Abstract
Vanadium, an important air pollutant derived from fuel product combustion, aggravates respiratory diseases and impairs cardiovascular function. In contrast, its effects on immune response are conflicting. The aim of our work was to determine if spleens of vanadium-exposed CD1 mice showed histological lesions that might result in immune response malfunction. One hundred and twelve CD-1 male mice were placed in an acrylic box and inhaled 0.02 M vanadium pentoxide (V2O5); actual concentration in chamber ≈1.4 mg V2O5/m3) for 1 hr/d, twice a week, for 12 wk. Control mice inhaled only vehicle. Eight mice were sacrificed prior to the exposures. Eight control and eight V2O5-exposed mice were sacrificed 24 hr after the second exposure of each week until the 12-wk study was over. Another 8 mice that completed the 12-wk regimen were immunized with recombinant Hepatitis B surface antigen (HBsAg; three times over an 8-wk period) before sacrifice and analyses of their levels of anti-HBsAg antibody (HBSAb) using ELISA. In all studies, at sacrifice, blood samples were obtained by direct heart puncture and the spleen was removed, weighed and processed for H-E staining and quantitation of CD19 cells. The results indicated that the spleen weight of V2O5-exposed animals peaked at 9 wk (546 ± 45 vs. 274 ± 27 mg, p < 0.0001) and thereafter progressively decreased (321 ± 39 mg at 12 wk, p < 0.001; control spleen = 298 ± 35 mg). Spleens of V2O5-exposed animals showed an increased number of very large and non-clearly delimited germinal centers (that contained more lymphocytes and megakaryocytes) compared to those of control mice. In addition, their red pulp was poorly delimited and had an increase in CD19+ cells within hyperplasic germinal nodes. The mean HBsAb levels in immunized control mice were greater than that in the exposed hosts (i.e., OD = 0.39 ± 0.03 vs. 0.11 ± 0.05, p < 0.01). HBsAb avidity dropped to a value of 40 in V2O5-exposed animals vs. 86 in controls (p < 0.0001). We conclude that the chronic inhalation of V2O5, a frequent particle (PM2.5) component, induces histological changes and functional damage to the spleen, each of which appear to result in severe effects on the humoral immune response.
Keywords :
INTRODUCTION
Vanadium (V) has recently become recognized as an important air pollutant (Fortoul et al., Citation1996, Citation2002) in the atmosphere of Mexican cities. There, as elsewhere, it is a component of residual oil fly ash (ROFA; Samet et al., Citation1999) that enters the organism, mainly by inhalation (Brook et al., Citation2004; Nemmar et al., Citation2004). Another important source of vanadium is as an atmospheric contaminant generated from combusted fuel products. Occupational exposure to one major chemical form that is also found in urban air, vanadium pentoxide (V2O5), occurs during the cleaning of oil-fired boilers and furnaces, during handling of catalysts in chemical plants, and during the refining, processing, and burning of vanadium-rich fossil fuels, especially Venezuelan or Mexican oils (Nriagu, 1998; Ivancsits et al., 2000).
There is ample epidemiological evidence showing that exposure to particulate matter with diameters less than 2.5 μ m (PM2.5)-bearing air pollution aggravate respiratory diseases and impairs the cardiovascular function (Dockery et al., Citation1993; Ackermann-Liebrich et al., Citation1997; Pope et al., Citation1999; Gold et al., Citation2000; Laden et al., Citation2000), especially if the individuals are exposed to peaks of air pollution (Peters et al., Citation1999). At this particle size, metals are adsorbed by inhalation and enter the systemic circulation, thus exerting its toxic effect in other organs and tissues (Dockery and Pope, Citation1994).
There are conflicting results in relation to vanadium exposure and the immune response. Inhalation of vanadium decreases the phagocytic index and the inducible production of interleukin (IL)-6 and interferon (IFN)-γ by rat pulmonary macrophages (Cohen et al., Citation1997). Similarly, the exposure of peripheral blood mononuclear cells to vanadium reduces the release of IFNγ and the proliferation of PHA-stimulated cultures, although IL-5 release shows a vanadium concentration-dependent bi-modal behavior (Di Gioacchino et al., Citation2002). Although there are slight differences between those studies probably related to species differences or method of administering the vanadium, the tendency is to suppress the immune response. We have previously shown that the chronic inhalation of V2O5 in CD1 mice induces an increase in the number and size of platelets (Gonzalez-Villalva et al., Citation2006). We have also seen that these vanadium-treated mice show macroscopic splenic lesions that might lead to histologic and functional alterations. Therefore, the aim of the work reported here was to determine if the spleens of vanadium-exposed CD1 mice showed histological lesions that might induce immune response malfunctions.
