Abstract
Pulmonary infections have been reported to be increased in welders. Previous animal studies have indicated that manual metal arc, stainless steel welding fume (MMA-SS) increased susceptibility to lung infections. MMA-SS is composed of a complex of metals (e.g., iron, chromium, nickel). The objective was to determine which metal component of MMA-SS welding fume alters lung defense responses. At Day 0, rats were intratracheally instilled one time with saline or MMA-SS at a concentration of 2 mg/rat. Additional rats were treated with the metal constituents, Fe2O3, NiO, or Cr2Na2O7 alone or in combination, at concentrations that are present in the dose used for MMA-SS treatment. At Day 3, rats were intratracheally inoculated with 5 × 103 Listeria monocytogenes. At Days 6, 8 and 10, homogenized left lungs were cultured, and colony-forming units were counted after an overnight incubation to assess pulmonary bacterial clearance. At Day 3 (prior to infection) and at Days 6, 8 and 10, right lungs were lavaged to recover cells and fluid from the airspaces to measure lung injury, inflammation, and cytokine secretion. The production of reactive oxygen species by phagocytes recovered from the lungs was measured. Exposure to MMA-SS, soluble Cr, or the mixture of all three metals before infection significantly increased bacterial lung burden and tissue damage when compared to control. Animals treated with NiO or Fe2O3 did not differ from control. Animals pre-treated with soluble Cr had alterations in inflammation and in the production of different cytokines (TNFα, IL-6, IL-2, and IL-12) involved in lung immune responses. This study indicates that soluble Cr present in MMA-SS is likely the primary component responsible for the suppression of lung defense responses associated with stainless steel welding fumes.
INTRODUCTION
The severity, frequency, and duration of upper and lower respiratory tract infections have been reported to be increased among welders (Howden et al., Citation1988), and excess mortality from pneumonia has been observed in welders (Doig and Challen, Citation1964; Coggon et al., Citation1994). Wergeland and Iversen (Citation2001) have described a potential lethal risk associated with the development of pneumonia after the inhalation of metal fumes. In addition, Palmer et al. (Citation2003) have reported that pulmonary exposure to ferrous and other metal fumes may increase the susceptibility to infection. Elucidation of the mechanisms that cause these observations is currently lacking.
Welding fumes are composed of complex metal oxides (Antonini, Citation2003). The chemical and physical properties of welding fume can be quite different, depending on the welding process and the type of materials used. Fumes generated using stainless steel (SS) welding fumes contain significant amounts of iron (Fe), chromium (Cr), and nickel (Ni). Fumes generated during shielded manual metal arc (MMA) welding processes, where fluxes are incorporated in the electrode, are generally more water-soluble than fumes formed during other welding processes.
Animal toxicology studies indicate that Cr-rich SS welding fumes induce more lung injury and inflammation compared to mild steel (MS) welding fumes, which are composed of predominately Fe (White et al., Citation1981; Antonini et al., Citation1996). Soluble metals associated with MMA-SS welding fumes, such as Cr, have been shown to play an important role in the pneumotoxicity observed in rats (White et al., Citation1982; Taylor et al., Citation2003). Pretreatment with SS fumes generated during MMA welding before infection altered lung defense responses, significantly slowed the pulmonary clearance of a bacterial pathogen, and increased morbidity of rats (Antonini et al., Citation2004).
It was the objective of the study to employ an animal infectivity model to determine which metal or metals may be responsible for the suppression in lung defense responses to infection caused by welding fume exposure. Before pulmonary inoculation with a bacterial pathogen, rats were pretreated with different metals that are present in SS welding fumes. It was hypothesized that the effects of SS welding fume on lung defenses after infection may be mediated by exposure to soluble Cr.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley [Hla:(SD) CVF] rats from Hilltop Lab Animals (Scottdale, PA), weighing 250–300 g and free of viral pathogens, parasites, mycoplasmas, Helicobacter, and CAR Bacillus, were used for all experiments. The rats were acclimated for at least six days after arrival and were housed in ventilated polycarbonate cages on Alpha-Dri cellulose chips and hardwood Beta-chips as bedding, and provided HEPA-filtered air, autoclaved Prolab 3500 diet, and tap water ad libitum. The animal facilities are specific pathogen-free, environmentally controlled, and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Welding Sample Collection and Characterization
A bulk sample of the MMA-SS welding fume was collected by Lincoln Electric Co. (Cleveland, OH) and has been used in a previous study (Antonini et al., Citation1999). MMA-SS fume was generated during manual metal arc welding using a flux-covered stainless steel E308-16 electrode. Particle size of the welding sample was previously characterized by scanning electron microscopy and was found to be of respirable size with a count mean diameter of < 1.5 μ m (Antonini et al., Citation1999).
