Abstract
Environmental impacts on autoimmunity have significant public health implications. Epidemiological studies have shown associations between exposure to airborne silicates, such as crystalline silica or asbestos, and autoimmunity, but the etiology remains unclear. The purpose of this study was to test the hypothesis that asbestos could lead to a specific pattern of autoantibodies and pathology indicative of systemic autoimmune disease (SAID). Female C57Bl/6 mice were instilled intratracheally with 2 doses × 60 μ g/mouse of amphibole asbestos (tremolite), wollastonite (a non-fibrogenic control fiber), or saline alone. Serum samples were collected and urine was checked for protein bi-weekly for 7 months. By 26 weeks, the asbestos-instilled animals had a significantly higher frequency of positive anti-nuclear antibody (ANA) tests compared to wollastonite and saline groups. The majority of positive ANAs showed homogeneous or combined homogeneous/speckled patterns, and tested positive for antibodies to dsDNA and SSA/Ro 52. Serum isotyping showed no significant changes in IgM, IgA, or IgG subclasses. However, there was an overall decrease in the mean IgG serum concentration in asbestos-instilled mice. IgG immune complex deposition was demonstrated in the kidneys of asbestos-instilled mice, with evidence of glomerular and tubule abnormalities suggestive of glomerulonephritis. Flow cytometry demonstrated moderate changes in the percentages of CD25+ T-suppressor cells and B1a B-cells in the superficial cervical lymph nodes of the asbestos-instilled mice. These data demonstrate that asbestos leads to immunologic changes consistent with the development of autoimmunity. This study provides a non-autoimmune prone murine model for use in future elucidation of mechanisms involved in asbestos-induced autoimmune disease.
Keywords :
INTRODUCTION
Asbestos-related lung disease continues to be a serious and significant problem despite increasing awareness of health hazards of asbestos inhalation. Exposures continue to occur in a variety of occupations, including extraction industries and building construction, particularly heating, insulation, and cement products (Niklinski et al., Citation2004). In addition, environmental exposures have occurred in communities such as Libby MT where asbestos-contaminated vermiculite from the nearby vermiculite mine was used throughout the community (Peipins et al., Citation2003) and shipped to processing plants in many locations throughout the United States. The form of asbestos contamination in the Libby vermiculite has been characterized in the amphibole family of fibers (Meeker et al., Citation2003). This family includes tremolite, crocidolite, winchite and, amosite, and may be more pathogenic than serpentine forms such as chrysotile, possibly due to poor clearance of the straight fibers characteristic of the amphiboles.
Asbestos exposure is associated with various lung diseases, including fibrosis, pleural plaques, and cancer. However, silica and asbestos exposures also appear to exacerbate autoimmune responses. Epidemiological studies have shown strong associations between silica exposure and several autoimmune diseases, including scleroderma (SSc), systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA) (Koeger et al., Citation1995; Steenland and Goldsmith, Citation1995; Powell et al., Citation1999). In addition, increased serum immunoglobulins, positive antinuclear antibody (ANA) tests, and immune complexes have been reported in small cohorts of individuals exposed to asbestos (Lange, Citation1980; Zerva et al., Citation1989; Nigam et al., Citation1993). We have recently demonstrated that the asbestos-exposed population of Libby MT has higher frequencies and titers of positive ANA's, antibodies to extractable nuclear antigens (ENA), and higher levels of serum IgA, compared to a matched reference population (Pfau et al., Citation2005b).
Despite the epidemiologic evidence linking autoimmunity with silica or asbestos exposure, there has been little work done in mouse models to explore possible mechanisms or health implications of the autoimmune responses. However, several studies using autoimmune prone New Zealand Mixed mice (NZM2410) have demonstrated that silica increases the production of autoantibodies, as well as proteinurea and immune complex deposition in the kidneys (Brown et al., Citation2003, Citation2004a), suggesting that silica exposure exacerbated the lupus-like syndrome characteristic of these mice (Rudofsky and Lawrence, Citation1999). Although this is an excellent model for studying environmental impacts on autoimmunity in a genetically susceptible background, the predisposition to autoimmunity in this model makes it difficult to explore mechanisms whereby asbestos could trigger autoimmune responses. This study therefore uses C57Bl/6 mice, which are not considered to be autoimmune-prone (Hudson et al., Citation2003), to test the hypothesis that asbestos can induce autoimmune responses in a genetic background that does not spontaneously express an autoimmune phenotype. This model can therefore be used not only to trace the pathogenesis of the autoimmune responses, but also to explore how asbestos might trigger the response through modified immune regulation.