MATERIALS AND METHODS
Mice
Eight-week-old CD-1 male mice (bred in the Instituto de Investigaciones Biomédicas, UNAM) weighing 33 ± 2 g were housed in hanging plastic cages kept in an animal facility (with average 21°C temperature, 57% humidity, controlled lighting [12:12 hr light/dark regime]) and fed Purina rat chow and water ad libitum. The experimental protocol was in accordance to the Animal Act of 1986 for Scientific Procedures.
Exposure Regimens
Inhalation exposures were performed as described by (Avila-Costa et al., Citation2004). Briefly, exposures were performed with a 0.02 M V2O5 (99.99% purity, Sigma-Aldrich, St Louis, MO) suspension in deionized water containing 100 μ l Tween-20/100 ml of suspension. The aerosol inhalation chamber was an acrylic box measuring 45 cm × 21 cm × 35 cm that could house 25 mice/session and had a total volume of 3.3 L. The ultra-nebulization to generate the atmospheres was carried out using a DeVilbiss Ultraneb 99 (Somerset, PA) system that maintained a constant flow of 10 L/min; according to the manufacturer, about 80% of the aerosolized particles reaching the mice would be expected to have a mass median aerodynamic diameter (MMAD) of 0.5–5.0 μ m.
The concentration of V2O5 in the inhalation chamber was quantified as previously described (Fortoul et al., Citation2002). A 0.22 μ m filter was positioned at the external outlet of the ultranebulizer during each exposure period. At the end of each exposure, the filter was removed and weighed. Vanadium concentration was analyzed in each filter in a graphite furnace atomic absorption spectrometer (Perkin Elmer Mod. 2380, Shelton, CT), using a 318.4 nm wavelength and a slit of 0.7 nm. The assay detection limit was 0.37 ppm. Accuracy was assessed by three random determinations of seven different standard solutions, prepared with the same chemical reagents used during the metal analysis. Each sample was analyzed in triplicate.
In these studies, sets of 25 mice were placed in the acrylic box to inhale V2O5 for 1 hr/d, twice a week, for 12 wk. Previous experiments in our laboratories have shown that the V2O5 concentration and the particular regimen used here truly induced a model of chronic vanadium exposure (Avila-Costa et al., Citation2005). There were a total of 112 mice in the vanadium exposure cohort at start of the experiment; initially, five separate runs per day were performed to accommodate this number, decreasing thereafter as the numbers of mice in the cohort were reduced due to need for analyses of endpoints. Control mice (112 also) inhaled only the vehicle—deionized water/Tween—during each exposure.
Tissue Sampling and Preparation
Mice from each group (n = 8/cohort) were sacrificed 24 hr before the first V2O5 or vehicle exposure and, thereafter, 8 controls and 8 exposed mice were sacrificed 24 hr after the second V2O5 exposure of each week until the 12-wk period was over. Each time, the mice were anesthetized with sodium pentobarbital. Blood samples from direct heart puncture were obtained. The spleen was then removed, weighed, and processed for histological examina-tions (i.e., paraffin embedding and staining with Hematoxylin-Eosin for subsequent light micro-scopy evaluation). Images from the spleen of control and exposed animals were captured at 10X and 20X in a BX51 Olympus microscope. All samples were evaluated by two independent observers.
To identify B-lymphocytes in the spleens, immunohistochemical staining for CD-19 (Cell Signaling, Technology Inc., Danvers, MA) was performed on spleen frozen sections (5 μ m thickness) using previously-published protocols (Mussali-Galante et al., Citation2005). Immunoreactivity of the anti-CD19 antibody was visualized by incubation in a solution containing 0.05% 3,3′-diaminobenzidinetetrahydrochloride substrate (Zymed Labs Inc., San Francisco, CA)
Determination of Antibodies to Hepatitis B Surface Antigen (HBsAg)
To evaluate antibody production in the vanadium-exposed animals, the animals were inoculated with a well-defined T-dependent antigen (Woo et al., Citation2006), since these require an adequate B-lymphocyte-T-lymphocyte collaboration to initiate an immune response. Sixteen animals that had completed the 12-wk regimen (8 control and 8 V2O5-exposed mice) were immunized intraperitoneally with 4 μ g recombinant HBsAg (EngerixB, GlaxoSmithKline, México) in FCA (Freund's complete adjuvant). The first two doses were applied at 3-wk intervals and a final dose of the HBsAg without adjuvant was applied intravenously via the tail 5 wk after the second dose. All mice were then sacrificed three days later. Blood from eight non-immunized CD-1 mice (maintained under same conditions as the experimental animals) served as negative-HBsAg controls. Blood and spleen samples were isolated as previously described.