The MMA-SS sample was suspended in sterile phosphate-buffered saline (PBS), pH 7.4, and sonicated for 1 min with a Sonifier 450 Cell Disruptor (Branson Ultrasonics, Danbury, CT). The fume sample was found to be highly water-soluble in a previous study (Antonini et al., Citation1999). It was further divided into soluble and insoluble components. The complete particle suspension was incubated for 24 hr at 37°C, and then centrifuged at 12,000 x g for 30 min. The soluble portion of the MMA-SS sample was recovered and filtered with a 0.22 μ m filter (Millipore Corp., Bedford, MA). The pellet (insoluble portion) was resuspended in PBS. Analysis of the metal constituents of the soluble and insoluble fractions extracted from 2 mg of the MMA-SS fume was determined by inductively coupled argon plasma-atomic emission spectroscopy (ICP-AES) and is reported in .
TABLE 1 Concentrations of welding fume sample (mg/instillate)
Experimental Design
On Day 0, rats were pretreated by a single intratracheal instillation of the welding fume sample, individual metal constituents of the welding fume, or saline (vehicle control). On Day 3, animals from each group were intratracheally inoculated with 5 × 103 Listeria monocytogenes. At Days 3 (pre-infection), 6, and 10, the left lungs were tied off, and bronchoalveolar lavage (BAL) was performed on the right lungs of some animals from each group. The recovered cells were differentiated and chemiluminescence (CL) was determined as an index of macrophage function. Albumin and lactate dehydrogenase (LDH), two indices of lung injury, and a number of immunomodulatory cytokines were measured in the acellular bronchoalveolar lavage fluid (BALF). On Days 6, 8, and 10, non-lavaged left lungs were removed and homogenized, and the number of bacterial colony-forming units (CFUs) was determined to assess bacterial load. Animal weights and morbidity were monitored over the course of the treatment period to evaluate the general health status of the infected animals.
Welding Fume Treatment
Rats were lightly anesthetized by an intraperitoneal injection of 0.6 ml of a 1% solution of sodium methohexital (Brevital, Eli Lilly, Indianapolis, IN) and intratracheally instilled one time with 2 mg/rat of the intact MMA-SS welding fume in 300 μ l of saline, according to the method of Reasor and Antonini (Citation2001). The welding fume dose of 2.0 mg/rat was chosen based on results from a previous study (Antonini et al., Citation1996). Animals in the vehicle control group were intratracheally instilled with 300 μ l of sterile saline.
For the pulmonary treatment with the individual metals, rats were exposed to the approximate amount of each metal measured in 2 mg of welding fume (). Rats were intratracheally treated with 0.82 mg of insoluble ferric oxide (Fe2O3; Fisher Scientific, Pittsburgh, PA), 0.60 mg of soluble sodium dichromate dehydrate (Cr2Na2O7·2H2O; Aldrich Chemical Co., St. Louis, MO), and 0.06 mg of insoluble nickel(ous) oxide (NiO; Fisher Scientific) alone or in combination.
Intratracheal Bacterial Inoculation
L. monocytogenes (strain 10403S, serotype 1) was cultured overnight in brain heart infusion broth (Difco Laboratories, Detroit, MI) at 37°C in a shaking incubator. Following incubation, the bacterial concentration was determined at an optical density of 600 nm using a spectrometric method and diluted with sterile saline to a concentration of 5 × 103 of L. monocytogenes/500 μ l of sterile saline. At Day 3, rats from each treatment group were intratracheally inoculated with 5 × 103 of L. monocytogenes. In a previous pilot study, this bacterial dose gave a uniform pulmonary infection and did not have an effect on animal morbidity and mortality in untreated naive Sprague–Dawley rats (Antonini et al., Citation2001).
Pulmonary Burden of L. monocytogenes
At Days 6, 8, and 10, the left lungs were removed from rats in each treatment group. The excised tissues were suspended in 10 ml of sterile water, homogenized using a Polytron 2100 homogenizer (Brinkmann Instruments, Westbury, NY), and cultured on brain heart infusion agar plates (Becton Dickinson and Co., Cockeysville, MD). The number of viable CFUs was counted after an overnight incubation at 37°C.
Bronchoalveolar Lavage
The rats were deeply anesthetized with an intraperitoneal injection of sodium pentobarbital (> 100 mg/kg body weight, Butler Co., Columbus, OH) and then exsanguinated by severing the abdominal aorta. At Days 3 (pre-infection), 6, and 10, the left bronchus was clamped off, and bronchoalveolar lavage was performed on the right lungs of selected rats from each group. The right lungs were first lavaged with a 1 ml/100 g body wt aliquot of calcium- and magnesium-free PBS, pH 7.4. The first fraction of recovered bronchoalveolar lavage fluid was centrifuged at 500 × g for 10 min, and the resultant cell-free supernatant was analyzed for various biochemical parameters and cytokine levels. The lungs were further lavaged with 6 ml aliquots of PBS until 30 ml were collected. These samples also were centrifuged for 10 min at 500 × g and the cell-free bronchoalveolar lavage fluid discarded. The cell pellets from all washes for each rat were combined, washed, and resuspended in 1 ml of PBS buffer and evaluated as described next.