MATERIALS AND METHODS
Mice
Female 8-week-old C57Bl/6 mice used in these experiments were obtained from a barrier facility at The Jackson Laboratories and were maintained at The University of Montana-Missoula (UM). The UM animal care program has been accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International since 1967. All animal studies were approved by the UM Institutional Animal Care and Use Committee and were conducted in accordance with guidelines established by the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. Mice were housed under specific pathogen-free conditions with a 12-hour light and 12-hour dark cycle, constant temperature, and free access to food and water. A dirty bedding exposure sentinel program was used to ensure exclusion of mouse hepatitis virus, mouse parvovirus, minute virus of mice, Theiler's murine encephalomyelitis virus, epizootic diarrhea of infant mice virus, Syphacia obvelata, Aspicularis tetraptera, and agents of acariasis, including Myobia musculi, Radfordia affinis, Myocoptes musculinus, and Psorergates simplex. Euthanasia was performed by intraperitoneal (IP) injection of a lethal dose of sodium pentobarbital, consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
Treatment of Mice
Saline alone, or either wollastonite (NYCO) or Korean tremolite (NIST) were instilled intratracheally with a total of two doses, each dose being 60 μ g of the fibers sonicated in sterile phosphate-buffered saline (PBS), given 1 wk apart in the first 2 wk of the 7-mo experiment. Thirty weeks after the second instillation, the mice were euthanized by lethal overdose of Nembutol (5 mg/mouse, in 100 μ l IP). For the instillations, a total of 10 mice for each treatment group were anesthetized with ketamine and xylazine IP, and a 1 cm longitudinal incision was made in a shaved area from below the chin. Suspensions of 30 μ l were injected into the trachea via 25-gauge needles, and the incision was closed with 3M Vetbond tissue adhesive. The animals were monitored biweekly for proteinurea and autoantibody production. Blood samples were obtained through saphenous vein bleeds, and then by cardiac puncture after euthanization. Serum was collected from the blood by centrifugation following clotting at room temperature. Serum samples were stored at −20°C until analysis.
Detection of Serum Autoantibodies
ANA were detected by indirect immunofluorescence (IIF) using HEp-2000 cell slides (ImmunoConcepts, Sacramento, CA), following the manufacturer's protocol at a 1:40 screening dilution. Anti-dsDNA antibodies were detected by ELISA kit (Alpha Diagnostics, San Antonio, TX). Sera were diluted 100-fold before assay, and the manufacturer's protocol was followed for procedure and determination of positive vs. negative results. Specific autoimmune targets were identified by immunoblotting on nitrocellulose bound with known nuclear antigens (MarDx Marblot, SLR Research, Carlsbad, CA), according to manufacturer instructions, modified to detect mouse IgG using a goat anti-mouse IgG secondary antibody conjugated to alkaline phosphatase (SouthernBiotech, Birmingham, AL). Briefly, the serum samples were diluted 100-fold and incubated on blocked Marblot strips for 30 min, then washed and developed with the alkaline phosphatase (AP)-conjugated goat anti-mouse IgG secondary antibody. The bands were visualized using the AP substrate solution provided with the kit. Positive reactivity was determined by comparing reactive bands with a positive control. To test the hypothesis that the band at 52 kD was SSA/Ro52 (the 52 kD subunit of SSA, a common target for autoantibodies in patients with Sjogren's Syndrome and SLE), the sera were assayed using an SSA/Ro52 ELISA kit (INOVA Diagnostics, San Diego, CA) modified to detect mouse IgG using goat anti-mouse secondary antibody (Jackson ImmunoResearch, Philadelphia PA).
Serum Immunoglobulin Quantification
Total serum IgG was purifed by Melon Gel IgG purification kits (Pierce, Rockford, IL), and protein was quantified spectrophotometrically using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Quantification of antibody isotypes and subclasses was performed using Beadlyte Mouse Immunoglobulin Isotyping Kit, according to the manufacturer's instructions (Upstate Biotech, Lake Placid, NY). However, because C57Bl/6 mice express IgG2c rather than IgG2a (Martin et al., Citation1998), IgG2c was quantified using a mouse IgG2c-specific ELISA kit (Bethyl Laboratories, Montgomery TX) according to manufacturer's instructions.
Urinary Protein
Proteinuria was measured by Chemstrip 2 GP test strips as described by the manufacturer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Milligram protein per deciliter was measured between groups following the provided scale (0 = negative/trace, 1+ = 30 mg/dl, 2+ = 100 mg/dl, 3+ = 500 mg/dl).
Histology
Animals were euthanized with a lethal I.P. injection of pentobarbitol, and kidneys were removed and stored in Histochoice fixative (Amresco Inc., Solon, IL) until processing using an automated histology processor (ThermoShandon, Pittsburgh, PA). Tissues were embedded in paraffin wax and sectioned 5–7 μ m thick, then collected on poly L-lysine coated slides (Sigma Chemical, St. Louis, MO). Using an automated stainer (ThermoShandon), kidney samples were stained with haematoxylin and eosin for general cellular morphology. Samples were blinded and examined by light microscopy. Kidney sections were boiled in a 0.01M sodium citrate buffer for 10 min followed by washes in distilled water and PBS. The sections were then blocked with 4% fetal bovine serum in PBS. Goat anti-mouse IgG-FITC (ICN Biomedicals, Irvine, CA) and a goat anti-mouse C3-FITC (ICN Biomedicals) were used for the detection of immune complexes and complement deposition, respectively. A goat anti-rat IgG antibody (ICN Biomedicals) was used as an isotype control. Samples were blinded and examined by two readers using a Zeiss fluorescence microscope, and were scored on a scale of 0–4 based on fluorescence intensity and extent of glomerular involvement. Data is presented as the number of mice with positive staining with a score greater than zero.