ELISA plates (Maxisorp, NUNC) were incubated with 20 ng recombinant HBsAg/well in 0.05 M carbonate buffer (pH 9.2) for 2 hr at room temperature, followed by an extensive wash with PBS-0.3% Tween 20. The plates were then incubated another 90 min with 1% bovine sera albumin in PBS before another extensive wash was performed. Aliquots (100 μ l) of 1:100, 1:200, 1:400 and 1:800 dilutions of mice sera in PBS-0.1% Tween 20 (PBS-T) were then added to triplicate designated wells and the plates incubated for 2 hr at room temperature. At the end of the incubation, the plates were extensively washed with PBS-T before wells received 100 μ l of a 1:2000 dilution of peroxidase-labeled goat anti-mouse IgG (Sigma) in PBS-T for 1 hr at room temperature. The plates were then extensively washed with PBS-T and 0.05% o-phenylenediamine in 0.1 M citric acid substrate was then added to each well. After 20 min, the reaction was stopped with 3 N HCl and the plates read at 490 nm in a BioTek ELx808 ELISA reader with Gen5 software (BioTek Corp, Winooski, VT).
Avidity of antibody to HBs (HBs-Ab) was determined by the urea method as described by Wilson et al. (Citation2006). Briefly, a Maxisorp ELISA plate was incubated with 20 pg/well of recombinant HBs-Ag (diluted 1:100) as previously noted. After washing the plate, a 1:200 dilution of mice sera in PBS-T were incubated for 90 min before adding 150 μ l of 8 M urea for 10 min; a duplicate plate was made where no urea was added. Both plates were then extensively washed with PBS-T before wells received 100 μ l of a 1:2000 dilution of peroxidase-labeled goat anti-mouse IgG in PBS-T for 1 hr at room temperature; the plates were then processed as above. The avidity results were derived from the formula: 100% × (mean OD of urea-treated wells/mean OD of non-treated wells) and each value represents the percentage of specific antibody bound to the plate after urea treatment. Each serum sample was tested in triplicate.
Statistical Analysis
The results were analyzed by means of a Mann–Whitney U-test and an ANOVA test with a Tukey's post-hoc test (SPSS software, 10.0 release). The difference between control and exposed animals was considered significant at a p-value less than or equal to 0.05.
RESULTS
The V2O5 concentration in the inhalation chamber, based on the average concentration calculated from examination of all the filters from the 24 exposures was 1436 ± 273 μ g/m3. Levels of vanadium in the control systems were consistently below the levels of detection of the system (i.e., < 0.37 ppm).
Animal Health
Eight of the V2O5-exposed and five control mice perished of an apparent ear infection during Wk 5 of the regimen. The infections that led to these deaths in both control and V2O5-exposed mice appeared to be due to animal (male-male) aggression (i.e., ear biting is one of the tactics observed in these actions) that led to subsequent infection and ultimately death. None of the remaining mice in either treatment group showed signs of developing this or any other health condition such as asthma, upper respiratory tract infection, diarrhea. Importantly, no mice died as a direct result of the vanadium exposures.
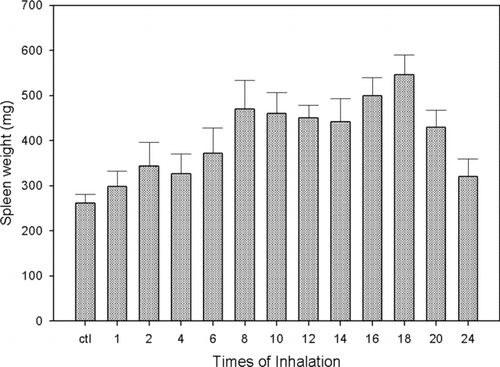
Spleen Weight
There was no difference in the basal versus the final weight of mice spleen in control animals (262 ± 19 mg vs. 298 ± 35 mg at 12 wk; p = 0.28). There was a gradual increase in the mice spleen weight as the number of exposures to vanadium was progressing (274 ± 27 mg to 546 ± 45 mg after 9 wk; p < 0.0001); afterwards the spleen weight started to diminish until it reached a 321 ± 39 mg value at the end of the experimental period (12 wk; p < .001) ().