Cellular Evaluation
Total cell numbers were determined using a Coulter Multisizer II and AccuComp software (Coulter Electronics, Hialeah, FL). Cells were differentiated using a Cytospin 3 centrifuge (Shandon Life Sciences International, Cheshire, England). Cell suspensions (5 × 104 cells) were spun for 5 min at 800 rpm and pelleted onto a slide. Cells (200/rat) were identified after labeling with Leukostat stain (Fisher Scientific).
Biochemical Parameters of Injury
Using the acellular first fraction of bronchoalveolar lavage fluid, albumin content, an index to quantify increased permeability of the bronchoalveolar-capillary barrier, and LDH activity, an indicator of general cytotoxicity, were measured. Albumin content was determined colorimetrically at 628 nm based on albumin binding to bromcresol green using an albumin BCG diagnostic kit (Sigma Chemical Co., St. Louis, MO). LDH activity was determined by measuring the oxidation of lactate to pyruvate coupled with the formation of NADH at 340 nm. Measurements were performed with a COBAS MIRA auto-analyzer (Roche Diagnostic Systems, Montclair, NJ).
Chemiluminescence
Luminol-dependent CL measures the light generated as reactive oxygen/nitrogen species are produced by activated cells. CL was performed with an automated Berthold Autolumat LB 953 luminometer (Wallace, Inc., Gaithersburg, MD) as previously described (Antonini et al., Citation1994). Luminol was used as an amplifier to enhance detection of the light, and 2 mg/ml of unopsonized zymosan (Sigma) was added immediately prior to the measurement of CL to activate the AM. Rat neutrophils (PMN) have not been observed to respond to unopsonized zymosan in our system, therefore, the zymosan-stimulated CL produced is generated by AM. Measurement of CL was recorded for 15 min at 37°C using 5 × 105 AM, and the integral of counts versus time was calculated. Zymosan-stimulated CL was calculated as the total counts of stimulated cells minus the total counts of the corresponding resting cells.
Lung Lavage Fluid Cytokine Analyses
Levels of cytokines, tumor necrosis factor (TNF)-α, interleukin (IL)-2, IL-6, IL-10, interferon (IFN)-γ, and IL-12p70, were assayed in the first fraction of bronchoalveolar lavage fluid at Days 3 (pre-infection), 6, and 10. The selection of the cytokines to be assayed was based on their potential role in lung inflammatory and immune responses to intracellular bacterial infection. Cytokine protein concentrations were determined using enzyme-linked immuno-absorbent assay (ELISA) kits (Biosource International, Inc., Camarillo, CA). The results of the colorimetric assay were obtained with a Spectramax 250 plate spectrophotometer using Softmax Pro 2.6 software (Molecular Devices Corp., Sunnyvale, CA).
Statistical Analysis
Results are expressed as means ± standard error of measurement. Comparisons between two means were analyzed using a Student's t-test; all other data were analyzed using a one-way analysis of variance (ANOVA) for multiple comparisons at a single timepoint followed by a Tukey–Kramer post-hoc test. For all analyses, the criterion of significance was set at p < 0.05.
RESULTS
Lung defense responses associated with SS welding fume and metal treatment were determined by measuring bacterial lung burden after L. monocytogenes infection. Pretreatment with MMA-SS welding fume before infection dramatically increased the pulmonary burden of L. monocytogenes (). There were significantly more bacteria in the lungs at each timepoint compared to control.
FIG. 1 Pulmonary clearance of bacteria by rats pre-exposed to (A) 2 mg of manual metal arc, stainless steel (MMA-SS) welding fume or (B) 0.82 mg insoluble Fe2O3, 0.60 mg soluble Cr2Na2O7, or 0.06 mg insoluble NiO alone or in combination. The MMA-SS and metal samples were intratracheally instilled 3 days prior to intratracheal inoculation with 5 × 103 L. monocytogenes. Control animals were pretreated with saline. Values are means in Log10 base units ± standard error of measurement (n = 4–12); *significantly greater than corresponding saline control at each timepoint (p < 0.05).
