Abstract
Glyoxylic acid, a small dicarboxylic acid, has been detected at measurable levels in the atmosphere and is suspected to be present in indoor air environments. It is generated through the ozonolysis of several high volume production compounds that are commonly found indoors. Glyoxylic acid was tested in a combined irritancy and local lymph node assay (LLNA). It tested positive in the LLNA with an EC3 value of 5.05%. Significant increases were observed in the B220+cell population in the draining lymph nodes. No changes were identified in the IgE+B220+ cell population in the draining lymph nodes or total serum IgE levels; this suggests that glyoxylic acid functions as a T-cell-mediated contact sensitizer. Exposure to volatile organic compounds (VOC), similar to glyoxylic acid, emitted from building materials, cleaning formulations or other consumer products, and /or indoor chemistry have been linked to adverse health effects. These results may provide an explanation for some of adverse health effects associated with indoor air exposure.
INTRODUCTION
Low-molecular weight dicarboxylic acids, such as glyoxylic acid (), have been identified as important constituents of the organic fraction of atmospheric particulate matter in remote and polluted regions (Rohrl and Lammel, Citation2001) with measurable levels documented during the summer months at a rural site in Georgia (Lee, Citation1995). Glyoxylic acid, a volatile organic compound (VOC), is generated from the ozonolysis of acrolein, acrylic acid, maleic acid, and cinnamic acid (Grosjean et al., Citation1994). Acrylic acid and acrolein are categorized as some of the most hazardous compounds with respect to human health and ecosystems (US EPA, 1990, 1994). They are both considered to be high production volume chemicals with production exceeding 1 million pounds annually (US EPA, 1990). Acrylic acid is used in the manufacturing of carpet resins and floor adhesive resins. It is also an intermediate in the production of acrolein, and other indoor sources include: polishes, paint coatings, plastics, textiles, and paper finishes. Acrylic acid and acrolein have also been identified in consumer products, building materials or furnishing that contribute to indoor air pollution. While considered to be less hazardous than acrylic acid or acrolein, maleic acid is also considered a high production volume chemical. It is used in the production of synthetic resins and rubber adhesives that contribute to indoor air pollution.
Glyoxylic acid has also been observed as an oxidation product of urocanic acid, a UV chromophore found in skin (Kammeyer et al., Citation2001. Although the specific yields of glyoxylic acid in the gas phase are unknown for these reactions, it is predicted that concentrations may be large enough to warrant further studies investigating the health effects associated with exposure to this compound. While the presence of glyoxylic acid has been widely documented in the outdoor environment, studies investigating its presence in the indoor environment are lacking. Since glyoxylic acid is a reaction product of ozone and its parent compounds are commonly found in products used indoors, it is predicted that glyoxylic acid will be found indoors but this research has yet to be conducted.
The focus of these studies is to further define the potential adverse effects associated with exposure to glyoxylic acid by examining the irritancy and sensitization potential after dermal exposure to glyoxylic acid using a combined murine LLNA. Subsequent studies were also conducted in an attempt to categorize glyoxylic as a T-cell-mediated or IgE-mediated sensitizer.
MATERIALS AND METHODS
Animals
Female BALB/c mice, 8–12-weeks old, were purchased from Taconic (Hudson, NY). Mice were quarantined for 1 wk upon arrival and maintained under conditions specified by NRC guidelines. Animals were fed a modified NIH-31 6% irradiated rodent diet (Harlan Teklad #7913) and provided tap water ad libitum. Animal facilities were maintained between 18–26°C and 30–70% relative humidity, with light–dark cycles at 12-hr intervals (light: 6:00–18:00). Cages, with hardwood chip bedding, were actively ventilated, cleaned and sanitized weekly. The NIOSH Animal Facility is an environmentally controlled barrier facility fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Mice were weighed, tail-marked for identification, and assigned to homogeneous weight groups (n = 5) before each experiment.
Chemicals
Glyoxylic acid (CAS 298-12-4) was purchased from Sigma Aldrich Chemical Company (St. Louis, MO). α -Hexylcinnamaldehyde (HCA, CAS 101-86-0) and toluene 2,4-diisocyanate (TDI, CAS 584-84-9) were purchased from Aldrich Chemical Company, Inc. (Milwaukee, WI).
