Abstract
Risk of developing certain diseases correlates with human personality. Cardiologists have defined Type “A” personalities as coronary-prone. Associated psychological peculiarities are easily angered, competitive, impatient and hard-driving. Psychologically-opposite people who are prone to suppress emotions and avoid conflicts (Type “C”), have a high risk of infectious diseases and certain forms of cancer. The development of contemporary biology and medicine determined an important role of the neuroendocrine and immune systems in these correlations. The peculiarity of human personality, as much as of animal behavioral patterns, is strongly expressed under stress conditions. The strategies of stress coping display a normal distribution in the human and wild animal populations, with truly passive and active coping styles located at the outermost regions of the curve. However, there are a number of strategies to breed experimental animals with extreme coping styles; animals selected for a passive coping style to acute stress show marked activation of the hypothalamic-pituitary-adrenal (HPA) axis and low stimulation of the sympathetic-adrenal system; both are associated with immunosuppression. An opposite reaction of the neuroendocrine system has been shown in animals with an active coping style to stress; this was associated with the signs of immunostimulation. Similarly, people with passive coping style (type “C”) might be at higher risk of infectious diseases and cancer, while people with active coping style (type “A”) might be predisposed to coronary, allergic, and autoimmune diseases. Furthermore, pain, decreased productivity, and anxiety, all common in patients with different diseases, are additional stressful entities. Thus, an adequate coping with a disease is an important approach to improve life quality and disease prognosis. Therefore, psychological and psychopharmacotherapeutic interventions that enhance effective coping should have beneficial effects in patients with immune-mediated diseases.
PERSONALITY AND COPING STYLES
It is well known from the time of Hippocrates that the risk of development of certain diseases correlates with the type of human personality. In this connection, Hippocrates marked out four types of temperament: melancholic, choleric, phlegmatic, and sanguine. Half a millennium later, Galenos picked out the melancholicus as being prone to cancer. Later, people with behavior patterns such as denial, suppression of emotions (anger primarily), avoidance of conflicts, social desirability, harmonizing behavior, high rationality were shown to have a high risk of infectious diseases, certain forms of cancer and they were termed type “C” (Baltrusch et al., Citation1991). In contrast, a Type “A” personality has been defined by cardiologists as coronary-prone. Psychologically, people of this type are hostile, easily angered, competitive, impatient and hard driving (Friedman et al., Citation1975; Irvine et al., Citation1982). The correlation between the people personality and their susceptibility to certain disorders has been supported by contemporary data on constitutional neurochemistry, neuroendocrinology, and neuroimmunology.
The development of contemporary biology and medicine allowed the characterization of the molecular mechanisms involved in the regulation of susceptibility of people with different personality types to a number of diseases and determined an important role of the neuroendocrine and immune systems in this relationship. Specific biological feature of type “A” people is the high sympathetic system activity caused, in part, by the elevated sensitivity of β-adrenoreceptors (Catipovič-Veselica et al., Citation1997; Le Melledo et al., 2001) and increased blood catecholamine levels (Friedman et al., Citation1975). High urine testosterone is also typical for type “A” people (Zumoff et al., Citation1984).
The specificity of human personality, as well as animal behavioral patterns, is strongly expressed under the stress conditions. There are plenty of behavioral and psychological methods to evaluate the stress coping strategy, i.e., coping style, in human and animals. These styles are distinguished as flight/fight, repressive/defensive, submissive/aggressive, anger-in/anger-out, blunting/monitoring, negative/positive, etc. (Jamner at al., Citation1988; De Boer et al., Citation1990; Zozulya et al., Citation1996; Devoino et al., Citation2003; Veenema et al., Citation2004). In general, coping styles might be grouped into “passive” and “active” patterns. The strategies of stress coping display normal distribution in the overall human and wild animal populations, with truly “passive” and “active” coping styles located at the outermost regions of the curve. In order to study the biological pattern of the phenomenon, researchers usually select animals with extreme coping styles by their behavior under the different stress conditions.