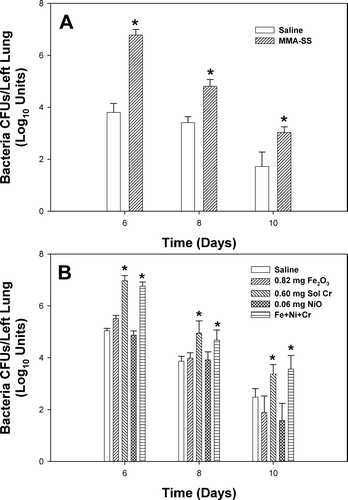
In examining which particular metal might be responsible for the effect, the animals were pretreated with the individual metals that compose the MMA-SS sample either alone or in combination before infection (). Pulmonary instillation of soluble Cr alone or a combination of all three metals at concentrations present in the MMA-SS sample before inoculation with L. monocytogenes significantly increased the bacteria in the lungs at Days 6, 8, and 10 compared to control. Neither Fe2O3 nor NiO alone (at concentrations present in SS welding fume) had an effect on L. monocytogenes lung burden.
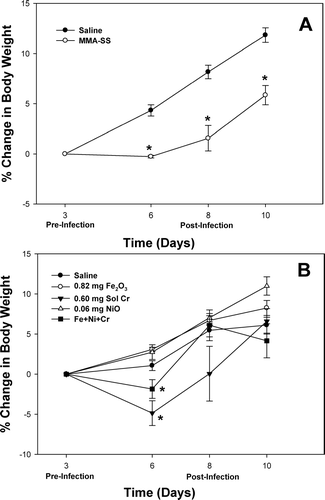
To assess the effect of infection on the general health status of the treated rats, body weight was monitored before and after infection. Rats from the MMA-SS group failed to gain weight as rapidly as controls at each timepoint after infection (). Pretreatment with soluble Cr or a combination of all three metals caused a significant decrease in body weight only at Day 6 as compared to the other groups ().
FIG. 2 Percent change in body weight of rats pre-exposed to (A) 2 mg of manual metal arc, stainless steel (MMA-SS) welding fume or (B) 0.82 mg insoluble Fe2O3, 0.60 mg soluble Cr2Na2O7, or 0.06 mg insoluble NiO alone or in combination. The MMA-SS and metal samples were intratracheally instilled 3 days prior to intratracheal inoculation with 5 × 103 L. monocytogenes. Control animals were pretreated with saline. Values are means ± standard error of measurement (n = 4–12); *significantly reduced than corresponding saline control at each time point (p < 0.05).
Lung lavage fluid and cells were recovered to assess pulmonary injury and inflammation after treatment with soluble Cr before (Day 3) and after infection (Days 6 and 10) with L. monocytogenes (). Soluble Cr pretreatment caused a significant increase in AMs at Day 10, but not Days 3 and 6, compared to control. Pretreatment with soluble Cr led to pulmonary inflammation and damage before and after infection as evidenced by an increase in PMN number and injury parameter levels (LDH and albumin) at all timepoints, respectively.
TABLE 2 Lung inflammation and injury
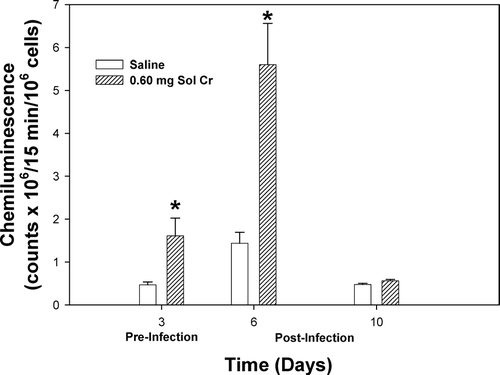
In the examination of AM activation and oxidant production, zymosan-stimulated CL was measured before and after infection in the different groups. Soluble Cr pretreatment caused a significant elevation in CL at Days 3 and 6 compared to control (). By Day 10, the CL level for the Cr group had returned to control value.
FIG. 3 Zymosan-stimulated chemiluminescence of macrophages recovered from rats pre-exposed to 0.60 mg soluble Cr2Na2O7. The Cr sample was intratracheally instilled three days prior to intratracheal inoculation with 5 × 103 L. monocytogenes. Control animals were pretreated with saline. Values are means ± standard error of measurement (n = 4–7); *significantly greater than corresponding saline control at Day 6 (p < 0.05).
A number of lung cytokines that have been shown to play important roles in inflammation and immune responses were measured in the acellular lavage fluid. At Days 3 and 6, a significant reduction in TNFα was observed after pretreatment with soluble Cr (). By Day 10, there was no difference in the TNFα levels when comparing the Cr and control groups. Soluble Cr treatment caused significant increases in IL-6 at Days 3 and 6, but not Day 10, compared to control (). No significant differences were observed in IL-10 levels at any timepoint when comparing the Cr and control groups (). Statistically significant decreases were observed in IL-2 for the Cr group at Days 3 and 6 compared to control (). Soluble Cr pretreatment caused a significant elevation in IL-12p70 only after infection at Day 6 compared to control (). IFNγ levels in the lavage fluid samples were below the limits of detection of the ELISA for each treatment group at all timepoints.