Spleen Histological Findings
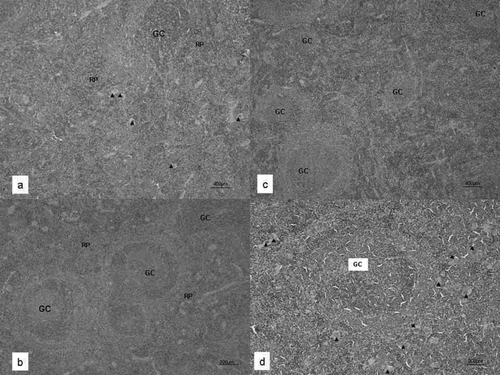
The spleens of control mice contained well-delimited areas of white and the red pulp (). In the white pulp, lymphocytes and well-structured germinal centers with their respective central artery were clearly defined, as were some megakaryocytes (). The spleens of animals exposed to vanadium showed an increased number of non-clearly delimited germinal centers that were larger and contained more lymphocytes than those in control spleens (). These samples also contained an important increase in numbers of megakaryocytes and their red pulp was poorly delimited (). The germinal centers boundaries were well defined in controls as compared to those in the exposed mice. Germinal centers from control spleens contained scanty CD19 B-lymphocytes; interestingly, spleens from vanadium-exposed animals showed an increased number of CD19 cells, with a well-defined location pattern within hyper-plastic germinal nodules ().
FIG. 2 (a) Spleen from control mice in which red (RP) and white pulp were clearly delimited. Megakaryocytes are also identified (arrowhead). (b) The boundaries of the lymphatic nodules with their germinal centers (GC) are well delimited by red pulp (RP). In exposed mice (c) lymphocytes and germinal centers increase in quantity and in size (GC) and red pulp is almost inexistent. (d) Lymphatic nodules increase in size as well as its germinal centers. Megakaryocytes also increase in size and in quantity (arrowhead). Hematoxylin and eosin staining was used throughout.
HBs-Ab Response
The HBs-Ab IgG mean OD value in the sera of non-immunized control animals was 0.070 ± 0.002, whereas the mean value for control HBs-Ag-immunized mice was 0.390 ± 0.030 (p < 0.001). Immunized mice exposed to vanadium had a mean OD value of 0.110 ± 0.050 (p < 0.01 vs. non-exposed animals) ().
TABLE 1 Hepatitis B surface antibody concentration and avidity in vanadium-exposed and control (non-vanadium-treated) CD1 mice
HBsAb Avidity
There was not only a difference in antibody concentration, but also in avidity. The mean OD value of antibodies bound to the plate before and after urea treatment in the control HBsAg-immunized group was 0.752 ± 0.249 and 0.682 ± 0.168, respectively; in contrast, these values were 0.608 ± 0.259 and 0.259 ± 0.128 in the vanadium-exposed mice (latter value significantly different from control at p < 0.003). The mean of the individual avidity values in the control HBsAg-immunized group was calculated to be 86; this value dropped to 40 in mice that had previously been exposed for 12 wk to vanadium (p < 0.0001) (). These results demonstrate that there is quite likely a deficiency in the histologically abnormal spleens of vanadium-exposed mice to induce VDJ recombination(s).
DISCUSSION AND CONCLUSIONS
It is an established fact that certain environmental heavy metals can alter the immune response of laboratory animals and probably humans as well. Both stimulation and suppression of immune responses have been demonstrated in contaminant-exposed animals (Exon, Citation1984). The source of heavy metals exposure is unlimited: paint, insecticides, fungicides, rat poisons, household disinfectants, automobile catalytic converters, diesel exhaust, coal-burning power plants, steel and metal foundries, sewage sludge, industrial wastes, fish, mercury amalgam fillings, and many others. Certain people risk increased exposure because of where they live, their occupation or their lifestyle; others may be genetically predisposed. Nevertheless, the apparent risk-free air pollutants have been linked to serious and life threatening problems such as sudden infant death syndrome (Dales et al., Citation2004).
The effects of inhaled pollutants in the bronchoalveolar tract have been widely analyzed. It has recently been shown that ultra fine particles are able to translocate from the lung into the systemic circulation in hamsters and humans (Nemmar et al., Citation2004). These observations have proven to be of such interest that the effect of inhaled pollutants on immune-related organs is emerging as a new area of research.
Vanadium (V) has become one of the foremost studied elements in the last few years, as its importance as an air pollutant has become increasingly apparent (i.e., see Fortoul et al., Citation2002; Cohen, Citation2004). It enters the organism primarily by inhalation (Brook et al., Citation2004); once entrained it can cause airway diseases in rats that are similar to the pathologies of asthma and bronchitis in humans (Bonner et al., Citation2000). For these reasons, in part, our laboratories have undertaken studies to better understand the toxicologic consequences from repeated exposures to airborne vanadium, as occurs regularly in the citizens exposed to urban atmospheres in many cities, including Mexico City.