Flow Cytometry of Lymph Node Cells
Superficial cervical lymph nodes were removed from the euthanized mice, ground between glass slides and washed in PBS. The cell preparations were then stained with anti-mouse antibodies from BD Pharmingen (San Diego, CA). For B-cells: anti-B220-FITC, anti-CD5 (ly-1)-CyChrome, and anti-CD23-PE. For T-cells: anti-CD3-CyChrome, anti-CD4-PE, and anti-CD25-FITC. Following wash steps in staining buffer, the cells were analyzed on a FACS Aria flow cytometer (BD Biosciences, San Jose, CA). All antibodies were used at 1 μ g/106 cells. Appropriate isotype control antibodies from BD Pharmingen were used to set regions for background staining. Cells that were CD23neg or low, B220+, CD5+ were classified as B1a B-cells (Stall et al., Citation1996; Mohan et al., Citation1998). Cells that were CD3+ and CD4+ were classified as T-helper cells, and cells that were CD3+, CD4+ and CD25+ were classified as regulatory T-cells (Sakaguchi et al., Citation1995).
Statistical Analyses
Scale level data analysis involved analysis of variance (ANOVA) followed by Bonferroni's correction for multiple pair-wise mean comparisons. Data are expressed graphically as mean ± SEM. Categorical or nominal level data analysis used chi-square testing for independence with frequency of occurrence in a contingency table. Data are expressed as raw counts within discrete categories. Statistical significance was determined with the probability of Type I error at p < 0.05. Experimental designs with directional hypotheses used one-tailed p-values, whereas designs with non-directional hypotheses used two-tailed p-values. Grubb's test was used to remove one statistical outlier. Sample size (n) refers to the number of mice used in an experimental condition; in some cases only samples from seven mice were available, while in other cases all 10 samples were used for each condition. Individual mice were considered to be independent observations. No biological samples were pooled. Data analysis and graphics were generated by PRISM software (GraphPad; San Diego, CA).
RESULTS
Frequency of Positive ANA Assays in C57Bl/6 Mice Treated with Tremolite Asbestos
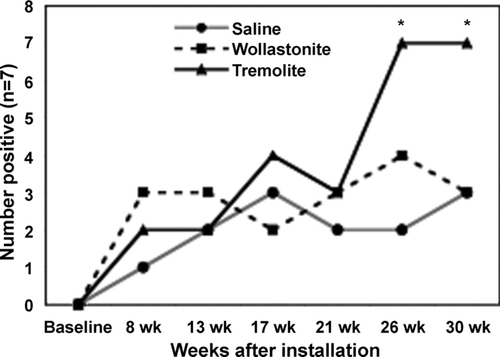
Nuclear antigens are common targets for autoantibodies in systemic autoimmune diseases in humans; therefore, detection of nuclear staining by serum antibodies provides a method to screen for autoantibodies indicative of a systemic autoimmune process. Female C57Bl/6 mice instilled intratracheally with tremolite asbestos developed a higher frequency of positive ANA tests compared to both saline and wollastonite-instilled mice (). Wollastonite, although somewhat inflammatory, is reportedly not fibrogenic (Koskinen, 1997; Maxim, 2005) and is therefore included as a non-fibrogenic control fiber.
FIG. 1 Frequency of positive ANA assays in C57Bl/6 mice treated with tremolite asbestos. At various time points following intratracheal instillation with saline, wollastonite or tremolite serum samples were assayed by IIF on HEp-2000 cells at a 1:40 serum dilution. n = 7 mice/group. *p < 0.05 by ANOVA followed by Bonferroni's correction for multiple pair-wise mean comparisons.
Identification of Autoantibody Antigen Targets
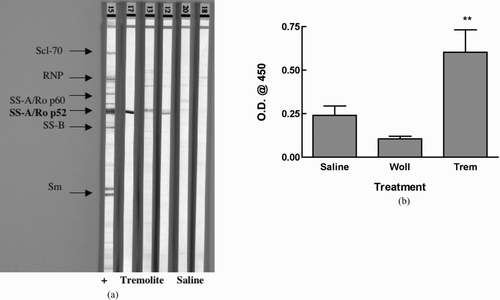
Because the autoantigens are located in various compartments of the nucleus, the pattern of IIF staining gives an initial indication of what the targeted autoantigens are. The most common staining pattern on HEp-2000 cells was homogeneous, often combined with speckled or nucleolar staining (). Measured by ELISA, antibodies to dsDNA, but not histone (not shown), were increased in the tremolite-exposed mice, with 5 of 7 tremolite-instilled mice having anti-dsDNA (). To identify other specific target antigens, the sera were incubated on commercial nitrocellulose strips bound with known nuclear antigens, including Scl-70, RNP, SSA, SSB, Jo-1, and Sm. shows that a possible target for the autoantibodies may be the p52 subunit of SS-A/Ro, further confirmed by ELISA.
FIG. 2 Representative picture of mixed homogeneous/speckled pattern on positive ANA test from tremolite-instilled mouse. Serum samples were assayed by IIF on HEp-2000 cells at a 1:40 serum dilution.