Range Finding Studies
Range finding studies were performed to select the concentrations of glyoxylic acid to be used for dermal exposures. Briefly, mice were exposed topically to acetone vehicle or increasing concentrations of glyoxylic acid in acetone on the dorsal surface of each ear (25 μ l per ear) for three consecutive days. Animals were allowed to rest for 2 d following the last exposure and then weighed and examined for signs of toxicity such as loss of body weight and ungroomed fur. For range finding studies glyoxylic acid was tested at the following concentrations: 10, 20, or 40%, with the highest concentration selected based on limits of solubility. There were no changes in body weight at the highest concentration tested.
Irritancy Measurement
Irritancy measurements were performed as previously described (Woolhiser et al., Citation1998). Briefly, before the first chemical administration, the thicknesses of the right and left ear pinnae of each mouse was measured using a modified engineer's micrometer (Mitutoyo Co., Kanagawa, Japan). Mice were exposed to 25 μ l of acetone or glyoxylic acid for 3 days. Ear thickness measure-ments were taken 24 hr following the final exposure. The mean percentage of ear swelling was calculated based on the equation: [(mean post-challenge ear thickness − mean pre-challenge ear thickness)/mean pre-challenge thickness] × 100. For these studies glyoxylic acid was tested at 10%, 20%, and 40%.
Local Lymph Node Assay
The local lymph node assay (LLNA) was performed following the method described in the ICCVAM Peer Review Panel report (NIEHS, Citation1999) with minor modifications. Briefly, mice were exposed topically to acetone, increasing concentrations of glyoxylic acid, or positive control (30% HCA) on the dorsal surface of each ear (25 μ l per ear) for three consecutive days. Animals were allowed to rest for 2 d following the last exposure. On Day 6, mice were injected intravenously via the lateral tail vein with 20 μ Ci [3H]-thymidine (Dupont NEN; specific activity 2 Ci/mmol).
Five hours after [3H]-thymidine injection, animals were euthanized via CO2 inhalation, and the left and right cervical draining lymph nodes (DLNs) located at the bifurcation of the jugular vein were excised and pooled for each animal. Single cell suspensions were made and following overnight incubation in 5% trichloroacetic acid (TCA), samples were counted using a Packard Tri-Carb 2500TR liquid scintillation analyzer. Stimulation indices (SI) were calculated by dividing the mean disintegrations per minute (DPM) per test group by the mean DPM for the vehicle control group. EC3 values (concentration of chemical required to induce a 3-fold increase over the vehicle control) were calculated based on the equation from Basketter et al. (Citation1999). In brief, The EC3 value was derived by interpolating between two points on the SI axis, one immediately above and one immediately below the SI value of 3. Where the data points lying immediately above and below and SI value of three have the coordinates (a,b) and (c,d), respectively, then the EC3 value may be calculated using the equation:
For these studies glyoxylic acid was tested at concentrations between 1.25–40%.
Phenotype Analysis
To further evaluate the mechanism of sensitization [T-cell-mediated (Type IV) vs. IgE-mediated hypersensitivity (Type I)], the draining lymph nodes were analyzed for B220+ and IgE+B220+ cells after dermal exposure to the compound. For the phenotyping analysis, glyoxylic acid was tested at concentrations of 10, 20, and 40%. Lymph node cell phenotypes were analyzed using flow cytometry (as described by Manetz and Meade, Citation1999). Mice were exposed to acetone or increasing concentration of glyoxylic acid topically on the dorsal surface of each ear (25 μ l per ear) for four consecutive days. Animals were allowed to rest for 6 d after the final exposure and were then euthanized on Day 10 by CO2 inhalation. Draining lymph nodes were collected (two nodes/animal/tube) in 2 ml phosphate-buffered saline (PBS) and were dissociated using the frosted ends of two microscope slides. Cell counts were performed using a Coulter Counter (Z2 model, Beckman Coulter, Fullerton, CA), and 1 × 106 cells per sample were added to the wells of a 96-well plate. Cells were washed using staining buffer (1% bovine serum albumin/0.1% sodium azide in phosphate buffered saline) and then incubated with Fc block (clone 2.4G2). The cells were then incubated with anti-CD45RA/B220 (PE, clone RA3-6B2) and anti-IgE antibodies (FITC, clone R-35-72) or the appropriate isotype controls, diluted in staining buffer, washed, and incubated with propidium iodide (PI). All antibodies and isotype controls were purchased from BD Pharmingen (San Jose, CA). After a final wash, cells were suspended in staining buffer and analyzed with a Becton Dickinson FACSVantage flow cytometer using a propidium iodine (PI) viability gate.