Because the route of administration/total numbers of exposures to vanadium can modify experimental outcomes (Avila-Costa et al., Citation2005), we feel it is critical to emphasize that these experiments were done with mice chronically exposed to airborne vanadium. The 0.02 M V2O5 atmospheric concentration was used because previous experiments with lower levels (e.g., 0.005 and 0.01 M) failed to induce any visible histologic damage. Similarly, these earlier studies indicated that clear differences in the extent of tissue damage seemed to be proportional to both the V2O5 concentration used and the number of inhalation exposures to any fixed dose of the agent.
The anatomical changes observed in vanadium-exposed animals were unanticipated. In contrast to the spleen, it was expected that the thymus would be a more preferential target for the toxicity of the inhaled vanadium. Unlike the spleen, the thymus has seemed to be a rather fragile organ; we have observed alterations in the percentage of T-lymphocyte subpopulations after just one V2O5 exposure (data not shown). Other Investigators have seen similar effects on the thymus/ related functions after host exposure to vanadium. For example, the introduction of vanadium into a phosphorus-deficient diet increased the number of CD4+ T-lymphocytes and antibody titer to SRBC (albeit afterwards, there was a diminution of the effect) in chicks (Qureshi et al., Citation1999).
Our results also differ from those of other previous studies. For example, Al-Bayati et al. (1989) showed that while the kidneys in vanadate-injected animals suffered severe dose- and time-dependent histological degenerative and necrotic changes that were accompanied by edema, vascular congestion, and glomerular membrane thickening, spleens of these hosts were normal. Similarly, a recent report by the U.S. National Toxicology Program (NTP, 2002) showed that mice and rats exposed to doubling doses of V2O5 (up to 32 mg V2O5/m3) for 6 hr/d, 5 d/wk, either for 16 d or for 3 mo, had an increase in lung weight as well as lung inflammation and hyperplasia. Interestingly, only rats exposed to the highest levels (i.e., 8 mg/m3) displayed significant effects on their spleens' weights and lymphocytic contents. These same doses had no comparable effects in the co-exposed mice.
Last, it is interesting to note that the mortality rates for the exposed rodents in the NTP study were very high relative to that in our studies (i.e., none died here). It is likely that the variables governing the NTP animals' exposures (i.e., combining the factors of exposure time, frequency, and vanadium concentrations, those rodents seeing ≈ 8 mg V2O5/m3 were ultimately exposed weekly to levels of V2O5≈ 85-fold greater than the mice in our experiments, i.e., 620 μ g [5 regimens] vs. 8.4 μ g [2 regimens]) were a major reason for these differences in mortality. However, this still leaves open the question as to why even with this much greater amount of exposure, these NTP mice failed to display any of the splenic changes that we note here.
We believe that the observed increases in organ weight and the numbers of germinal centers (together with their abnormal structures) are apparently a form of non-antigenic-based toxicity associated with chronic vanadium exposure. This is based, in part, on the fact that vanadium pentoxide has never been recognized to act as an antigen and as such, is not acting to induce a “host humoral response” to the compound itself. Interestingly, the increased amount of CD19+ cells found in the spleens of the chronically V2O5-exposed animals was also not related to antibody production; the concentrations of anti-HBsAg antibody in these mice was shown to be significantly lower in comparison with that in control HBsAg-treated hosts. Based on these two sets of findings, it is becoming increasingly possible that, similar to what is seen with B-lymphocytes from chronic lymphocytic leukemia patients with spleen manifestations, the CD19+ cells of V2O5-exposed mice have a higher expression of CD44 (Bairey et al., Citation2004) thus favoring their spleen “homing” through numerous ligands (Naor et al., Citation2002). Further studies are needed to elucidate the mechanism(s) underlying our observations and, more importantly, the physiological relevance of these (immune) changes in the spleens of the exposed hosts.
Beyond these effects on the spleen, the results of these studies also showed that chronic V2O5 inhalation affected the amounts of high avidity antibodies (i.e., these were greatly diminished) generated in the mice. These outcomes are somewhat akin to those that have been observed in studies examining effects on antibody affinity from exogenous (like tetrachlorodibenzo-p-dioxin; Inouye et al., Citation2003) or intrinsic (i.e., like aging; Han et al., Citation2003) factors. Antibody avidity, also called functional affinity, depends on both antibody affinity and its ability to engage multiple epitopes on the antigen. The former is the result of somatic mutation that is carried out in the germinal centers, mainly of the spleen, although somatic mutations can occur in the absence of germinal centers (Kato et al., Citation1998; Kim et al., Citation2006). It is clear that antibody avidity was severely affected by the histological changes seen in the spleen.