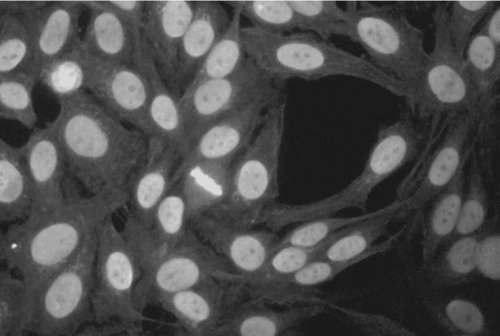
FIG. 3 Antibodies to dsDNA. Serum samples from the saline and tremolite-instilled mice were assayed at 7 months for antibodies to dsDNA by ELISA. Five of the 7 tremolite-instilled mice had antibodies to dsDNA, whereas none of the saline treated animals were positive for anti-dsDNA. Due to the small number of samples analyzed (n = 7), this difference was not statistically significant using two-way ANOVA followed by Bonferroni's correction for multiple pair-wise mean comparisons.
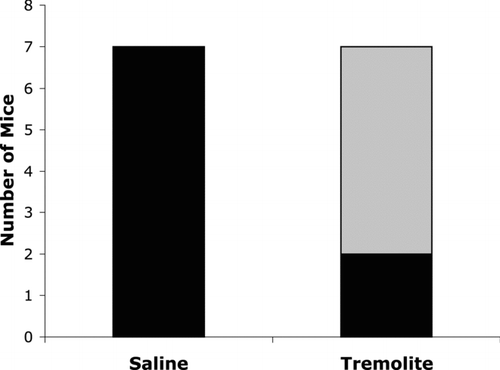
FIG. 4 Asbestos-induced Antibodies to SSA/Ro p52. Serum samples from saline and tremolite instilled mice were assayed for antibodies to various nuclear antigens using A: a line assay Western blot. Lane 1 is the positive control showing all of the antigens; Lanes 2-4 are probed with sera from tremolite-instilled mice; Lane 5-6 are from saline mice. B: Ro52 ELISA. n = 7 mice/group. **p < 0.01 compared to saline control by Dunnett's test. Wollastonite was not significantly different from saline treatment. One outlier in the saline treatment group was removed as determined by Grubb's test.
IgG and Complement (C3) Deposition in Kidneys
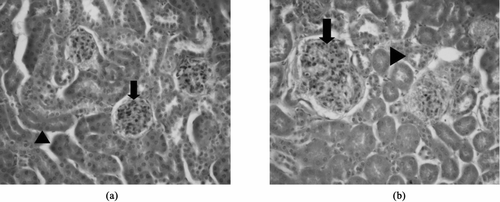
In order to determine whether there was any systemic autoimmune disease process associated with the autoimmune responses seen, urine was monitored for increased proteinurea using Chemstrip 2 GP test strips, but no increased levels of urine protein were detected by this method. Kidneys were subsequently stained for the deposition of IgG and complement C3 as evidence of immune complex deposition. Saline- or wollastonite-instilled mice showed little or no fluorescence staining for either IgG or C3. A representative picture is shown in . The tremolite-instilled mice had bright immunofluorescent staining of mesangial IgG deposits (), as well as C3 staining, also in the glomeruli (not shown). Graphs in show the number of mice staining positively for IgG () or C3 (). Neither saline nor wollastonite led to significant immune complex deposition, but 90% of tremolite-instilled mice had evidence of both IgG and C3 deposition (n = 10). Although none of the mice showed significantly elevated proteinurea seven months after instillation, there was evidence of glomerular damage including proliferation of the mesangium, scattered pycnotic nuclei, and focal glomerular sclerosis. Moderate variation in tubular size and slight cytoplasmic basophilia indicated tubular regeneration. Representative pictures of H&E stained kidneys are shown in .
FIG. 5 Immune complex deposition in kidneys from asbestos-instilled mice. Kidneys were fixed, sectioned, and stained with antibodies to mouse IgG or mouse complement factor C3, followed by a FITC-conjugated secondary antibody. Representative images using 40X objective are shown as A: Saline-instilled mice showed no fluorescence staining, B: Tremolite-instilled mice showed bright fluorescence staining, suggestive of mesangial IgG deposition. Graphs show the number of mice in each group that had positive staining in C: IgG and D: C3. *p < 0.05, ***p < 0.001, n = 10.
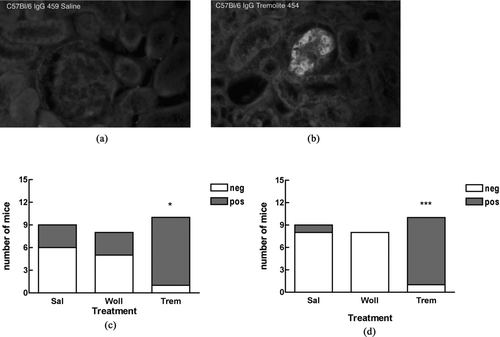
FIG. 6 Glomerular pathology in kidneys from asbestos-instilled mice. Kidneys were fixed, sectioned, and stained with H&E. A: Saline instilled mice had normal glomeruli (arrow), and tubules (head arrow) with no evidence of pathology. B. Tremolite instilled mice showed evidence of glomerular damage including proliferation of mesangial cells (arrow), scattered pycnotic nuclei, and focal glomerular sclerosis. Moderate variation in tubular size and slight cytoplasmic basophilia indicated tubular regeneration (head arrow).