Total Serum IgE
Blood samples were collected via cardiac puncture from the animals used in the phenotypic assays. Sera were separated by centrifugation and frozen at −20°C for later analysis (within 2 wk) of IgE by ELISA. All antibodies were purchased from BD Pharmingen. In brief, 96-well flat bottom plates (Dynatec Immulon-2) were coated with (2 μ g/ml in PBS) purified monoclonal Rat anti-mouse IgE antibody (clone R35-72), sealed with plate sealers, and incubated overnight at 4°C. The plates were washed 3 times with PBS/Tween 20 and then blocked for 1 hr with 2% Newborn Calf Serum (NCS) and 0.05% sodium azide at room temperature.
An initial dilution (1:10) was made from the serum samples and IgE control standards were prepared at 500 ng/ml. All dilutions were made in 2% NCS and 0.05% sodium azide. Serum samples and IgE control standard (mouse IgE anti-TNP, clone C38-2) were then serially diluted (1:2), added to the coated plates in a 100 μ l volume and incubated at room temperature for 1 hr. The plates were washed three times with PBS/Tween 20 and biotin-conjugated rat anti-mouse IgE (clone R35-92) was added in a 100 μ l volume and incubated at room temperature for 1 hr. The plates were then washed three times with PBS/Tween 20 and Streptavidin-alkaline phosphatase (Pharmingen Cat# 554065) was added (100 μ l of a 1:400 dilution) and plates were incubated for 1 hr at room temperature. p-Nitrophenyl phosphate (Sigma Cat# N-9389) was used as the alkaline phosphatase substrate and added to the plates in a 100 μ l volume. The plates were allowed to develop for up to 30 min at room temperature or until the OD reading of the highest standard reached 3.0. Absorbance was determined using a Spectramax Vmax plate reader (Molecular Devices, Sunnyvale, CA) at 405–605 nm. Data analysis was performed using the IBM Softmax Pro 3.1 (Molecular Devices), and the IgE concentrations for each sample were interpolated from a standard curve using multipoint analysis.
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism version 3.0 (San Diego, CA). All data were analyzed by a one-way analysis of variance (ANOVA) and when significant differences were detected (p = 0.05), Dunnett's test was used to compare treatment groups with the appropriate control group. If the assumptions were not able to be met by parametric analysis, the nonparametric Kruskal-Wallis k-sample test was utilized followed by the Mann-Whitney U– test for pairwise comparisons with the control. Statistical significance is designated by *p ≤ 0.05 and **p ≤ 0.01.
RESULTS
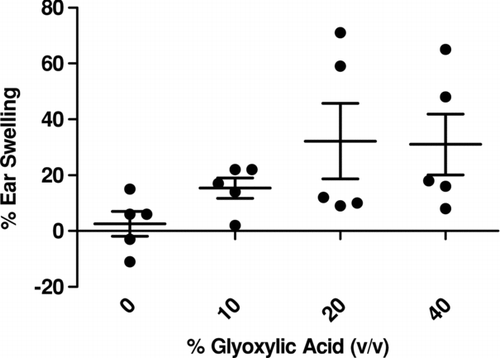
Irritancy as Indicated by Ear Swelling
Although not statistically significant, a trend in ear swelling 24 hr post-final chemical exposure was observed after exposure to 20% and 40% glyoxylic acid (). Observations of the 20% and 40% exposure groups did identify two of the five animals in each group to display common signs of irritation including redness and swelling. In several of the mice exposed to 40% glyoxylic acid, one or both of the ears were red and blistered after the dermal exposure. These observations were consistent with the observations after animal exposure for the LLNA and phenotype studies. TDI (2.5%) was used as a positive control for irritancy studies and resulted in an averaged significant increase of 68% ear swelling post exposure.