Although the molecular basis of somatic mutation remains elusive, recent discoveries suggest a role for transcription or transcription-related factors (Diaz et al., Citation2001; Peters and Storb, Citation1996). Short-term exposure to vanadium can stimulate activation of multiple transcription factors (Chen et al., Citation1999; Ding et al., Citation1999; Huang et al., Citation2000; Wang et al., Citation2003), whereas the long-term exposure to V2O5 suppresses more than 1000 genes, including 298 genes related to regulation of transcription (Ingram et al., Citation2007), especially interferon regulatory factors. It has been postulated that low-affinity B-lymphocyte clone recruitment and selection for both long-lived plasma cells and memory B-lymphocyte pathways in the germinal center depends on the transcription factor IRF-4 (interferon regulatory factor-4; Benson et al., 2007). IRF-4 is necessary for class switch recombination and the plasma cell differentiation at exit from the germinal center (Cattoretti et al., Citation2006); its absence would impact B-lymphocyte somatic mutation (as our results seem to suggest) and the long-term humoral immune response. Preliminary results seem to support the latter.
In summary, the chronic inhalation of vanadium pentoxide, a frequent component of PM2.5 ambient pollution, induces histological changes and functional damage to the spleen, thus severely affecting the humoral immune response of the exposed host. This could be of particular interest to those who seek to understand the adverse health outcomes or the low response to vaccines (Idel et al., Citation1994) among children with strong environmental exposures to PM2.5.
ACKNOWLEDGMENTS
The authors are grateful to Ms. Veronica Rodriguez and Ms. Judith Reyes Ruiz, for technical assistance. This work was partially supported by grants IN-224906, IN-200606 and IN-225106-2, from DGAPA-UNAM.
REFERENCES
- Ackermann-Liebrich U., Leuenberger P., Schwartz J., Schindler C., Monn C., Bolognini G., Bongard J. P., Brandli O., Domenighetti G., Elsasser S., Grize L., Karrer W., Keller R., Keller-Wossidlo H., Kunzli N., Martin B. W., Medici T. C., Perruchoud A. P., Schoni M. H., Tschopp J. M., Villiger B., Wuthrich B., Zellweger J. P., Zemp E. Lung function and long-term exposure to air pollutants in Switzerland. Study on Air Pollution and Lung Diseases in Adults (SAPALDIA) Team. Am. J. Resp. Crit. Care Med. 1997; 155: 122–129
- Al-Bayati M. A., Giri S. N., Raabe O. G., Rosenblatt L. S., Shifrine M. Time and dose-response study of the effects of vanadate on rats: Morphological and biochemical changes in organs. J. Environ. Pathol. Toxicol. Oncol. 1989; 9: 435–455
- Avila-Costa M. R., Colin-Barenque L., Zepeda-Rodríguez A., Antuna S. B., Saldivar L., Espejel-Maya G., Mussali-Galante P., Avila-Casado M. C., Reyes-Olivera A., Anaya-Martínez V., Fortoul T. I. Ependymal epithelium disruption alter vanadium pentoxide inhalation. A mice experimental model. Neurosci. Lett. 2005; 381: 21–25
- Avila-Costa M. R., Montiel Flores E., Colin-Barenque L., Ordonez J. L., Gutierrez A. L., Nino-Cabrera H. G., Mussali-Galante P., Fortoul T. I. Nigrostriatal modifications after vanadium inhalation: An immunocytochemical and cytological approach. Neurochem. Res. 2004; 29: 1365–1369
- Bairey O., Zimra Y., Rabizadeh E., Shaklai M. Expression of adhesion molecules on leukemic B-cells from chronic lymphocytic leukemia patients with predominantly splenic manifestations. Isr. Med. Assoc. J. 2004; 6: 147–151
- Benson M. J., Erickson L. D., Gleeson M. W., Noelle R. J. Affinity of antigen encounter and other early B-cell signals determine B-cell fate. Curr. Opin. Immunol. 2007; 19: 275–280
- Bonner J. C., Rice A. B., Moomaw C. R., Morgan D. L. Airway fibrosis in rats induced by vanadium pentoxide. Am. J. Physiol. 2000; 278: L209–L216
- Brook J. R., Johnson D., Mamedov A. Determination of source areas contributing to regionally high warm season PM2.5 in eastern North America. J. Air Waste Manag. Assoc 2004; 54: 1162–1169