Coping Styles and Neuroimmunoendocrinology
Using a number of behavioral models, it has been shown that coping style determines, to a large degree, the reaction of the neuroendocrine system to stress. For instance, animals selected for the “passive” coping style (in the “open field,” “shuttle-box” or some zoo social models) show marked activation of the hypothalamic-pituitary-adrenal axis (HPA) and low stimulation of the sympathetic adrenal system (SAS) in response to acute stress (). This has been demonstrated by analyzing the levels of adrenocorticotropic hormone (ACTH), corticosterone and norepinephrine in the blood of animals under the stress conditions. It should be noted that under the rest conditions, basal levels of corticosterone in “passive” animals might be lower as compared to the levels in “active” animals. However, acute stress induces more pronounced rise of the hormone level, which is accompanied by rapid exhaustion of HPA in “passive” animals (De Boer et al., Citation1990; Seredenin, Citation2003; Steimer and Driscoll, Citation2003; Veenema et al., Citation2004; Frank et al., Citation2006). An opposite reaction of the HPA and SAS systems has been demonstrated in animals with the “active” coping style. They show relatively low release of ACTH and corticosterone, but with high levels of catecholamines in the blood following acute stressful stimulus (; De Boer et al., 1990; Sgoifo et al., 1996).
TABLE 1 The reaction of the HPA-axis and SAS to acute experimental stress in animals selected to “active” (A) and “passive” (P) coping styles by different behavioral patterns
The stress reaction of the immune system has being studied since the past century. In many experimental models it was shown that, as a rule, acute stress induced some signs of immune stimulation, while chronic stress induced immunosuppression (Korneva and Shkhinek, Citation1989; Shurin et al., Citation1994). A meta-analytic study of 30-year inquiry permitted Segerstrom and Miller (Citation2004) to pick out different stages of the human immune system changes under the psychological stress. Acute stressors (lasting minutes) were associated with potentially adaptive up-regulation of natural immunity and down-regulation of adaptive immune responses. Brief psychological stressors (such as an exam) tended to suppress cellular immunity while preserving humoral immunity. Chronic stressors were associated with suppression of both cellular and humoral immune responses. One might forecast that stress perception depends on the human personality, stress coping style, which, as has been shown above, might determine the neuroendocrine stress reaction.
Neuroendocrine immunomodulation is achieved through both autonomic innervation of immune system organs and hormonal receptors on immune cells (Sternberg, Citation2006). For example, dendritic cells, which are the key regulators of innate and adaptive immunity, bear receptors to biogenic amines (i.e., α1A, β1, β2-adrenoreceptors (Maestroni, Citation2005), 5-HT-receptors (Idzko et al., Citation2004)), steroids (i.e., glucocorticoid receptors) (Bellinghausen et al., Citation2001; Freeman et al., Citation2005), and to regulatory peptides (i.e. δ-, μ-, κ-opioid receptors) (Eshe et al., Citation1999; Kirst et al., Citation2002). Therefore, the balance between the levels of different stress-realizing hormones should be important for the degree and even direction of the immunomodulative stress reaction.
Animal Studies
It would be important now to analyze the associations between the neuroendocrine reaction to stress in human and animals with “passive” and “active” stress coping style and the stress-reaction of the immune system. We have shown that under the stress conditions Wistar rats with “passive” strategy in “AutoTrack System” have low proliferative responses of blood lymphocytes to the mitogen Conconavalin A as compared to “active” ones. It should be mentioned that treatment with a synthetic analog of the opioid peptide Leu-enkephalin (dalargin) normalized this stress reaction, meaning that lymphocyte proliferation was heightened in “passive” animals and decreased in “active” rats (Zozulya et al., Citation1996). This fact provides additional support for a homeostatic role of regulatory peptides under stress conditions. Similarly, low-active in “open field” Lewis rats have lower mitogen-induced interleukin (IL)-10 and interferon (IFN)-γproduction by splenocytes (Sajti et al., Citation2004). It is well known that Balb/c mice display “passive” behavior in “open field” and have lower humoral and higher cellular immune responses than the “active” in “open field” C57Bl/6 mice (Seredenin, Citation2003). Also, the exploratory behavior of (CBA × C57Bl/6)F1 mice in “open field” correlates with a delayed-type hypersensitivity, proliferative activity of immune cells, and expression of the IL-1β type I IL-1 receptor, and erythropoietin receptor genes in brain cells (Markova et al., Citation2000, Citation2004).
