Abstract
Inflammatory bowel disease (IBD) is a condition of the intestine with significant morbidity. Although hereditary, environmental, immunologic, and bacterial factors have been implicated, the etiology of IBD remains unknown. Since opioid peptides modulate inflammatory cytokine production and opioid antagonists promote tissue growth and repair, we hypothesized the opioid antagonist naltrexone could reduce inflammation of the bowel. Using a chemically-induced mouse model of IBD, C57BL/6J mice received either untreated drinking water or water containing 2% dextran sulfate sodium (DSS) in two parallel regimens modeling moderate and severe colitis. After colitis was established, animals in the moderate colitis study were administered either saline (control) or naltrexone (NTX; 8 or 400 μ g/kg) daily, while those in the severe colitis study received 0.1 or 10 mg/kg NTX. DSS-treated animals had significant weight loss (p = 0.006) and higher disease activity index (DAI) scores (p < 0.001) compared to water controls. However, NTX treatment of mice with moderate colitis resulted in less weight loss, lower DAI scores, and less histologic evidence of inflammation compared to controls. Significantly, elevated levels of colonic RNA for pro-inflammatory cytokines interleukin (IL)-6 and IL-12 were also decreased toward normal with NTX. Similar to patients with severe and unresponsive disease, animals in the severe colitis study did not significantly respond to treatment. Thus, NTX therapy reverses physical symptoms, histologic evidence, and molecular markers of inflammation in moderate colitis. The mechanism by which NTX acts to reverse colitis is related in part to the decreased expression of pro-inflammatory cytokines.
| Abbreviations | ||
| DSS | = | Dextran sulfate sodium, |
| DAI | = | Disease activity index, |
| IBD | = | Inflammatory bowel disease, |
| NTX | = | Naltrexone, |
INTRODUCTION
Inflammatory bowel disease (IBD) includes two idiopathic conditions termed ulcerative colitis and Crohn's disease which affect approximately 1 million people in North America (Cominelli, Citation2004). The pathogenesis of IBD appears to involve the dysregulation of the immune system in the intestine in response to either commensal bacteria or environment in a genetically predisposed host (Cominelli, Citation2004; Sands, Citation2007). Cytokines have been implicated in the pathogenesis of Crohn's disease, in particular the pro-inflammatory/TH1 cytokines interleukin-1 (IL-1), IL-2, IL-6, IL-12, IL-18, interferon (INF)-γ, and tumor necrosis factor-alpha (TNFα) (Pizarro and Cominelli, Citation2007).
Medical treatment of this condition has focused on targeting the inflammatory response with immunosuppressive drugs (i.e., corticosteroids, azathioprine) or immune-specific biological drugs, such as monoclonal antibodies against TNFα (Targan et al., Citation1997; Sandborn and Hanauer, Citation1999; Hanauer and Present, Citation2003; Navarro and Hanauer, Citation2003; Bell and Kamm, Citation2000; Kamm, 2006). Unfortunately, chronic immunosuppression may increase the risk of developing infections such as tuberculosis (Keane et al., Citation2001) and lymphoma (Kandiel et al., Citation2005). Since the risks may at times outweigh the benefits when immunomodulating drugs are combined with biologic agents (Hanauer, Citation2007), novel therapeutic approaches to treat IBD are needed.
Accumulating evidence points to roles for endogenous opioid peptides in the development or perpetuation of inflammation. Immune cells have been shown to express μ, κ, and δ -opioid receptors that bind both opioid agonists and antagonists (McCarthy et al., Citation2001). In vivo treatments with opioids have been shown to induce the release of pro-inflammatory cytokines, such as IL-12 and TNFα, by mouse peritoneal macrophages (Tomassini et al., Citation2003). Studies have verified similar roles with endogenous opioids, demonstrating that opioids, including [Met5]-enkephalin, stimulate peritoneal macrophages in rodents systems (Vujic et al., Citation2004). Opioid receptors are found throughout the gastrointestinal tract in the myenteric and submucosal plexus as well as in epithelial cells (Jimenez et al., Citation2006).
Furthermore, in these studies, cytokine production was decreased by simultaneous treatment with the opioid receptor antagonist, naltrexone, indicating the effects were mediated by opioid receptors expressed by peripheral and intestinal immune cells. Pre-treatment with naltrexone (10 mg/kg) has also been shown to block TNFα synthesis and induction of septic shock in LPS/d-galactosamine-treated mice (Greeneltch et al., Citation2004). Recently, a clinical study in human subjects with active Crohn's disease demonstrated that naltrexone therapy improved Crohn's inflammatory scores and quality of life (Smith et al., Citation2007).
