrexate. In the present study we have shown that cyclophosphamide, cyclosporin, prednisolone and methotrexate suppressed IgM antibody response to SRBC in rats. There are differences in the mechanism of immunosuppression between cyclophosphamide (an alkylating agent; Brock et al., Citation1996), cyclosporine (a calcineurin inhibitor; Stähelin et al., Citation1996), prednisolone (a synthetic corticosteroid; Okazaki et al., Citation1992) and methotrexate (a folic acid antagonist; Moreland et al., Citation1997). Therefore, further studies using known immunosuppressive agents are necessary to confirm the broad usefulness of our improved ELISA method in the T-dependent antibody response assay.
In conclusion, we have established an improved whole SRBC-ELISA method that is an easy and reproducible method to detect SRBC-specific antibodies in serum. Our method can detect anti-SRBC antibody responses in rat serum to monitor the T-dependent antibody response. It is hoped that further studies on various immunosuppressants, as well as non-immunosuppres- sants, will clarify the specificity and sensitivity of the improved ELISA in T-dependent antibody response assays.
ACKNOWLEDGMENTS
The authors thank Mr. H. Watanabe for his contributions to the work presented here.
REFERENCES
- Andrade-Mena C. E., Sardo-Olmedo J. A., Ramirez-Lizardo E. J. Effects of phenytoin administration on murine immune function. J. Neuroimmunol. 1994; 50: 3–7
- Brock N. The history of the oxazaphosphorine cytostatics. Cancer 1996; 78: 542–547
- Doi T., Nagai H., Tsukuda R., Suzuki T. Dose-response relationships of cytotoxicity, PFC response and histology in the spleen in rats treated with alkylating agents. Toxicology 1996; 107: 47–60
- Fan F., Wierda D., Rozman K. K. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on humoral and cell-mediated immunity in Sprague–Dawley rats. Toxicology 1996; 106: 221–228
- Gore E. R., Gower J., Kurali E., Sui J. L., Bynum J., Ennulat D., Herzyk D. J. Primary antibody response to keyhole limpet hemocyanin in rat as a model for immunotoxicity evaluation. Toxicology 2004; 197: 23–35
- Heyman B., Holmquist G., Borwell P., Heyman U. An enzyme-linked immunosorbent assay for measuring anti-sheep erythrocyte antibodies. J. Immunol. Meth. 1984; 68: 193–204
- Immunochemistry in Practice, A. Johnstone, R. Thorpe. Blackwell, London 1982; 81
- Ladics G. S., Smith C., Elliott G. S., Slone T. W., Loveless S. E. Further evaluation of the incorporation of an immunotoxicological functional assay for assessing humoral immunity for hazard identification purposes in rats in a standard toxicology study. Toxicology 1998; 126: 137–152
- Ladics G. S., Smith C., Heaps K., Elliott G. S., Slone T. W., Loveless S. E. Possible incorporation of an immunotoxicological functional assay for assessing humoral immunity for hazard identification purposes in rats on standard toxicology study. Toxicology 1995; 96: 225–238
- Luster M. I., Portier C., White K. L., Jr., Gennings C., Munson A. E., Rosenthal G. J. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 1992; 18: 200–210
- Moreland L. W., Heck L. W., Jr., Koopman W. J. Biologic agents for treating rheumatoid arthritis: Concepts and progress. Arthr. Rheum. 1997; 40: 397–409
- Mori H., Sakamoto O., Xu Q. A., Daikoku M., Koda A. Solid phase enzyme-linked immunosorbent assay (ELISA) for anti-sheep erythrocyte antibody in mouse serum. Int. J. Immunopharmacol. 1989; 11: 597–606
- Okazaki S., Yamazaki E., Tamura K., Hoshiya T., Anabuki K., Tanaka H., Tanaka G. A 13-week subcutaneous toxicity study of prednisolne farnesylate (PNF) in rats. J. Toxicol. Sci. 1992; 17: 1–48
- Putman E., Van Loveren H., Bode G., Dean J., Hastings K., Nakamura K., Verdier F., Van der Laan J. W. Assessment of the immunotoxic potential of human pharmaceuticals: A workshop report. Drug Inform. J. 2002; 36: 417–427
- Roman D., Ulrich P., Paul G., Court M., Vit P., Kehren J., Mahl A. Determination of the effect of calcineurin inhibitors on the rat's immune system after KLH immunization. Toxicol. Lett. 2004; 149: 133–140
- Rosenberg C. E., Salibian A., Fink N. E. An enzyme-linked immunosorbent assay for measuring anti-sheep red blood cells antibodies in lead-exposed toads. J. Pharmacol. Toxicol. Meth. 2002; 47: 121–128
- Shea J. A., Peachee V. L., White K. L. Characterization of keyhole limpet hemocyanin (KLH) as an alternative T-dependent antigen for ELISA immunotoxicological evaluations in mice. Toxicol. Sci. 2003; 72(S1)104, Toxicologist
- Smith H. W., Winstead C. J., Stank K. K., Halstead B. W., Wierda D. A predictive F344 rat immunotoxicology model: Cellular parameters combined with humoral response to NP-Cγ G and KLH. Toxicology 2003; 194: 129–145
- Stähelin H. F. The history of cyclosporin A (Sandimmune®) revisited: Another point of view. Experientia 1996; 52: 5–13
- Stocker J. W., Heusse C. H. Method for binding cells to plastic: Application to a solid-phase radioimmunoassay for cell-surface antigens. J. Immunol. Meth. 1979; 26: 87–95
- Temple L., Butterworth L., Kawabata T. T., Munson A. E., White K. L. ELISA to measure SRBC specific serum IgM: Method and data evaluation;. Methods in Immunotoxicology, Vol. 1, G. R. Burleson, J. H. Dean, A. E. Munson. Wiley-Liss, New York 1995; 137–157
- Temple L., Kawabata T. T., Munson A. E., White K. L. Comparison of ELISA and plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with benzo[a]pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 1993; 21: 412–419
- Van Loveren H., Verlaan A. P., Vos J. G. An enzyme-linked immunosorbent assay of anti-sheep red blood cell antibodies of the classes M, G, and A in the rat. Int. J. Immunopharmacol. 1991; 13: 689–695