Aggression is another behavioral pattern to select the strategy of stress coping in animals. It has been shown that low-aggressive (“passive” strategy) wild house mice have faster thymus involution under defeat stress (Veenema et al., Citation2004). Moreover, acute stress induces immune stimulation in “aggressive” and immunosuppression in “submissive” C57Bl/6J mice (Devoino et al., Citation2003).
Human Studies
An interconnection of personality, coping style and immune status has been also demonstrated in human. The “passive” coping style is associated with decreased monocyte numbers, elevated eosinophile counts (Jamner et al., Citation1988), and a stress-induced decrease in T-helper lymphocytes (Sakami et al., Citation2004). The type of character accentuation relates to humoral immunity (Zabrodskii and Timofeev, Citation1997; Abramov et al., Citation2001). The personality tested by MMPI is connected to NK activity and the ratio of T-lymphocyte subpopulations (Biondi et al., Citation1994). High introversion, together with high neuroticism, which is typical for a melancholic temperament (Eysenck, 1967) and for “passive” coping style, is associated with low IFN production by blood lymphocytes (Surkina et al., Citation2001).
Personality, Coping Style and Immune-Mediated Diseases
Thus, the “passive” acute stress coping style exhibits marked activation of HPA axis and low stimulation of SAS, both associated with signs of immunosuppression. This type of personality in people is a risk factor for infectious diseases and some forms of cancer (Jamner et al., Citation1988; Baltrusch et al., Citation1991; Sakami et al., Citation2004). An opposite reaction of the HPA and SAS systems to stress has been demonstrated in animals with an “active” stress coping style. It was associated with up-regulated function of the immune system, as well. Depletion of SAS on a background of low cortisol under chronic stress might however induce hyper activation of the immune system in people with “active” coping style. This can raise the risk of inflammatory and autoimmune diseases, including arthritis, systemic lupus erythematosus, allergy, asthma, atopic dermatitis, etc. (Wilder, Citation2002; Marques-Deak et al., Citation2005).
In fact, particular features of temperament and behavior have been found in children with allergy and asthma in a pre-morbid period (Kim et al., Citation1980; Lilljeqvist et al., Citation2002; Stevenson et al., Citation2003). High liability was shown to be typical for adults with atopic dermatitis (Scheich et al., Citation1993). One study, performed with a large sampling (n = 11,540), has shown that high extra-version is a risk factor for asthma in women (Huovinen et al., Citation2001). A reduced activity of the HPA axis in patients predisposed to allergic diseases was confirmed by low basal and stress-induced blood cortisol levels (Buske-Kirschbaum et al., Citation2003) and saliva (Ball et al., Citation2006). A peculiarity of the SAS function has been shown in children with asthma (Miller and Chen, Citation2006) and patients with systemic lupus erythematosus (Pawlak et al, Citation1999). A high basal level of β2-receptors expression was detected in lymphocytes; a reduction of this parameter was more pronounced in above-mentioned patients under the psychoemotional stress as compared to healthy volunteers. Moreover, the immunomodulating effect of adrenalin in patients with rheumatoid arthritis differed from that in healthy people. This was shown during the analyses of levels of NK cell activity, T-lymphocyte subpopulations, and pro-/anti-inflammatory cytokine production in these populations (Kittner et al., Citation2002).
CONCLUSIONS
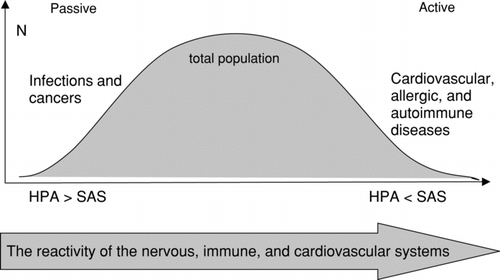
Thus, the reactivity of the nervous system to stress determines, to certain degree, a stress reaction of other systems such as the cardiovascular and the immune system (). This reaction is mediated by the neuroendocrine system, partly by the ratio of HPA axis and SAS activity. As a result, people with a “passive” coping style (type “C”) might be under a higher risk of infectious diseases and cancer, while people with an “active” coping style (type “A”) might be predisposed to coronary, allergic and autoimmune diseases.