Immunologic Assessment of Draining Lymph Nodes
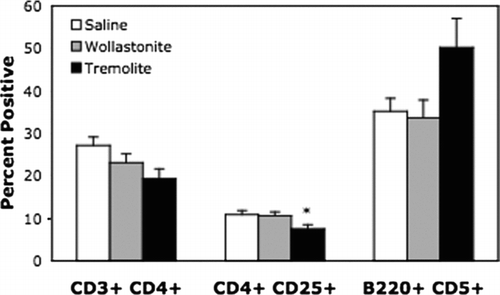
In order to detect any changes in the local immune responses, the size and cell composition of superficial cervical lymph nodes were assessed. Lymph node diameters were significantly increased as well as total cell numbers (), but percentages of total T- and B-cells were not significantly different in the wollastonite or tremolite-instilled mice compared to saline. Interestingly, there was a decrease in the percentage of CD4+ CD25+ T-cells (), which might represent a loss of regulatory T-cells. There was a non-significant increase in the percentage of CD5+ B-cells, which are characterized as B1a Bcells.
FIG. 7 Lymph node diameters and cell count. Superficial cervical lymph nodes from the treated and untreated mice were removed following euthanasia, measured by caliper, and then disaggregated for counting. For diameter, the largest lymph node is reported, and for cell number the total for right- and left-side nodes was counted. *p < 0.05, **p < 0.01, n = 7.
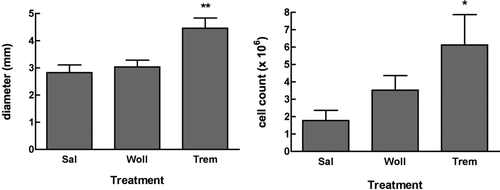
FIG. 8 Lymph node subsets. Superficial cervical lymph nodes were prepared as in , and then stained for flow cytometry as described in the Materials & Methods. CD4+ indicates that the cells were positive for CD3 and CD4 = helper T-cells. CD25+ indicates cells were positive for CD3, CD4 and CD25. CD5+ indicates cells were gated as low positive for CD23, and positive for B220 and CD5. *p < 0.05, n = 7.
Serum Immunoglobulin Isotyping
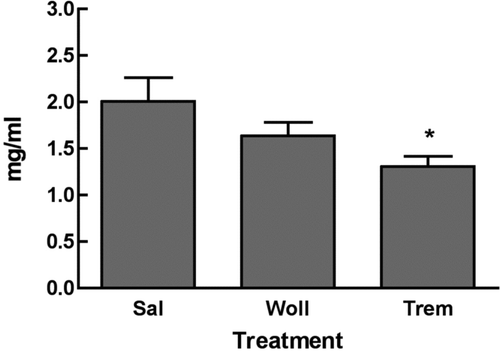
In order to determine whether asbestos led to changes in serum immunoglobulin isotypes, isotypes and the IgG subclasses were measured in the tremolite and saline treated mice as described in Materials and Methods. Total serum IgG was decreased in the tremolite-treated mice (). IgA, IgE, IgM, and relative values for IgG subclasses IgG1, IgG2b, IgG2c, and IgG3 were statistically unchanged (n = 7). In these mice, the mean serum concentrations of IgG subclasses ranged as follows: IgG2c (tremolite = 960 ± 80 μ g/ml, saline = 933 ± 89 μ g/ml) > IgG2b (tremolite = 737 ± 60 μ g/ml, saline = 986 ± 124 μ g/ml) > IgG3 (tremolite = 577 ± 149 μ g/ml, saline = 591 ± 29 μ g/ml) > IgG1 (tremolite = 414 ± 33 μ g/ml, saline = 384 ± 27 μ g/ml).
DISCUSSION
Previous work in this laboratory and by others have suggested that asbestos exposure in humans can lead to autoimmune responses and possibly increase the risk of systemic autoimmune disease (Zerva et al., Citation1989; Nigam et al., Citation1993; Pfau et al., Citation2005b; Noonan et al., Citation2006). This study used C57Bl/6 mice, which are not considered to be autoimmune-prone (Hudson et al., Citation2003), to test the hypothesis that asbestos would lead to autoimmune responses and pathology indicative of systemic autoimmune disease in mice. The results show that tremolite asbestos led to the production of antinuclear antibodies, immune complex deposition in the kidneys, and kidney pathology consistent with this hypothesis. This study therefore provides a non-autoimmune prone murine model for use in future elucidation of mechanisms involved in asbestos-induced autoimmune disease.
C57Bl/6 mice have been characterized as having a primarily TH1 type of immune responsiveness and are susceptible to silica- and asbestos-induced lung fibrosis (Davis et al., Citation1998; Warshamana et al., Citation2002). Studies in our laboratories have demonstrated that tremolite asbestos leads to lung pathology by 6 months after instillation that is indicative of a fibrotic process (Pfau et al., Citation2005a; Putnam et al., 2007). This included localized accumulation of multinucleate giant cells and polymorphonuclear cells, and localized peribroncheolar collagen deposition, seen by trichrome staining. A significant increase (p < 0.01) in the amount of collagen deposition in the lungs at six months after tremolite instillation in C57Bl/6 mice was shown using Lucifer Yellow staining (Putnam et al., 2007).
The asbestos used in this study was a well-characterized sample of tremolite, obtained from the National Institute of Standards and Technology (NIST). This material was chosen because of its availability as a representative of the amphibole family of asbestos fibers, and because early studies described the contaminant of the Libby, MT vermiculite as tremolite or actinolite (Bassett, Citation1959). However, newer studies have reclassified the material as a mixture of amphiboles of which tremolite is only a single component (Meeker et al., Citation2003). We have therefore initiated a new study in this mouse model using a sample of the Libby amphibole mixture provided by the U.S. Geological Survey, in which both fibrotic and autoimmune processes can be assessed.