- Cattoretti G., Shaknovich R., Smith P. M., JÃgck H. M., Murty V. V., Alobeid B. Stages of germinal center transit are defined by B-cell transcription factor coexpression and relative abundance. J. Immunol. 2006; 177: 6930–6939
- Chen F., Demers L. M., Vallyathan V., Ding M., Lu Y., Castranova V., Shi X. Vanadate induction of NF-κ B involves Iκ B kinase-β and SAPK/ERK kinase 1 in macrophages. J. Biol. Chem. 1999; 274: 20307–20312
- Cohen M. D. Pulmonary immunotoxicology of select metals: Aluminum, arsenic, cadmium, chromium, copper, manganese, nickel, vanadium, and zinc. J. Immunotoxicol. 2004; 1: 39–70
- Cohen M. D., Becker S., Devlin R., Schlesinger R. B., Zelikoff J. T. Effects of vanadium upon poly-l:C-induced responses in rat lung and alveolar macrophages. J. Toxicol. Environ. Health 1997; 51: 591–608
- Dales R., Burnett R. T., Smith-Doiron M., Stieb D. M., Brook J. R. Air pollution and sudden infant death syndrome. Pediatrics 2004; 113: e628–631
- Di Gioacchino M., Sabbioni E., Di Giampaolo L., Schiavone C., Di Sciascio M. B., Reale M., Nicola V., Qiao N., Paganelli R., Conti P., Boscolo P. In vitro effects of vanadate on human immune functions. Ann. Clin. Lab. Sci. 2002; 32: 148–154
- Diaz M., Verkoczy L. K., Flajnik M. F., Klinman N. R. Decreased frequency of somatic hypermutation and impaired affinity maturation but intact germinal center formation in mice expressing antisense RNA to DNA polymerase ζ. J. Immunol. 2001; 167: 327–335
- Ding M., Li J. J., Leonard S. S., Ye J. P., Shi X., Colburn N. H., Castranova V., Vallyathan V. Vanadate-induced activation of activator protein-1: Role of reactive oxygen species. Carcinogenesis 1999; 20: 663–668
- Dockery D. W., Pope C. A., 3rd. Acute respiratory effects of particulate air pollution. Annu. Rev. Publ. Health 1994; 15: 107–132
- Dockery D. W., Pope C. A., 3rd, Xu X., Spengler J. D., Ware J. H., Fay M. E., Ferris B. G., Jr, Speizer F. E. An association between air pollution and mortality in six U.S. cities. New Engl. J. Med. 1993; 329: 1753–1759
- Exon J. H. The immunotoxicity of selected environmental chemicals, pesticides and heavy metals. Prog. Clin. Biol. Res. 1984; 161: 355–368
- Fortoul T. I., Osorio L. S., Tovar A. T., Salazar D., Castilla M. E., Olaiz-Fernandez G. Metals in lung tissue from autopsy cases in Mexico City residents: Comparison of cases from the 1950s and the 1980s. Environ. Health Perspect. 1996; 104: 630–632
- Fortoul T. I., Quan-Torres A., Sanchez I., Lopez I. E, Bizarro P., Mendoza M. L., Osorio L. S., Espejel-Maya G., del Avila-Casado M. C., Avila-Costa M. R., Colin-Barenque L., Villanueva D. N., Olaiz-Fernandez G. Vanadium in ambient air: Concentrations in lung tissue from autopsies of Mexico City residents in the 1960s and 1990s. Arch. Environ. Health 2002; 57: 446–449
- Gold D. R., Litonjua A., Schwartz J., Lovett E., Larson A., Nearing B., Allen G., Verrier M., Cherry R., Verrier R. Ambient pollution and heart rate variability. Circulation 2000; 101: 1267–1273
- Gonzalez-Villalva A., Fortoul T. I., Avila-Costa M. R., Pinon-Zarate G., Rodriguez-Lara V., Martinez-Levy G., Rojas-Lemus M., Bizarro-Nevarez P., Diaz-Bech P., Mussali-Galante P., Colin-Barenque L. Thrombocytosis induced in mice after subacute and sub-chronic V2O5 inhalation. Toxicol. Ind. Health 2006; 22: 113–116
- Häggqvista B., Havarinasaba S., Björnb E., Hultman P. The immunosuppressive effect of methylmercury does not preclude development of autoimmunity in genetically susceptible mice. Toxicology 2005; 208: 149–164
- Han S., Yang K., Ozen Z., Peng W., Marinova E., Kelsoe G., Zheng B. Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice. J. Immunol. 2003; 170: 1267–1273
- Huang C., Zhang Z., Ding M., Li J., Ye J., Leonard S. S., Shen H. M., Butterworth L., Lu Y., Costa M., Rojanasaku Y., Castranova V., Vallyathan V., Shi X. Vanadate induces p53 transactivation through hydrogen peroxide and causes apoptosis. J. Biol. Chem. 2000; 275: 32516–32522