FIG. 1 Personality, coping style and predisposition to immune-mediated diseases. HPA = hypothalamic-pituitary-adrenal axis; SAS = sympathetic-adrenal system.
Furthermore, symptoms such as pain, decreased productivity, and anxiety, which are common in patients with different diseases, comprise additional stressful entities. Thus, adequate and efficient coping with the disease is an important approach to improve the life quality of patients and the disease prognosis. For instance, the global health-related quality of life positively correlates with an “active” coping style in patients with different types of cancer (Zhou et al., Citation2005). Psychological and psycho-pharmacotherapeutic interventions that enhance an effective coping have shown beneficial effects in cancer patients (Reiche et al., Citation2005). Moreover, one could suggest that the stress of environment pollution would induce different immune-mediated disease development in persons with different personalities.
Since the coping style is the result of the interplay between an innate predisposition and environmental and social factors, it can be corrected. Psychological and psychopharmacological correction of a particular coping style may be beneficial for both decreasing the susceptibility to immune-mediated diseases and optimizing the efficacy of immunotherapy.
REFERENCES
- Abramov V. V., Markova E. V., Poveshchenko A. F., Gontova I. A., Yakushenko E. V., Korotkova N. A., Abramova T. Y., Kozlov V. A. The interdependence of behavior and immunity: Possible mechanisms and significance. Russ. J. Immunol. 2001; 6: 215–220
- Ball T. M., Anderson D., Minto J., Halonen M. Cortisol circadian rhythms and stress responses in infants at risk of allergic disease. J. Allergy Clin. Immunol. 2006; 117: 306–311
- Baltrusch H. J., Stangel W., Titze I. Stress, cancer and immunity. New developments in biopsychosocial and psychoneuroimmunologic research. Acta Neurol. (Napoli) 1991; 13: 315–327
- Bellinghausen I., Brand U., Steinbrink K., Enk A. H., Knop J., Saloga J. Inhibition of human allergic T-cell responses by IL-10-treated dendritic cells: Differences from hydrocortisone-treated dendritic cells. J. Allergy Clin. Immunol. 2001; 108: 242–249
- Biondi M., Peronti M., Pacitti F., Pancheri P., Pacifici R., Altieri I., Paris L., Zuccaro P. Personality, endocrine and immune changes after eight months in healthy individuals under normal daily stress. Psychother. Psychosom. 1994; 62: 176–184
- Buske-Kirschbaum A., von Auer K., Krieger S., Weis S., Rauh W., Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: A general feature of atopic disease?. Psychosom. Med. 2003; 65: 806–810
- Catipovič-Veselica K., Durijancek J., Bracič-Kalan M., Amidzič V., Mrdenovič S., Kozmar D., Burič D., Catipovič B. Heart rate and heart-rate variability in patients with acute coronary heart disease classified on Bortner's scale as type A and type B. Psychol. Rep. 1997; 80: 775–784
- De Boer S. F., Slangen J. L., Van der Gugten J. Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: Effects of chlordiazepoxide. Physiol. Behav, 1990; 47: 1089–1098
- Devoino L., Alperina E., Pavina T. Immunological consequences of the reversal of social status in C57Bl/6J mice. Brain Behav. Immunol. 2003; 17: 28–34
- Eshe C., Makarenkova V. P., Kost N. V., Lotze M. T., Zozulya A. A., Shurin M. R. Functional δ -type opioid receptors on dendritic cells. Ann. N.Y. Acad. Sci. 1999; 885: 387–390
- The Biological Basis of Personality, H. J. Eysenck. Charles C. Thomas Publishers, Springfield, IL 1967