Sensitization Potential Determined by the LLNA
Initial concentrations tested (10–40%) generated a greater than 3-fold stimulation index, therefore the experiment was repeated using concentrations between 1.25–10% ( and ). No changes in body weight were observed following exposure to the highest concentration of 40%. Exposure to glyoxylic acid resulted in a calculated EC3 value of 5.05% (). HCA (30%) was used as a positive control for the LLNA and resulted in an average stimulation index (SI) value of 23.4.
FIG. 3 Sensitization potential after dermal exposure. Analysis of the sensitization potential of glyoxylic acid using the LLNA. [3H]-thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle or increasing concentrations of glyoxylic acid. Bars represent means ± SE of 5 mice per group. Numbers above bars represent stimulation indices. Level of statistical significance is denoted as **p ≤ 0.01 as compared to acetone vehicle.
![FIG. 3 Sensitization potential after dermal exposure. Analysis of the sensitization potential of glyoxylic acid using the LLNA. [3H]-thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle or increasing concentrations of glyoxylic acid. Bars represent means ± SE of 5 mice per group. Numbers above bars represent stimulation indices. Level of statistical significance is denoted as **p ≤ 0.01 as compared to acetone vehicle.](/cms/asset/a4a2b953-4254-4e28-a22c-3d3941adf5dc/iimt_a_308734_uf0003_b.gif)
TABLE 1 Phenotypic analysis and total IgE dose-response studies
Lymph Node Phenotyping and Analysis of Total Serum IgE
Phenotype analysis of the lymphocytes from draining lymph nodes following exposure to glyoxylic acid resulted in significant increases in the B220+ cell population at all concentrations tested (). However, there was no significant increase in the IgE+B220+ cells or total serum IgE (). This result suggests that the glyoxylic acid response is a TH1 response rather than a TH2 response (Manetz and Meade, Citation1999). TDI (2.5%) was used as a positive control for phenotyping experiments and resulted in significant elevations of IgE+B220+ (17.8%) and B220+ (33.3%) cell populations. TDI (2.5%) was also used as a positive control for the total IgE ELISA and resulted in a significant elevation of total IgE (1752 ng/ml) when compared to vehicle (607 ng/ml).
DISCUSSION
In this study, the irritancy and sensitization potential of glyoxylic acid was examined. Although not statistically significant, the results suggest that glyoxylic acid may be an irritant. This compound was found to significantly elevate the lymphocyte population in the draining lymph nodes at concentrations of 10% and higher. Phenotypic analysis of the draining lymph nodes identified significant increases in the B220+ cell population at all concentrations tested. Exposure to glyoxylic acid did not cause an elevation in the IgE+B220+ cells in the draining lymph nodes or total serum IgE levels. These results suggest that exposure to glyoxylic acid elicits a T-cell mediated hypersensitivity response. Glyoxylic acid is a high production volume chemical (US EPA, Citation1990), but specific reports of hazard and safety information about this chemical are lacking. In 1983 the National Institute of Occupational Safety and Health (NIOSH) estimated 4,281 workers are potentially exposed to glyoxylic acid in the United States (NIOSH, 1983). Occupational exposure to glyoxylic acid may occur through inhalation and dermal contact with this compound at workplaces where glyoxylic acid is produced or used. These workers primarily consist of chemists, hairdressers, and cosmetologists. Since glyoxylic acid is used in cosmetic preparations, the general population may also be exposed to this compound through the use of these products.
Recently, the Cosmetic Ingredient Review (CIR) expert panel has identified glyoxylic acid as a cosmetic ingredient that should be further evaluated for adverse effects. Allantoin, an agent used for skin-softening and rapid cell regeneration, functions by precipitating proteins on skin and is the diureide of glyoxylic acid. In the industrial field, it is used as a base chemical for the synthesis of other chemical products of acids, esters, cyclic compounds. Applications include aroma compounds, agrochemicals, pharmaceuticals and polymers.