FIG. 4 TNFα measured within the bronchoalveolar lavage fluid (BALF) recovered from rats pre-exposed to 0.60 mg soluble Cr2Na2O7. The Cr sample was intratracheally instilled 3 days prior to intratracheal inoculation with 5 × 103 L. monocytogenes. Control animals were pretreated with saline. Values are means ± standard error of measurement (n = 4–7); *significantly less than corresponding saline control at Days 3 and 6 (p < 0.05).
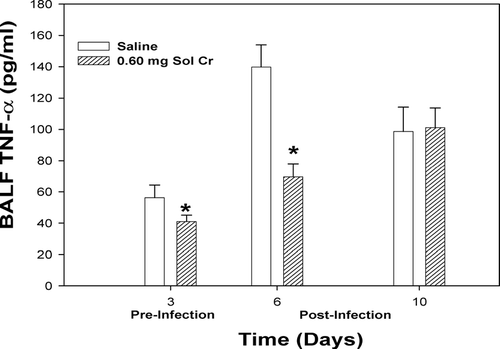
FIG. 5 IL-6 measured within the bronchoalveolar lavage fluid (BALF) recovered from rats pre-exposed to 0.60 mg soluble Cr2Na2O7. The Cr sample was intratracheally instilled 3 days prior to intratracheal inoculation with 5 × 103 L. monocytogenes. Control animals were pretreated with saline. Values are means ± standard error of measurement (n = 4–7); *significantly greater than corresponding saline control at Days 3 and 6 (p < 0.05); n.d. = not detected.
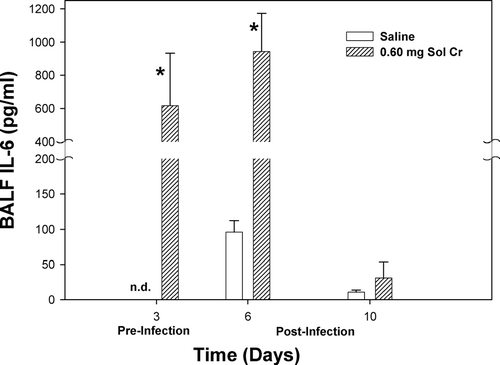
FIG. 6 IL-10 measured within the bronchoalveolar lavage fluid (BALF) recovered from rats pre-exposed to 0.60 mg soluble Cr2Na2O7. The Cr sample was intratracheally instilled 3 days prior to intratracheal inoculation with 5 × 103 L. monocytogenes. Control animals were pretreated with saline. Values are means ± standard error of measurement (n = 4–7).
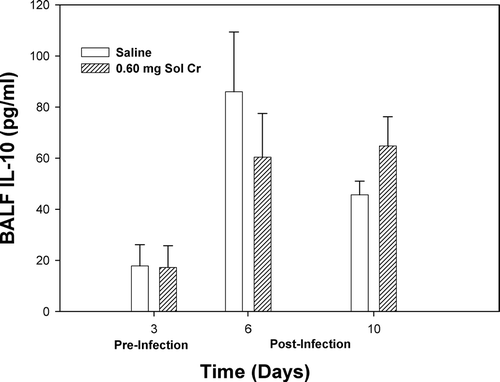
FIG. 7 IL-2 measured within the bronchoalveolar lavage fluid (BALF) recovered from rats pre-exposed to 0.60 mg soluble Cr2Na2O7. The Cr sample was intratracheally instilled three days prior to intratracheal inoculation with 5 × 103 L. monocytogenes. Control animals were pretreated with saline. Values are means ± standard error of measurement (n = 4–7); *significantly less than corresponding saline control at Days 3 and 6 (p < 0.05).
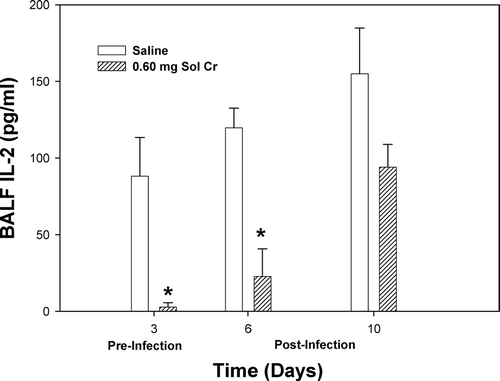
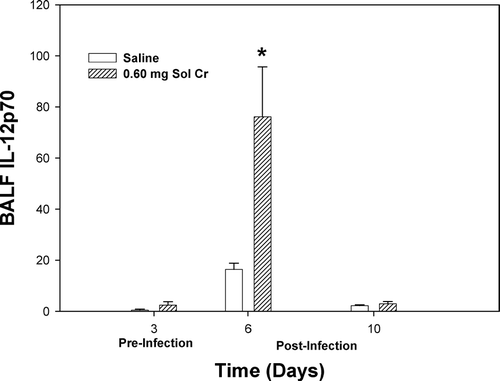
FIG. 8 IL-12p70 measured within the bronchoalveolar lavage fluid (BALF) recovered from rats pre-exposed to 0.60 mg soluble Cr2Na2O7. The Cr sample was intratracheally instilled 3 days prior to intratracheal inoculation with 5 × 103 L. monocytogenes. Control animals were pretreated with saline. Values are means ± standard error of measurement (n = 4–7); *significantly greater than corresponding saline control at Day 6 (p < 0.05).