Given the role of endogenous opioids in inflammation and the inhibition of these effects by the opioid receptor antagonist, naltrexone, we hypothesized that naltrexone therapy could improve colitis in mice. In order to test this hypothesis we used the DSS (dextran sulfate sodium) model of experimental colitis in mice to examine symptoms, tissue histology, and RNA profiling of cytokines in response to naltrexone. The addition of DSS to the drinking water induces hematochezia, weight loss, intestinal shortening, and infiltration of neutrophils, and thus serves as a model for human inflammatory bowel diseases, particularly ulcerative colitis (Okayasu et al., Citation1990). Breakdown of epithelial barrier function in DSS-treated mice leads to an induction of pro-inflammatory cytokines, which are thought to play a central role in disease progression (Strober et al., Citation2002). Treatments aimed at reducing this excessive inflammatory response have demonstrated therapeutic promise in DSS models; therefore, DSS-treated mice are particularly suitable for proof-of-concept studies of novel IBD therapeutics and treatments that may reduce the cytokine-induced inflammatory state (Pizarro et al., Citation2003).
METHODS
Animals and Experimental Procedures
Two separate studies were performed to test the effects of naltrexone: chemically induced moderate or severe colitis. The research protocol was approved by the Institutional Animal Care and Usage Committee of the Pennsylvania State University College of Medicine and animals were housed in accordance with the AAACCR guidelines for veterinary medicine.
In the moderate colitis study, 6- to 8-wk-old male C57BL/6J mice (Charles River, Wilmington, MA) were randomly allocated into one of two groups of 24 mice each. Food and water were provided ad libitum. Individual mice were housed in separate cages for accurate measurement of food and water intake to determine whether NTX alone affected animal weight, water intake, and food consumption, each measured daily. The first group (Normal) received untreated drinking water and the second group (DSS) received water containing 2% dextran sulfate sodium (DSS; TDB Consultancy AB, Uppsala, Sweden; molecular weight, 40,000) for six days followed by untreated water for three additional days (See treatment schedule, ).
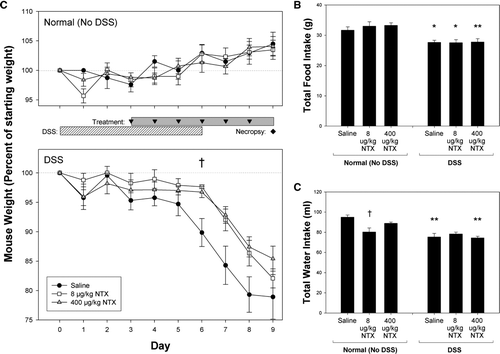
FIG. 1 Daily weight, food and water consumption. (A) Mean weight (expressed as % starting weight ± SEM) of animals receiving either normal drinking water (upper panel) or 2% DSS in drinking water (lower panel) is shown. DSS-induced weight loss was lessened by treatment with naltrexone (NTX) in moderate colitis by Day 6. (B) Mean daily food intake in grams per mouse and (C) total water intake in ml per mouse over the 9-day course of study is shown. Asterisks indicate significantly different values between corresponding DSS and Normal (No DSS) treatment groups (*p < 0.025; **p < 0.005). Significant differences in water intake between saline- and 8 μ g/kg NTX-treated mice are indicated by a †(p = 0.022).
Mice in each group (Normal or DSS) were randomly subdivided into three treatment groups of 8 mice each. Starting on Day 3, mice were treated once daily for six consecutive days with a subcutaneous (SC) injection (0.1 ml) of one of the following: saline (control), 8.0 μ g/kg naltrexone (NTX), or 400 μ g/kg NTX. These doses were selected to bracket the approximate clinical dosing reported with efficacy in humans with Crohn's disease (Smith et al. Citation2007). On Day 9, all animals were necropsied and their colons resected and analyzed.
In the severe colitis study, C57BL/6J mice were placed into one of four groups of 10 mice each. The first group received normal water (control). The remaining groups received water containing 2% DSS (30 mice) for the duration of the study (9 days). Beginning on Day 3, the normal water mice and 10 of the DSS-treated mice were injected with vehicle (water; 0.1 ml SC daily). For NTX treatment in this study, an intermediate dose (100 μ g/kg NTX) and an escalated dose (10 mg/kg NTX) were used to test efficacy against the more severe symptoms anticipated with extended DSS treatment. Mice were injected (0.1 mg/kg or 10 mg/kg; 0.1 ml SC daily; n = 10) until the end of the experiment on Day 9 (See treatment schedule, ).