- Idel H., Stiller-Winkler R., Malin E. M., Kragmer U. Antibodies to tetanus toxoid in children from areas with different heavy levels of air pollution. Zentr. Hyg. Umweltmed. 1994; 195: 457–462
- Ingram J. I., Antao-Menezes A., Turpin E. A., Wallace D. G., Mangum J. B., Pluta L. J., Thomas R. S., Bonner J. C. Genomic analysis of human lung fibroblasts exposed to vanadium pentoxide to identify candidate genes for occupational bronchitis. Resp. Res. 2007; 8: 34–46
- Inouye K., Ito T., Fujimaki H., Takahashi Y., Takemori T., Pan X., Tohyama C., Nohara K. Suppressive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on the high-affinity antibody response in C57BL/6 mice. Toxicol. Sci. 2003; 74: 315–324
- Ivancsits S., Pilger A., Diem E., Schaffer A., Rüdiger H. W. Vanadate induces DNA strand break in cultured human fibroblasts at doses relevant occupational exposure. Gen. Toxicol. Environ. Mutagen. 2002; 519: 25–35
- Kato J., Motoyama N., Taniuchi I., Takeshita H., Toyoda M., Masuda K., Watanabe T. Affinity maturation in Lyn kinase-deficient mice with defective germinal center formation. J. Immunol. 1998; 160: 4788–4795
- Kim J. H., Kim J., Jang Y. S., Chung G. H. Germinal center-independent affinity maturation in tumor necrosis factor receptor 1-deficient mice. J. Biochem. Mol. Biol. 2006; 39: 586–594
- Laden F., Neas L. M., Dockery D. W., Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ. Health Perspect. 2000; 108: 941–947
- Mussali-Galante P., Rodríguez-Lara V., Hernandez-Tellez B., Ávila-Costa M. R., Colín-Barenque L., Bizarro-Nevarez P., Martínez-Levy G., Rojas-Lemus M., Piñón Zarate G., Saldivar-Osorio L., Diaz-Bech P., Herrera-Enriquez M. A., Tovar-Sánchez E., Fortoul T I. Inhaled vanadium alters γ -tubulin of mouse testes at different exposure times. Toxicol. Ind. Health 2005; 21: 215–222
- Naor D., Nedvetzki S., Golan I., Melnik L., Faitelson Y. CD44 in cancer. Crit. Rev. Clin. Lab. Sci. 2002; 39: 527–579
- Nemmar A., Hoylaerts M. F., Hoet P. H., Nemery B. Possible mechanisms of the cardiovascular effects of inhaled particles: Systemic translocation and pro-thrombotic effects. Toxicol. Lett. 2004; 149: 243–253
- Vanadium in the Environment, J. O. Nriagu. Wiley Interscience Publishers, New York 1998
- NTP. National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Vanadium Pentoxide (CAS No. 1314-62-1) in F344/N Rats and B6C3F1 Mice (inhalation). National Toxicology Program Technical Report Series 2002; 507: 1–343
- Peters A., Storb U. Somatic hyper-mutation of immunoglobulin genes is linked to transcription initiation. Immunity 1996; 4: 57–64
- Peters A., Perz S., Doring A., Stieber J., Koenig W., Wichmann H. E. Increases in heart rate during an air pollution episode. Am. J. Epidemiol. 1999; 150: 1094–1098
- Pope M., Ashley M. J., Ferrence R. The carcinogenic and toxic effects of tobacco smoke: Are women particularly susceptible?. J. Gend. Specif. Med. 1999; 2: 45–51
- Qureshi M. A., Hill C. H., Heggen C. L. Vanadium stimulates immunological responses of chicks. Vet. Immunol. Immunopathol. 1999; 68: 61–71
- Samet J. M., Silbajoris R., Weidong W., Graves L. M. Tyrosine phosphatases as targets in metal-induced signaling in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 1999; 21: 68–76
- Wang Y. Z., Bonner J. C. Mechanism of extracellular signal-regulated kinase (ERK)-1 and ERK-2 activation by vanadium pentoxide in rat pulmonary myofibroblasts. Am. J. Respir. Cell Mol. Biol 2000; 22: 590–596
- Wang Y. Z., Ingram J. L., Walters D. M., Rice A. B., Santos J. H., Van Houten B., Bonner J. C. Vanadium-induced STAT-1 activation in lung myofibroblasts requires H2O2 and p38 MAP kinase. Free Rad. Biol. Med. 2003; 35: 845–855
- Wilson K. M., Di Camillo C., Doughty L., Dax E. M. Humoral immune response to primary rubella virus infection. Clin. Vaccine Immunol. 2006; 13: 380–386
- Woo W. P., Doan T., Herd K. A., Netter H. J., Tindle R. W. Hepatitis B surface antigen vector delivers protective cytotoxic T-lymphocyte responses to disease-relevant foreign epitopes. J. Virol. 2006; 80: 3975–3984