DISCUSSION
Studies have indicated that workers exposed to welding fumes are more susceptible to airway infections compared to the general population (Doig and Challen, Citation1964; Coggon et al., Citation1994; Wergeland and Iversen, Citation2001; Palmer et al., Citation2003). Boshnakova et al. (Citation1989) observed decrements in cell-mediated immunity in a significantly higher proportion of welders compared to controls. Tuschl et al. (Citation1997) reported that welders had recurrent respiratory infections due to reduced cytotoxic activity of immune cells. Animal studies have shown that SS welding fumes that contain significant amounts of water-soluble Cr can alter lung defense responses, slow the pulmonary clearance of a bacterial pathogen, and increase morbidity (Antonini et al., Citation2004). The mechanisms involved in the suppression of lung defenses caused by exposure to welding fumes are lacking.
The current study indicates that soluble Cr present in MMA-SS is likely the primary component responsible for the suppression of lung defense responses associated with exposure to stainless steel welding fume. Pretreatment with MMA-SS or soluble Cr before infection significantly increased bacterial lung burden as well as inflammation and tissue damage when compared to control. Animals treated with other metals found in MMA-SS fume, insoluble NiO or insoluble Fe2O3 at concentrations found in the fume, before infection did not differ from control. The lung immunosuppressive effects of Cr have been well documented (as reviewed by Cohen, Citation2004). Previous animal studies also have demonstrated an increase in lung inflammation and injury after exposure to soluble Cr (Glaser et al., Citation1985; Cohen et al., Citation1998; Derelanko et al., Citation1999).
One mechanism by which lung phagocytes kill microbes and control the spread of L. monocytogenes infection is to produce biologically reactive oxygen (e.g., superoxide anion, hydroxyl radical, hydrogen peroxide) and nitrogen (e.g., NO) species (Ohya et al., Citation1998; Ogawa et al., Citation2001). It was our hypothesis that lung defenses, such as macrophage function, would be altered after exposure to the metals associated with welding fumes. Soluble Cr treatment increased the production of reactive oxygen species by macrophages as measured by chemiluminescence. This observed increase in oxidant production caused by Cr treatment likely played only a minor role in the killing of the bacteria. The elevation in oxidative stress instead may have injured lung cells and affected the way they responded to the bacterial challenge. The increase in oxidants may explain the elevations observed when measuring LDH and albumin levels in the lavage fluid after Cr treatment.
In agreement with the findings from the current studies, in vitro exposure of J774A.1 macrophages to soluble Cr(VI) resulted in increased production of reactive oxygen species, nitric oxide and superoxide anion (Hassoun and Stohs, Citation1995). However, Galvin and Oberg (Citation1984) indicated that pulmonary exposures to soluble Cr(VI) had no effect on macrophage chemiluminescence or oxygen consumption. Importantly, the effect of Cr on macrophage function may be related to dose. Glaser et al. (Citation1985) demonstrated that exposure to soluble Cr at low levels may enhance macrophage activity, whereas exposure to high levels of Cr may suppress macrophage function.
To determine if soluble Cr altered immune cell signaling, the production of a number of cytokines important in lung defense was measured. TNFα and IL-6, two cytokines with prominent pro-inflammatory properties, were assessed. IL-6 was significantly elevated after infection in the Cr group compared to control. This result was not surprising because IL-6 is an important mediator of acute phase reactions and has been shown to be involved in the development of oxidative stress and tissue damage (Borish et al., Citation1989; Taga and Kishimoto, Citation1997). However, TNFα was significantly reduced in the Cr group before and at three days after infection compared to control. Cohen et al. (Citation1998) also observed a reduction in TNFα, but not IL-6, produced by macrophages recovered from rats after treatment with soluble Cr. Alterations in TNFα and IL-6 production are clear indications that the Cr treatment is affecting the ability of the macrophage to respond to bacterial challenge.