These data show that tremolite induced the production of antinuclear antibodies. The low level of positive ANA tests in the saline and wollastonite treated animals may have resulted from the low screening dilution used, may simply mirror the increase in low titer ANAs seen in aging mammals, or may be a response to the surgical instillation procedure. While not ideal, the intratracheal instillation is a well-defined model for particulate/fiber instillation in the absence of an inhalation facility (Brown et al., Citation2003; Gharaee-Kermani et al., Citation2005; Fattman et al., Citation2006). In future studies, it will nevertheless be interesting to compare this model to inhalation exposure in terms of mechanisms of fibrosis and autoimmunity.
The results suggest that the dominant target antigens in this model may be dsDNA and the p52 subunit of the SSA/Ro antigen (Ro52). The combination of these two specificities may explain the homogeneous and speckled staining patterns seen on IIF. Autoantibodies to dsDNA are very common in SLE and are often associated with glomerular immune complex deposition and lupus nephritis, although the mechanisms are unclear (Bagavant et al., Citation2004). In this model, tremolite asbestos also led to immune complex deposition and kidney pathology, suggestive of mild autoimmune glomerulonephritis. Further studies will be necessary to define by electron microscopy the location of the complexes, the extent of the glomerular lesions, and to establish a time-line for development of glomerulonephritis in this model. Our finding of specific antibodies to Ro52 in the sera from tremolite-treated mice is interesting as these antibodies are also found in SLE and other systemic autoimmune diseases (Peene et al., Citation2002). In addition, the Ro52 protein may play a role in T-cell activation (Ishii et al., Citation2003). Further characterization of the specific target antigens in asbestos-induced autoantibodies in this model is needed, as this may provide clues regarding the mechanisms of lost tolerance to self antigens.
All autoantibodies were detected using secondary antibodies to mouse IgG, demonstrating that the autoantibodies are primarily of IgG isotype, consistent with an antigen-driven, T-cell-dependent immune response. However, since there were not changes in the relative serum frequencies of each of the IgG subclasses, it remains unclear whether any particular B-cell subclass or T-helper cell predominance is involved in the autoimmune response. IgG2c and IgG2b were the predominant subclasses in the sera of C57Bl/6 mice, with and without treatment, which is consistent with the tendency of these mice to show a TH10phenotype (Martin et al., Citation1998). Both of these antibody subclasses actively bind complement and therefore can be considered potentially pathogenic antibodies (Karachunski et al., Citation2000; Latham et al., Citation2003).
Local lymph nodes were examined to determine whether asbestos altered the cell numbers or relative frequencies of lymphocyte subsets. Although the lymph nodes were enlarged and had increased total numbers of cells, the relative frequencies of total T- and B-cells, or CD4+ T-cells, were not significantly altered. An increase in the percentage of CD5+ B-cells was observed in the superficial cervical lodes of asbestos-treated mice, although this was not statistically significant. It will be an interesting cell type to follow in this model, since these CD5+ (B1a) B-cells are characterized as being more active antigen presenting cells and may play a role in loss of tolerance in some autoimmune models (Mohan et al., Citation1998; Youinou and Lydyard, Citation2001). Their exact role remains unclear, as does their functional location and ontogeny; however, there is some evidence that, in lymph node germinal centers, CD5+ B-cells express recombination activating genes (RAG), which could lead to the production of high-affinity, pathogenic autoantibodies (Hillion et al., Citation2005; Morbach et al., Citation2006). Their presence in this model of asbestos-induced autoimmunity could indicate that activation and/or recruitment of CD5+ B-cells in lymph nodes may have a mechanistic role either in self-antigen presentation or in the maturation of self-reactive immunoglobulin.
There was also an apparent decrease in the percentage of CD4+ CD25+ T-cells, which have previously been described as regulatory or suppressor T-cells. These cells have been implicated in the regulation of certain autoimmune responses (Shevach, Citation2000; Chatenoud et al., Citation2001), and were decreased in the lymph nodes of lupus prone NZM mice following intratracheal instillation of silica, concurrent with significant exacerbation of the lupus-like phenotype of these mice (Brown et al., Citation2003). More recently, however, it has become more accepted to include measurement of the transcription factor, FoxP3, in the phenotyping of suppressor T-cells since the CD4+ CD25+ characteristic can include other types of T-cells. In addition, it remains unclear whether suppressor T-cells play a role in regulation of lupus-glomerulonephritis, or only in certain organ-specific autoimmune diseases (Bagavant et al., Citation2004). In view of these issues, the significance of our observed change in the percentage of CD4+ CD25+ T-cells needs further clarification and characterization of the lymph node T-cells in this model.