FIG. 2 Naltrexone treatment reduces Disease Activity Index (DAI) scores. Mice that received normal drinking water (saline or NTX-treated) showed no evidence of colitis by DAI score (A, upper panel; mean ± SEM). DAI scores increased over time in both the moderate (A, lower panel) and the severe colitis (B) studies. Treatment with 400 μ g/kg NTX significantly lowered DAI scores in moderate colitis on Day 6 († p = 0.015). In contrast, NTX failed to improve DAI scores of the severe colitis model (B).
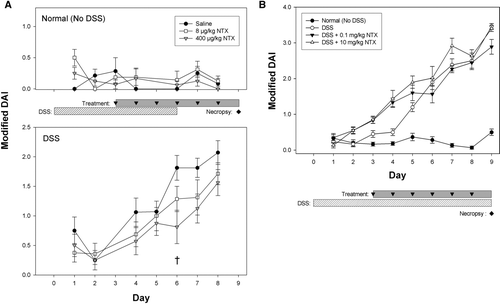
The colitis disease activity index (DAI) was calculated for each mouse according to the system established by Murthy and colleagues (1993) using animal weight, stool occult blood, and stool consistency. Overt changes in stool consistency were rarely discerned in the moderate colitis study so a modified DAI was calculated based on percent weight loss and stool hemoccult or presence of gross bleeding.
Histologic Evaluation
At necropsy, the entire colon was excised, measured in length, and bisected into proximal and distal portions. The proximal and distal colons were additionally divided for RNA extraction and histology. Each histology specimen was fixed in 10% neutral buffered formalin, paraffin embedded and sectioned for hematoxylin and eosin (H&E) staining. Specimens were examined microscopically and scored based upon the criteria established by Williams et al. (Citation2001) by an investigator blinded to the treatment groups. Briefly, a representative longitudinal section from each mouse was scored at six random fields for inflammation severity, extent of inflammation (mucosa, submucosa, transmural) and crypt damage. Each of these scores was weighted to reflect the percent involvement of the overall section and the weighted scores from each of the six fields were averaged to achieve an overall inflammation score for each mouse.
Statistical Analysis
Results were calculated as mean ± SEM. To eliminate undo influence of abnormally sick or resistant mice, statistical outliers from normally distributed data in weight loss and histological sectioning were determined (Minitab 13, State College, PA; below Q1 – 1.5× IQR, above Q3 + 1.5× IQR) and excluded. Pairwise Student t-tests were performed (Minitab 13) using a modified Bonferroni method to correct for multiple comparisons to controls. Statistical comparisons were performed between NTX treatment sub-groups and their corresponding vehicle control, as well as between the Normal and DSS groups, comparing corresponding vehicle or NTX treatments.
Quantitative Real-Time PCR
Total RNA was extracted from the distal colon samples (Trizol; Invitrogen, Carlsbad, CA) and subjected to analysis by Real-Time RT-PCR. RNA (18S and 28S bands) was visualized using the Agilent 2100 Bioanalyzer (Agilent Technologies) and concentrations were adjusted. First strand cDNA was then produced from 1.0 μg of total RNA using random hexamer primers and the SuperScript III Reverse Transcription kit (Invitrogen). The concentration and quality of resulting cDNA was quantified and analyzed using the Agilent 2100 Bioanalyzer or spectroscopically with the NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE). Samples were standardized to 30 ng/μl, and 60 ng of cDNA per sample was then utilized as a template for Real-Time RT-PCR using a SYBR Green Master Mix (Qiagen, Valencia, CA). 18S rRNA primers (Eurogentec, San Diego, CA) and the following gene-specific primer sequences obtained from PrimerBank (Wang and Seed, Citation2003) were utilized: β -actin, 6671509a; IL-5, 6754336a; IL-6, 13642311a; IL-12, 6680395a; STAT3, 13277852a; STAT4, 6755670a; Muc2, 3452503a2; TFF3, 6755773a1; Palladin, 9828173a1; TGF-β BP, 7305243a1; and, TNFα, 202093a3. To exclude the possibility of genomic DNA contamination, control reactions with no cDNA template were also performed for each gene-specific primer set. PCR amplification and analysis were performed with the Applied Biosystems Sequence Detection System 7300 using the Relative Quantification (ddCt) Plate setup. At least six replicates were performed for each target gene set. Amplification data for the target genes were calibrated using the 18S rRNA endogenous control within each sample. The resulting mean Δ CT values were then normalized to the β-actin expression (corresponding treatment groups) to ensure observed differences were biologically robust. Pairwise Student t-tests were performed on the normalized mean Δ CT values for each group using a modified Bonferroni method to correct for multiple comparisons.