IL-10 has diverse effects on different cell types, but is considered to be primarily inhibitory in nature, and to be an anti-inflammatory cytokine (Taga et al., Citation1993; Moore et al., Citation2001). IL-10 has been observed to inhibit activation of CD4+ TH1 cells, CD8+ T-lymphocytes, macrophages and neutrophils, and have varying effects on natural killers cells. It was previously observed that MMA-SS pretreatment caused a significant increase in IL-10 after infection with L. monocytogenes, indicating SS welding fume treatment may be indirectly affecting cell-mediated immune responses (Antonini et al., Citation2004). Interestingly, levels of IL-10 were not different between the control and Cr groups before and after infection in the current study. The effect observed with the MMA-SS treatment may be due to the combination of metals present in the fume sample as opposed to Cr alone. Additional studies are needed to test this hypothesis.
Effective clearance of the bacterial infection requires both the innate (non-specific) and adaptive (pathogen-specific) immune responses (Pamer, Citation2004). Activation of the cell-mediated arm of the immune system is vital in the elimination of L. monocytogenes. IL-12 induces the expression of IFNγ by CD4+ TH1 cells, CD8+ T-lymphocytes, and natural killer cells, and in the presence of IFNγ, in turn, drives the differentiation of naïve CD4+ T-lymphocytes toward the CD4+ TH1 subset (Hsieh et al., Citation1993; Mosmann and Sad, Citation1996). IL-2 is produced primarily by CD4+ TH1 cells and by naïve CD4+ and some CD8+ T-lymphocytes and is involved in T-lymphocyte growth and differentiation (Mosmann, Citation1992). Despite the notable increase in IL-12, which should lead to an increase in T-lymphocyte activity, T-lymphocyte production of IL-2 was significantly decreased in the Cr group at timepoints before and after infection compared to control.
This reduction in IL-2 suggests that the soluble Cr may be altering T-lymphocyte activity directly, resulting in a slowing in the clearance of the bacteria over time. Thus, the necessary TH1 cell-stimulation of macrophages to further kill and clear L. monocytogenes from the lungs, as well as the killing of infected cells by CD8+ T-lymphocytes may have been impaired. A similar response was observed in a previous study when animals were pretreated with MMA-SS fume before infection (Antonini et al., Citation2004).
Studies are ongoing in the laboratory to further characterize the effects of welding fume and their associated metals on lung defenses. In the current experimental model, animals have been treated with welding particles and metals by intratracheal instillation. It is our goal to use a more physiological method of exposure when examining the effects of welding fume on lung immune responses in animals. An automated, robotic welding fume generation and animal inhalation system recently has been constructed to assess the pulmonary effects after more chronic exposures to inhaled welding particles (Antonini et al., Citation2006).
Disclaimer: The findings and conclusions of this paper have not been formally disseminated by NIOSH and should not be construed to represent any agency determination or policy.
REFERENCES
- Antonini J. M. Health effects of welding. CRC Crit. Rev. Toxicol. 2003; 33: 61–103
- Antonini J. M., Van Dyke K., Ye Z., DiMatteo M., Reasor M. J. Introduction of luminol-dependent chemiluminescence as a method to study silica inflammation in the tissue and phagocytic cells of rat lung. Environ. Health Perspect. 1994; 102(S10)37–42
- Antonini J. M., Krishna Murthy G. G., Rogers R. A., Albert R., Ulrich G. D., Brain J. D. Pneumotoxicity and pulmonary clearance of different welding fumes after intratracheal instillation in the rat. Toxicol. Appl. Pharmacol. 1996; 140: 188–199
- Antonini J. M., Lawryk N. J., Krishna Murthy G. G., Brain J. D. Effect of welding fume solubility on lung macrophage viability and function in vitro. J. Toxicol. Environ. Health 1999; 58: 343–363
- Antonini J. M., Roberts J. R., Clarke R. W. Strain-related differences of nonspecific respiratory defense mechanisms in rats using a pulmonary infectivity model. Inhal. Toxicol. 2001; 13: 85–102
- Antonini J. M., Taylor M. D., Millecchia L., Bebout A. R., Roberts J. R. Suppression in lung defenses after bacterial infection in rats pretreated with different welding fumes. Toxicol. Appl. Pharmacol. 2004; 200: 206–218
- Antonini J. M., Afshari A. A., Stone S., Chen B., Scwegler-Berry D., Fletcher W. G., Goldsmith W. T., Vandestouwe K. H., McKinney W., Castranova V., Frazer D. G. Design, construction, and characterization of a novel robotic welding fume generator and inhalation exposure system for laboratory animals. J. Occup. Environ. Hyg. 2006; 3: 194–203