These data clearly demonstrate asbestos-induced autoimmune responses and initiation of a systemic autoimmune disease process in C57Bl/6 female mice, and opens up many directions for studies to elucidate the mechanism by which these responses occur. It will be important to characterize specific antigen targets being recognized by the autoantibodies to find clues to the mechanism of lost tolerance. Based on many studies linking apoptosis to autoimmune responses (Casciola-Rosen et al., Citation1994; Huggins et al., Citation1999; van Nieuwenhuijze et al., Citation2003; Hall et al., Citation2004), we have hypothesized that silica-associated autoimmunity may be triggered through apoptotic events that expose new or modified epitopes that become antigenic (Brown et al., Citation2004b; Pfau et al., Citation2004). It is possible that there is a similar mechanism with asbestos since it has also been reported to cause apoptosis by defined pathways (Hamilton et al., Citation1996; Shukla et al., Citation2003). It may therefore be revealing to compare the autoimmune responses in this model to both silica and asbestos. Exploration of the specific cellular events within the exposed lung is ongoing in our laboratory. Finally, further exploration of the lymphocyte and dendritic cell population changes in local lymph nodes as well as systemically will lead to a better understanding of the overall immune dysfunction leading to asbestos-induced autoimmunity.
ACKNOWLEDGMENT
This work was supported by CDC Grant #CCR822091-01, NIH R01/ES04804-08, and NCRR P20 RR-017670. We gratefully acknowledge the statistical analyses performed by Raymond Hamilton, Jr., CEHS Biostatistician.
Dr. Brown is currently at The National Institute of Allergy & Infectious Diseases, NIH, Bethesda, MD.
REFERENCES
- Bagavant H., Deshmukh U. S., Gaskin F., Fu S. M. Lupus glomerulonephritis revisited 2004: Autoimmunity and end-organ damage. Scand. J. Immunol. 2004; 60: 52–63
- Bassett W. A. The origin of the vermiculite deposit at Libby, Montana. Am. Mineralogist 1959; 44: 282–299
- Brown J. M., Archer A. J., Pfau J. C., Holian A. Silica accelerated systemic autoimmune disease in lupus-prone New Zealand mixed mice. Clin. Exp. Immunol. 2003; 131: 415–421
- Brown J. M., Pfau J. C., Holian A. Immunoglobulin and lymphocyte responses following silica exposure in New Zealand mixed mice. Inhal. Toxicol. 2004a; 16: 133–139
- Brown J. M., Pfau J. C., Pershouse M. A., Holian A. Silica, apoptosis, and autoimmunity. J. Immunotoxicol. 2004b; 1: 177–187
- Casciola-Rosen L. A., Anhalt G., Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 1994; 179: 1317–1330
- Chatenoud L., Salomon B., Bluestone J. A. Suppressor T-cells–they're back and critical for regulation of autoimmunity!. Immunol. Rev. 2001; 182: 149–163
- Davis G. S., Leslie K. O., Hemenway D. R. Silicosis in mice: Effects of dose, time, and genetic strain. J. Environ. Pathol. Toxicol. Oncol. 1998; 17: 81–97
- Fattman C. L., Tan R. J., Tobolewski J. M., Oury T. D. Increased sensitivity to asbestos-induced lung injury in mice lacking extracellular superoxide dismutase. Free Rad. Biol. Med. 2006; 40: 601–607
- Gharaee-Kermani M., Ullenbruch M., Phan S. H. Animal models of pulmonary fibrosis. Meth. Mol. Med. 2005; 117: 251–259
- Hall J. C., Casciola-Rosen L., Rosen A. Altered structure of autoantigens during apoptosis. Rheum. Dis. Clin. North Am. 2004; 30: 455–471, vii
- Hamilton R. F., Iyer L. L., Holian A. Asbestos induces apoptosis in human alveolar macrophages. Am. J. Physiol 1996; 271(5)L813–L819, Pt 1
- Hillion S., Rochas C., Youinou P., Jamin C. Expression and re-expression of recombination activating genes: Relevance to the development of autoimmune states. Ann. N. Y. Acad. Sci. 2005; 1050: 10–18
- Hudson C. A., Cao L., Kasten-Jolly J., Kirkwood J. N., Lawrence D. A. Susceptibility of lupus-prone NZM mouse strains to lead exacerbation of systemic lupus erythematosus symptoms. J. Toxicol. Environ. Health A 2003; 66: 895–918
- Huggins M. L., Todd I., Cavers M. A., Pavuluri S. R., Tighe P. J., Powell R. J. Antibodies from systemic lupus erythematosus (SLE) sera define differential release of autoantigens from cell lines undergoing apoptosis. Clin. Exp. Immunol. 1999; 118: 322–328
- Ishii T., Ohnuma K., Murakami A., Takasawa N., Yamochi T., Iwata S., Uchiyama M., Dang N. H., Tanaka H., Morimoto C. SS-A/Ro52, an autoantigen involved in CD28-mediated IL-2 production. J. Immunol. 2003; 170: 3653–3661
- Karachunski P. I., Ostlie N. S., Monfardini C., Conti-Fine B. M. Absence of IFN-γ or IL-12 has different effects on experimental myasthenia gravis in C57BL/6 mice. J. Immunol. 2000; 164: 5236–5244
- Koeger A. C., Lang T., Alcaix D., Milleron B., Rozenberg S., Chaibi P., Arnaud J., Mayaud C., Camus J. P., Bourgeois P. Silica-associated connective tissue disease. A study of 24 cases. Medicine (Baltimore) 1995; 74: 221–237