Exposure to VOCs, similar to glyoxylic acid, emitted from building materials, cleaning formulations, other consumer products, and indoor chemistry have been linked to adverse health effects (Weschler, Citation2006). Several studies have been conducted in an attempt to assess the health effects caused by exposures to VOC oxidation products (e.g., aldehydes, ketones, dicarbonyls, and carboxylic acids) in the indoor environment. Recently, Anderson et al. (Citation2007) explored the respiratory sensitization potential of glyoxal, methylglyoxal, glycolaldehyde, and diacetyl. Results from a combined irritancy and local lymph node assay (LLNA) identified all compounds except glyoxal to be irritants. These compounds were also identified as sensitizers in the LLNA with EC3 values ranging from 0.42 to 1.9%. Doyle et al. observed increased cytotoxicity, IL-8, and IL-6 gene expression when cells were exposed to 1,3-butadiene, ozone, and their oxidation products in vitro (Doyle et al., Citation2004). Several studies have also documented significant changes in airway responsiveness with an increase in sensory irritation in mice after inhalation exposures to mixtures of isoprene and ozone (Wilkins et al., Citation2001; Rohr et al., Citation2003). Additional studies have also found carboxylic acids to have the potential to be strong lung irritants (for example, formic acid) (Nair, Citation1997). Furthermore, when two or more additional functional groups are present (i.e., dicarbonyls) the calculated irritancy potential can increase (Jarvis et al., Citation2005).
Urocanic acid that is present in the outermost layer of the skin is a key precursor contributing to glyoxylic acid generation. Research investigating the gas-phase products and health complaints resulting from aircraft ozone exposure found reactions between ozone and the passengers to be responsible for more than 50% of the gas-phase products that were generated (Weschler et al., Citation2007). Because the precursors for these reactions are present in the skin, the inhalation of volatilized glyoxylic acid is enhanced. Specific measurements of glyoxylic acid produced by reaction of ozone and acrolein, cinnamic acid, maleic acid, urocanic acid, and acrylic acid have yet to be determined as well as the indoor measurements of these precursors. Total measurements from all glyoxylic acid precursor reactions may yield quantities large enough to be partially responsible for the rising number of health complaints resulting from indoor air exposure. The above-mentioned results have prompted investigations into low-molecular weight dicarboxylic acids such as glyoxylic acid.
Although these studies have identified glyoxylic acid as a contact sensitizer after dermal exposure, the most common route of exposure to glyoxylic acid is predicted to be through inhalation. Dermal exposure to glyoxylic acid does exist through cosmetic application, pharmaceutical use and occupational exposures. Recent data suggests that dermal exposure of glyoxylic acid in the gas phase may be responsible for certain health complaints. An increased sensation of lip and skin dryness was observed in individuals exposed to ozone in a simulated aircraft compared to individuals exposed to the identical relative humidity conditions without ozone (Strøm-Tejsen et al., Citation2007). These symptoms could potentially be explained by the increase in oxidation products, such as glyoxylic acid, on the skin. Although the studies described in this paper did not identify glyoxylic acid as an IgE-mediated sensitizer, they suggest glyoxylic acid is an irritant. Irritation resulting from dermal or inhalation glyoxylic acid exposure could potentially exacerbate pre-existing health conditions.
The exposure regimes employed in these investigations delivered a relatively high dose of glyoxylic acid to the skin of a mouse. Although normal exposure levels to glyoxylic acid in the indoor environment is probably much lower than the concentrations used in these studies, hazard identification of the immunological effects caused by glyoxylic acid exposure may encourage investigations into risk assessment and the long-term health effects associated with chronic exposure.
In summary, glyoxylic acid was identified as a sensitizer using a combined LLNA. The results from the phenotypic analysis suggest glyoxylic acid functions as a T-cell-mediated sensitizer. These findings raise concern for exposure of the general public to glyoxylic acid in both an indoor air environment and through the use of cosmetics and pharmaceuticals. The high production volume of the parent chemicals increases the likelihood that glyoxylic acid may be generated in indoor air settings such as office spaces or janitorial closets. These findings in animal models suggest glyoxylic acid can alter biological systems and potentially have an impact on human health.