RESULTS
Effects of Naltrexone on Animal Weight, Food and Water Consumption
Over the 9-day course of the moderate colitis study, Normal control animals given untreated drinking water exhibited steady weight gain (, upper panel) while DSS mice showed weight loss beginning between Days 4 and 6 (, lower panel) and continuing until necropsy (Day 9). Animals treated with naltrexone exhibited less weight loss compared to DSS + saline mice, reaching statistical significance on Day 6 (p = 0.02). NTX-treated mice also had a tendency toward decreased weight loss on Days 7 and 8, although they did not reach statistical significance.
Food consumption was significantly decreased in the animals with DSS-induced colitis compared to animals without colitis (). Naltrexone treatment alone had no effect on food consumption in both normal animals and DSS-treated animals. Animals treated with NTX (8 μg/kg) had slightly decreased water consumption (). DSS-treated mice drank less water that their treatment counterpart, but there was no difference between the DSS groups treated with saline or NTX ().
Disease Activity Index (DAI) Scores are Reduced by Naltrexone Treatment
To monitor disease progression, a disease activity index (DAI) was assessed daily for each mouse including weight and stool hemoccult. DAI scores for all Normal mice (no DSS), regardless of saline or NTX treatment, showed no clinical evidence of colitis (, upper panel), suggesting that NTX alone has no deleterious effects on the colon. All DSS-treated mice developed colitis symptoms (hemoccult-positive stools and increased DAI scores) by Day 4, which continued to increase through the end of each study (moderate colitis, lower panel; severe colitis, ). A reduction in the DAI scores was evident with NTX treatment in animals with moderate colitis; on Day 6, DSS + 400 μ g/kg NTX animals had significantly lower (55%) DAI scores than DSS + saline mice (p = 0.015). DAI scores for 400 μ g/kg NTX mice at Day 7 were also improved, but did not reach significance (p = 0.038). In contrast, neither dose of NTX (0.1 mg/kg or 10 mg/kg) improved the DAI score in severe colitis ().
Colon Length
Reduced colon length, another indicator of colitis, was also evident in all DSS-treated animals. In the moderate colitis study, control mice drinking normal water and injected with saline had colon length 26% longer than DSS-treated animals (9.32 ± 0.39 cm vs. 6.95 ± 0.43 cm, respectively; p = 0.002). DSS-mice treated with naltrexone had more typical colon lengths (7.86 ± 0.31 cm; p = 0.1), although they were still 15% shorter than in the Normal (no DSS) animals.
Histological Evidence for Reduced Colonic Inflammation in Naltrexone-Treated Mice
Histologic inflammation scores and H&E stains of the distal colon in untreated and treated animals are shown in . No inflammation was observed in control animals drinking normal water (saline or NTX-treated), indicating that naltrexone alone did not alter the mucosal integrity of the colon (). All DSS animals had increased inflammation scores, and exhibited crypt damage and increased leukocyte infiltration. In moderate colitis ( and ), the DSS + NTX animals had a significant decrease in inflammation and damage. The DSS + 400 μ g/kg NTX treated mice had reduced histology scores (p = 0.018; ), with improved crypt architecture and fewer invading leukocytes than were observed in the DSS + saline mice ().
FIG. 3 Histologic scores are improved in moderate colitis mice treated with naltrexone. At necropsy, longitudinal sections of the distal colon were H&E stained and evaluated using the scoring system of Williams and colleagues (2001). Histology scores are expressed as the mean ± SEM. Normal histology was observed in animals drinking untreated water compared to high inflammatory scores in DSS-treated animals (A). Naltrexone treatment improved the histologic scores in animals with moderate colitis (A). Representative H&E sections of distal colon from animals with moderate colitis are shown in B. Compared to the normal appearance of healthy murine colon (Normal + saline; top), leukocyte infiltration and an absence of normal crypt architecture are evident in the DSS + saline mice (middle). Improved architecture and less inflammation are clearly discernable in DSS mice treated with 400 μ g/kg NTX (bottom). Cross indicates significantly different values between DSS + saline and DSS + 400 μ g/kg NTX (p = 0.018). In contrast, NTX was unable to reverse the inflammatory response observed in the severe colitis model (C, D).