- Frank E., Salchner P., Aldag J. M., Salomé N., Singewald N., Landgraf R., Wigger A. Genetic predisposition to anxiety-related behavior determines coping style, neuroendocrine responses, and neuronal activation during social defeat. Behav. Neurosci. 2006; 120: 60–71
- Freeman L., Hewison M., Hughes S. V., Evans K. N., Hardie D., Means T. K., Chakraverty R. Expression of 11β -hydroxysteroid dehydrogenase type 1 permits regulation of glucocorticoid bioavailability by human dendritic cells. Blood 2005; 106: 2042–2049
- Friedman M., Byers S. O., Diamant J., Rosenman R. H. Plasma catecholamine response of coronary-prone subjects (typeA) to a specific challenge. Metabolism 1975; 24: 205–210
- Huovinen E., Kaprio J., Koskenvuo M. Asthma in relation to personality traits, life satisfaction, and stress: A prospective study among 11,000 adults. Allergy 2001; 56: 971–977
- Idzko M., Panther E., Stratz C., Müller T., Bayer H., Zissel G., Dürk T., Sorichter S., Di Virgilio F., Geissler M., Fiebich B., Herouy Y., Elsner P., Norgauer J., Ferrari D. The serotoninergic receptors of human dendritic cells: Identification and coupling to cytokine release. J. Immunol. 2004; 172: 6011–6019
- Irvine J., Lyle R. C., Allon R. Type A personality as the psychopathology: Personality correlates and an abbreviated scoring system. J. Psychosom. Res. 1982; 26: 183–189
- Jamner L. D., Schwartz G. E., Leigh H. The relationship between repressive and defensive coping styles and monocyte, eosinophile, and serum glucose levels: support for the opioid peptide hypothesis of repression. Psychosom. Med. 1988; 50: 567–575
- Kim S. P., Ferrara A., Chess S. Temperament of asthmatic children. A preliminary study. J. Pediatr. 1980; 97: 483–486
- Kirst A., Wack C., Lutz W. K., Eggert A., Kämpgen E., Fischer W. H. Expression of functional κ -opioid receptors on murine dendritic cells. Immunol. Lett. 2002; 84: 41–48
- Kittner J. M., Jacobs R., Pawlak C. R., Heijnen C. J., Schedlowski M., Schmidt R. E. Adrenaline-induced immunological changes are altered in patients with rheumatoid arthritis. Rheumatology 2002; 41: 1031–1039
- Korneva E. A., Shkhinek E. K. Stress and immune system function. Usp. Fiziol. Nauk. 1989; 20: 3–20
- Le Mellédo J. M., Arthur H., Dalton J., Woo C., Lipton N., Bellavance F., Koszycki D., Boulenger J. P., Bradwejn J. The influence of type A behavior pattern on the response to the panicogenic agent CCK-4. J. Psychosom. Res. 2001; 51: 513–520
- Lilljeqvist A. C., Smørvik D., Faleide A. O. Temperamental differences between healthy, asthmatic, and allergic children before onset of illness: A longitudinal prospective study of asthma development. J. Genet. Psychol. 2002; 163: 219–227
- Maestroni G. J. Adrenergic modulation of dendritic cells function: Relevance for the immune homeostasis. Curr. Neurovasc. Res. 2005; 2: 169–173
- Markova E. V., Chernova T. G., Fillimonov P. N., Korotkova N. A., Abramov V. V., Kozlov V. A. Immunomorphological characteristics of animals with different levels of orientation and exploratory behavior. Bull. Exp. Biol. Med. 2004; 138: 415–417
- Markova E. V., Gromykhina N. Y., Abramov V. V., Kozlov V. A. The peculiarities of the immune status in mice with different level of behavioral reactions. Russ. J. Immunol. 2000; 5: 89–94
- Marques-Deak A., Cizza G., Sternberg E. Brain-immune interactions and disease susceptibility. Mol. Psych. 2005; 10: 239–250
- Miller G. E., Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and β 2-adrenergic receptor in children with asthma. Proc. Natl. Acad. Sci. USA 2006; 103: 5496–5501
- Pawlak C. R., Jacobs R., Mikeska E., Ochsmann S., Lombardi M. S., Kavelaars A., Heijnen C. J., Schmidt R. E., Schedlowski M. Patients with systemic lupus erythematosus differ from healthy controls in their immunological response to acute psychological stress. Brain Behav. Immunol. 1999; 13: 287–302