- Lange A. An epidemiological survey of immunological abnormalities in asbestos workers. II. Serum immunoglobulin levels. Environ. Res. 1980; 22: 176–183
- Latham K. A., Zamora A., Drought H., Subramanian S., Matejuk A., Offner H., Rosloniec E. F. Estradiol treatment redirects the isotype of the autoantibody response and prevents the development of autoimmune arthritis. J. Immunol. 2003; 171: 5820–5827
- Martin R. M., Brady J. L., Lew A. M. The need for IgG2c-specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Meth. 1998; 212: 187–192
- Meeker G. P., Bern A. M., Brownfield I. K., Lowers H. A., Sutley S. J., Hoefen T. M., Vance J. S. The composition and morphology of amphiboles from the Rainy Creek Complex, near Libby, Montana. Am. Mineralogist 2003; 88: 1955–1969
- Mohan C., Morel L., Yang P., Wakeland E. K. Accumulation of splenic B1a cells with potent antigen-presenting capability in NZM2410 lupus-prone mice. Arthritis Rheum. 1998; 41: 1652–1662
- Morbach H., Singh S. K., Faber C., Lipsky P. E., Girschick H. H. J. Analysis of RAG expression by peripheral blood CD5+ and CD5- B-cells of patients with childhood systemic lupus erythematosus. Ann. Rheum. Dis. 2006; 65: 482–487
- Nigam S. K., Suthar A. M., Patel M. M., Karnik A. B., Dave S. K., Kashyap S. K., Venkaiah K. Humoral immunological profile of workers exposed to asbestos in asbestos mines. Indian J. Med. Res. 1993; 98: 274–277
- Niklinski J., Niklinska W., Chyczewska E., Laudanski J., Naumnik W., Chyczewski L., Pluygers E. The epidemiology of asbestos-related diseases. Lung Cancer 2004; 45(Suppl 1)S7–S15
- Noonan C. W., Pfau J. C., Spence M., Larson T. C. Nested case-control study of autoimmune disease in an asbestos-exposed population. Environ. Health Perspect. 2006, On-line ehp.9203
- Peene I., Meheus L., De Keyser S., Humbel R., Veys E. M., De Keyser F. Anti-Ro52 reactivity is an independent and additional serum marker in connective tissue disease. Ann. Rheum. Dis. 2002; 61: 929–933
- Peipins L. A., Lewin M., Campolucci S., Lybarger J. A., Miller A., Middleton D., Weis C., Spence M., Black B., Kapil V. Radiographic abnormalities and exposure to asbestos-contaminated vermiculite in the community of Libby, Montana, USA. Environ. Health Perspect. 2003; 111: 1753–1759
- Pfau J. C., Brown J. M., Holian A. Silica-exposed mice generate autoantibodies to apoptotic cells. Toxicology 2004; 195: 167–176
- Pfau J. C., Sentissi J. J., Brown J. M., Thompson R. Asbestos-induced autoimmunity in C57BL/6 mice. The Toxicologist 2005a; 79: 186
- Pfau J. C., Sentissi J. J., Weller G., Putnam E. A. Assessment of autoimmune responses associated with asbestos exposure in Libby, Montana, USA. Environ. Health Perspect. 2005b; 113: 25–30
- Powell J. J., Van de Water J., Gershwin M. E. Evidence for the role of environmental agents in the initiation or progression of autoimmune conditions. Environ. Health Perspect. 1999; 107(Suppl 5)667–672
- Putnam E. A., Smartt A., Brezinski M., Groves A., Schwanke C. M., Pershouse M. A. Histologic and gene expression changes after amphibole exposures in a mouse model. J. Immunotoxicol. 2008, In Press
- Rudofsky U. H., Lawrence D. A. New Zealand mixed mice: A genetic systemic lupus erythematosus model for assessing environmental effects. Environ. Health Perspect 1999; 107(Suppl 5)713–721
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T-cells expressing IL-2 receptor α -chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995; 155: 1151–1164
- Shevach E. M. Regulatory T-cells in autoimmmunity. Annu. Rev. Immunol. 2000; 18: 423–449
- Shukla A., Stern M., Lounsbury K. M., Flanders T., Mossman B. T. Asbestos-induced apoptosis is protein kinase C delta-dependent. Am. J. Respir. Cell Mol. Biol. 2003; 29: 198–205
- Stall A. M., Wells S. M., Lam K. P. B-1 cells: Unique origins and functions. Sem. Immunol. 1996; 8: 45–59
- Steenland K., Goldsmith D. F. Silica exposure and autoimmune diseases. Am. J. Ind. Med. 1995; 28: 603–608
- van Nieuwenhuijze A. E., van Lopik T., Smeenk R. J., Aarden L. A. Time between onset of apoptosis and release of nucleosomes from apoptotic cells: Putative implications for systemic lupus erythematosus. Ann. Rheum. Dis. 2003; 62: 10–14
- Warshamana G. S., Pociask D. A., Sime P., Schwartz D. A., Brody A. R. Susceptibility to asbestos-induced and transforming growth factor-beta1-induced fibroproliferative lung disease in two strains of mice. Am. J. Respir. Cell Mol. Biol. 2002; 27: 705–713
- Youinou P., Lydyard P. M. CD5+ B-cells in nonorgan-specific autoimmune diseases: A fresh look. Lupus 2001; 10: 523–525
- Zerva L. V., Constantopoulos S. H., Moutsopoulos H. M. Humoral immunity alterations after environmental asbestos exposure. Respiration 1989; 55: 237–241