- Borish L., Rosenbaum R., Albury L., Clark S. Activation of neutrophils by recombinant interleukin-6. Cell. Immunol. 1989; 121: 280–289
- Boshnakova E., Divanyan S., Zlatarov I., Marovsky S. V., Kisyova K., Zanev D., Karev G., Marinova T. Z. Immunological screening of welders. J. Hyg. Epidemiol. Microbiol. Immunol. 1989; 33: 379–382
- Cohen M. D. Pulmonary immunotoxicology of select metals: Aluminum, arsenic, cadmium, chromium, copper, manganese, nickel, vanadium, and zinc. J. Immunotoxicol. 2004; 1: 39–69
- Cohen M. D., Zelikoff J. T., Chen L. C., Schlesinger R. B. Immunotoxicological effects of inhaled chromium: Role of particle solubility and co-exposure to ozone. Toxicol. Appl. Pharmacol. 1998; 152: 30–40
- Coggon D., Inskip H., Winter P., Pannett B. Lobar pneumonia: An occupational disease in welders. Lancet 1994; 344: 41–43
- Derelenko M. J., Rinehart W. E., Hilaski R. J., Thompson R. B., Loser E. Thirteen-week Subchronic rat inhalation toxicity study with a recovery phase of trivalent chromium compounds, chromic oxide and basic chromium sulfate. Toxicol. Sci. 1999; 52: 278–288
- Doig A. T., Challen P. J. R. Respiratory hazards in welding. Ann. Occup. Hyg. 1964; 7: 223–231
- Galvin J. B., Oberg S. G. Toxicity of hexavalent chromium to alveolar macrophages in vitro and in vivo. Environ. Res. 1984; 33: 7–16
- Glaser U., Hochrainer D., Kloppel H., Kuhmen H. Low level chromium(VI) inhalation effects on alveolar macrophages and immune functions in Wistar rats. Arch. Toxicol. 1985; 57: 250–256
- Hassoun E. A., Stohs S. J. Chromium-induced production of reactive oxygen species, DNA single-strand breaks, nitric oxide production, and lactate dehydrogenase leakage in J744A.1 cell cultures. J. Biochem. Toxicol. 1995; 10: 315–321
- Howden D. G., Desmeules M. J. A., Saracci R., Sprince N. L., Herber P. I. Respiratory hazards of welding: Occupational exposure characterization. Am. Rev. Respir. Dis. 1988; 138: 1047–1048
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T-cells through IL-12 produced by Listeria-induced macrophages. Science 1993; 260: 547–549
- Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Ann. Rev. Immunol. 2001; 19: 683–765
- Mosmann T. R. T lymphocyte subsets, cytokines, and effector functions. Ann. New York Acad. Sci. 1992; 664: 89–92
- Mosmann T. R., Sad S. The expanding universe of T-cell subsets: TH1, TH2, and more. Immunol. Today 1996; 17: 138–146
- Ogawa R., Pacella R., Espey M. G., Miranda K. M., Friedman N., Kim S., Cox G., Mitchell J. B., Wink D. A., Russo A. Comparison of control of listeria by nitric oxide redox chemistry from murine macrophages and NO donors: Insights into Listeriocidal activity of oxidative and nitrosative stress. Free Rad. Biol. Med. 2001; 30: 268–276
- Ohya S., Tanabe Y., Makino M., Nomura T., Xiong H., Arakawa M., Mitsuyama M. The contributions of reactive oxygen intermediates and reactive nitrogen intermediates to listericidal mechanisms differ in macrophages activated pre- and post-infection. Infect. Immun. 1998; 66: 4043–4049
- Palmer K. T., Poole J., Ayres J. G., Mann J., Burge P. S., Coggon D. Exposure to metal fume and infectious disease. Am. J. Epidemiol. 2003; 157: 227–233
- Pamer E. G. Immune responses to Listeria monocytogenes. Nature Rev. 2004; 4: 812–823
- Reasor M. J., Antonini J. M. Pulmonary responses to single versus multiple intratracheal instillations of silica in rats. J. Toxicol. Environ. Health 2001; 62: 9–21
- Taga K., Mostowski H., Tosata G. Human interleukin-10 can directly inhibit T-cell growth. Blood 1993; 81: 2964–2971
- Taga T., Kishimoto T. GP130 and the interleukin-6 family of cytokines. Ann. Rev. Immunol. 1997; 15: 797–819
- Taylor M. D., Roberts J. R., Leonard S. S., Shi X., Antonini J. M. Effects of welding fumes of differing composition and solubility on free radical production and acute lung injury and inflammation in rats. Toxicol. Sci. 2003; 75: 181–191
- Tuschl H., Weber E., Kovac R. Investigations on immune parameters in welders. J. Appl. Toxicol. 1997; 17: 377–383
- Wergeland E., Iversen B. G. Deaths from pneumonia after welding. Scand. J. Work Environ. Health 2001; 27: 353
- White L. R., Hunt J., Richards R. J., Eik-Nes K. B. Biochemical studies of rat lung following exposure to potassium dichromate or chromium-rich welding fume particles. Toxicol. Lett. 1982; 11: 159–163
- White L. R., Hunt J., Tetley T. D., Richards R. J. Biochemical and cellular effects of welding fume particles in the rat lung. Ann. Occup. Hyg. 1981; 24: 93–101