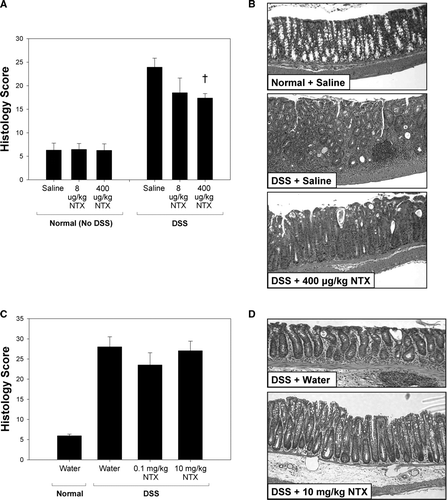
Animals with severe colitis had no improvement in their histology scores with NTX. Although those mice treated with the lower dose of NTX (0.1 mg/kg) had less inflammation than saline-treated mice and high-dose NTX (10 mg/kg) mice, this difference was not statistically significant ( and ).
No histologic changes consistent with colitis were observed in the proximal colons of the mice (data not shown).
Expression of DSS-Induced, Pro-inflammatory Cytokine Genes is Decreased by Naltrexone Treatment
Because naltrexone reduced the inflammatory histology of DSS-induced colitis, the expression of several genes of interest, including both cytokines and downstream mediators, was examined by Real-Time RT-PCR. Expression of β -actin, cytokines IL-5, IL-6, IL-12, and transcription factors STAT3 and STAT4 were assessed using 18S rRNA as an endogenous control. After normalizing to β -actin, IL-5 levels were not significantly changed by either DSS + saline or by DSS + NTX (). Similarly, levels of TNFα were not significantly changed in DSS-treated animals compared to normal, uninflamed tissue (data not shown). Because TNFα is an early mediator of inflammation, transcript levels may have already subsided during the post-DSS recovery that preceded necropsy.
By contrast, mRNA encoding the cytokines IL-6 and IL-12, known to be up-regulated in IBD, were increased in DSS + saline animals in comparison to Normal controls ( and ). The increase in IL-6 mRNA was 66-fold, while the increase in IL-12 was more modest (2.7-fold). Upon treatment with naltrexone, levels of IL-6 and IL-12 were reduced and, for IL-12, naltrexone treatment restored mRNA expression to that seen in the colitis-free, Normal mice. The reduction in IL-6 was also significant, although levels were not completely restored to those seen in the colitis-free animals.
FIG. 4 Gene expression of DSS-induced pro-inflammatory cytokines is decreased by naltrexone. Relative mRNA levels for (A) beta-actin and the cytokine IL-5; (B) cytokine IL-12 and downstream mediator STAT4; and (C) cytokine IL-6 and downstream mediator STAT3, were determined by Real-time RT-PCR using total RNA from the distal colon. Target genes were calibrated to 18S rRNA, and means were normalized to beta-actin expression for each corresponding treatment group. Histogram columns represent the mean relative quantity (RQ = 2- Δ Δ CT) and bars represent a 95% confidence interval (CI; RQ = 2- (Δ Δ CT± CI)). DSS induced significant elevations in the RNA of pro-inflammatory cytokines IL-6 and IL-12. However, mice treated with naltrexone exhibited restoration toward normal levels. Asterisks indicate significantly different values between corresponding Normal + saline and each DSS treatment group (*p < 0.0008). Significant differences between DSS-Saline and DSS-NTX mice are indicated by a † (IL-12, p < 0.005; STAT4, p < 0.024; IL-6, p < 0.0008).