- Reiche E. M., Morimoto H. K., Nunes S. M. Stress and depression-induced immune dysfunction: Implications for the development and progression of cancer. Int. Rev. Psych. 2005; 17: 515–527
- Sajti E., Van Meeteren N., Kavelaars A., van der Net J., Gispen W. H., Heijnen C. Individual differences in behavior of inbred Lewis rats are associated with severity of joint destruction in adjuvant-induced arthritis. Brain Behav. Immunol. 2004; 18: 495–496
- Sakami S., Maeda M., Maruoka T., Nakata A., Komaki G., Kawamura N. Positive coping up- and down-regulates in vitro cytokine productions from T-cells dependent on stress levels. Psychother. Psychosom. 2004; 73: 243–251
- Scheich G., Florin I., Rudolph R., Wilhelm S. Personality characteristics and serum IgE level in patients with atopic dermatitis. J. Psychosom. Res. 1993; 37: 637–642
- Segerstrom S. C., Miller G. E. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004; 130: 601–630
- Seredenin S. B. Genetic differences in response to emotional stress and tranquilizers. Psichofarmacol. Biol. Narcol. 2003; 3: 494–509
- Seredenin S. B., Badyshtov B. A., Nikitina M. M., Rozen V. B. Changes in the corticosterone content in the blood plasma of inbred mice after stress exposure. Bull. Eksp. Biol. Med. 1982; 94: 36–37
- Sgoifo A., De Boer S. F., Haller J., Koolhaas J. M. Individual differences in plasma catecholamine and corticosterone stress responses of wild-type rats: Relationship with aggression. Physiol. Behav. 1996; 60: 1403–1407
- Shurin M. R., Zhou D., Kusnecov A., Rassnick S., Rabin B. S. Effect of one or more footshocks on spleen and blood lymphocyte proliferation in rats. Brain Behav. Immunol. 1994; 8: 57–65
- Steimer T., Driscoll P. Divergent stress responses and coping styles in psychogenetically selected Roman high-(RHA) and low-(RLA) avoidance rats: Behavioral, neuroendocrine, and developmental aspects. Stress 2003; 6: 87–100
- Sternberg E. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006; 6: 318–328
- Stevenson J., The ETAC Study Group. Relationship between behavior and asthma in children with atopic dermatitis. Psychosom. Med. 2003; 65: 971–975
- Surkina I. D., Yu Sokolov O, Gabaeva M. V., Gurevich K. G., Alfimova M. V., Akatova E. V., Pak L. S. Relationship between IFNγ production by blood lymphocytes and constitutional personality features of patients with idiopathic mitral valve prolapse. Bull. Exp. Biol. Med. 2001; 131: 389–391
- Veenema A. H., Koolhaas J. M., De Kloet E. R. Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Ann. N.Y. Acad. Sci. 2004; 1018: 255–265
- Wilder R. L. Neuroimmunoendocrinology of the rheumatic diseases: Past, present, and future. Ann. N.Y. Acad. Sci. 2002; 966: 13–19
- Zabrodskii P. F., Timofeev D. A. Relationship between the type of character accentuation and indices of humoral immunity. Bull. Eksp. Biol. Med. 1997; 124: 70–72
- Zhou F. L., Zhang W. G., Wei Y. C., Xu K. L., Hui L. Y., Wang X. S., Li M. Z. Impact of comorbid anxiety and depression on quality of life and cellular immunity changes in patients with digestive tract cancers. World J. Gastroenterol. 2005; 11: 2313–2318
- Zozulya A. A., Kost N. V., Toropov A. V., Meshavkin V. K., Butenko O. B., Barsegyan G. G. The dependence of immunomodulative and behavioral effects of opioid peptide dalargin on the parameters of higher nervous activity in rats. Immunologia 1996; 5: 25–29
- Zumoff B., Rosenfeld R. S., Friedman M., Byers S. O., Rosenman R. H., Hellman L. Elevated daytime urinary excretion of testosterone glucuronide in men with the type A behavior pattern. Psychosom. Med. 1984; 46: 223–225