The Authors would like to thank Leon Butterworth and Laurel Jackson of the Hazard Identification Core lab for their technical assistance. The Authors also thank the following for their suggestions and critical reading of the paper: Laurel Jackson and Charles Weschler. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
REFERENCES
- Anderson S. E., Wells J. R., Fedorowicz A., Butterworth L. F., Meade B. J., Munson A E. Evaluation of the contact and respiratory sensitization potential of volatile organic compounds generated by simulated indoor air chemistry. Toxicol. Sci. 2007; 97: 355–363
- Basketter D. A., Lea L. J., Dickens A., Briggs D., Pate I., Dearman R. J., Kimber I. A comparison of statistical approaches to the derivation of EC3 values from local lymph node assay dose responses. J. Appl. Toxicol. 1999; 19: 261–266
- Doyle M., Sexton K. G., Jeffries H., Bridge K., Jaspers I. Effects of 1,3-butadiene, isoprene, and their photochemical degradation products on human lung cells. Environ. Health Perspect. 2004; 112: 1488–1495
- Grosjean E., Williams E. L., Grosjean D. Atmospheric chemistry of acrolein. Sci. Total Environ. 1994; 153: 195–202
- Jarvis J., Seed M. J., Elton R., Sawyer L., Agius R. Relationship between chemical structure and the occupational asthma hazard of low molecular weight organic compounds. Occup. Environ. Med. 2005; 62: 243–250
- Kammeyer A., Eggelte T. A., Overmars H., Bootsma A., Bos J. D., Teunissen M. B. Oxidative breakdown and conversion of urocanic acid isomers by hydroxyl radical generating systems. Biochim. Biophys. Acta 2001; 1526: 277–285
- Lee Y. N., Hallock K. Atmospheric carbonyl compounds at a rural southeastern United States site. J. Geophys. Res. Atmos. 1995; 100: 25933–25944
- Manetz T. S., Meade B. J. Development of a flow cytometry assay for the identification and differentiation of chemicals with the potential to elicit irritation, IgE-mediated, or T-cell-mediated hypersensitivity responses. Toxicol. Sci. 1999; 48: 206–217
- Nair B. Final report on the safety assessment of formic acid. Int. J. Toxicol. 1997; 16: 221–234
- NIEHS; National Institute of Environmental Health Sciences. The Murine Local Lymph Node Assay: A Test Method for Assessing the Allergic Contact Dermatitis Potential of Chemicals/Compounds. Fed. Reg. 1999; 64: 14006–14007
- NIOSH; National Institute for Occupational Safety and Health. National Occupational Exposure Survey 1981–1983. Washington, DC 1983
- Rohr A. C., Weschler C. J., Koutrakis P., Spengler J. D. Generation and quantification of ultrafine particles through terpene/ozone reaction in a chamber setting. Aerosol Sci. Technol. 2003; 37: 65–78
- Rohrl A., Lammel G. Low-molecular weight dicarboxylic acids and glyoxylic acid: Seasonal and air mass characteristics. Environ. Sci. Technol. 2001; 35: 95–101
- Strøm-Tejsen P., Weschler C. J., Wargocki P., Myków D., Zarzycka J. The influence of ozone on self-evaluation of symptoms in a simulated aircraft cabin. J. Exposure Sci. Environ. Epidemiol. 2007, doi:10.1038/sj.jes.7500586
- USEPA; Environmental Protection Agency. 1990 HPV Challenge Program Chemical List. Environmental Protection Agency, Washington, DC 1990
- USEPA; Environmental Protection Agency. Chemical Summary for Acrylic Acid. Office of Pollution Prevention and Toxics, Washington, DC 1994
- Weschler C. J. Ozone's impact on public health: Contributions from indoor exposures to ozone and products of ozone-initiated chemistry. Environ. Health Perspect. 2006; 114: 1489–1496
- Weschler C. J., Wisthaler A., Cowlin S., Tamás G., Strøm-Tejsen P., Hodgson A. T., Destaillats H., Herrington J., Zhang J., Nazaroff W. W. Ozone-initiated chemistry in an occupied simulated aircraft cabin. Environ. Sci. Technol. 2007; 41: 6177–6184
- Wilkins C. K., Clausen P. A., Wolkoff P., Larsen S. T., Hammer M., Larsen K., Hansen V., Nielsen G. D. Formation of strong airway irritants in mixtures of isoprene/ozone and isoprene/ozone/nitrogen dioxide. Environ. Health Perspect. 2001; 109: 937–941
- Woolhiser M. R., Hayes B. B., Meade B. J. A combined murine local lymph node and irritancy assay to predict sensitization and irritancy potential of chemicals. Toxicol. Meth. 1998; 8: 245–256