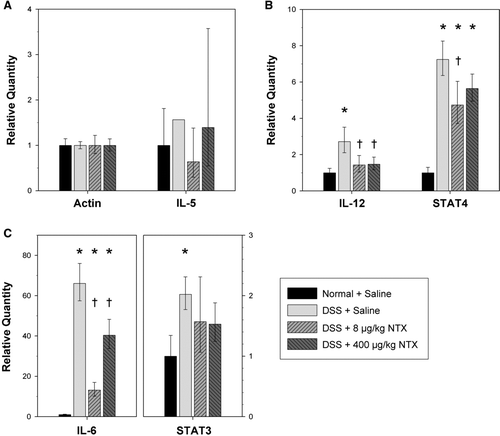
The mRNA for cytokine signaling intermediates STAT3, downstream of IL-6, and STAT4, downstream of IL-12 (Mudter et al., Citation2005), also were increased in DSS + saline animals (2.20- and 8.03-fold, respectively) ( and ). However, STAT3 and STAT4 mRNA levels were not decreased to as great an extent by naltrexone treatment. This may reflect the fact that STAT 3 and STAT4 activities are regulated both at the level of transcription and post-transcriptionally by phosphorylation and nuclear relocalization in response to cytokine signaling (Mudter et al., Citation2005). In the severe colitis study, inflammatory cytokine markers, as measured by Real-Time RT-PCR, were also significantly elevated by DSS treatment. However, in these animals exhibiting severe, rather than moderate colitis, neither NTX dose significantly affected RNA levels (data not shown).
DISCUSSION
This study is the first to report improvement of colitis in a murine model upon treatment with an opioid antagonist. In both the moderate and severe colitis studies, we administered DSS for three days prior to either vehicle or naltrexone injections, emulating a condition of established bowel inflammation preceding treatment. Naltrexone treatment resulted in a rapid mitigation of moderate colitis symptoms in DSS mice, including weight loss and bleeding. Furthermore, the modest differences in histology (DSS ± NTX) may have been even greater if the diseased animals were not allowed potential time for recovery (post-DSS) prior to necropsy.
A robust, impact on pro-inflammatory gene expression by naltrexone was also confirmed. Characteristic of IBD, significant elevations in the gene expression of IL-6 and IL-12 cytokines were induced with DSS. However, naltrexone treatment significantly decreased their expression in DSS mice to normal or near-normal levels. This suggests a possible pathway by which NTX improves colitis. Pro-inflammatory NF-κ B signaling is associated with opioid receptor activity in immune cells (Chen et al., Citation2006). When opioid receptor signaling is blocked by naltrexone, the over-stimulation of immune system is moderated, cytokine levels are restored, and a more normal mucosal structure reappears.
There is ample evidence that opioid peptides, particularly delta opioid receptor agonists, can regulate immune responses (House et al., Citation1996). First, immune cells secrete opioid peptides (Cabot, Citation2001; McCarthy et al., Citation2001) and activation of murine T-lymphocytes increases the synthesis and secretion of [Met5]-enkephalin (Zurawski et al., Citation1986). Second, immune cells express delta, mu, and kappa opioid receptors that respond to endogenous and synthetic opioids (Cabot, Citation2001; McCarthy et al., Citation2001). In fact, [Met5]-enkephalin knock-out mice exhibit a defect in T-lymphocyte activation and a reduced ability of T-lymphocytes to proliferate (Hook et al., Citation2003).
Both endogenous and exogenous opioids have been shown to intensify the production of pro-inflammatory cytokines by immune cells and, in some cases, this opioid-stimulated increase in cytokines has been abrogated by opioid receptor antagonists (Vujic et al., Citation2004). Third, while opioid peptides do not, in themselves, induce inflammatory responses, they can sensitize T-lymphocytes and macrophages to other pro-inflammatory stimuli (Hucklebridge et al., Citation1990; Kamphuis et al., Citation1998). Thus, because opioids can enhance the immune response to pro-inflammatory stimuli and thereby contribute to the escalation of inflammation, it is reasonable to suggest that naltrexone's action on both T-lymphocytes and macrophages could effectively moderate the excessive pro-inflammatory response in IBD. Consistent with this notion, the DSS-induced pro-inflammatory response in this study was down-regulated by opioid receptor blockade.
Although naltrexone is a competitive antagonist at delta, mu and kappa opioid receptors, the effects on immune function reported herein are consistent with delta receptor blockade. In a broad sense, while delta opioid receptor stimulation is pro-inflammatory, signaling through mu opioid receptors has been shown to be immunosuppressive (McCarthy et al., Citation2001). Indeed, the use of specific mu receptor agonists for treatment of IBD has shown therapeutic benefit both in mouse models and in human mucosal explants (Philippe et al., Citation2003, Citation2006). Studies with delta and mu receptor-specific antagonists will further clarify the role of these receptors in colonic inflammation. Conceivably, a delta receptor antagonist and mu receptor agonist may function synergistically with maximal therapeutic benefit.
It has yet to be determined whether naltrexone blocks an overactive opioid peptide system which is aggravating the inflammatory response, or whether it augments the release of endogenous opioids to alter diarrhea and pain through specific receptors in the gut. In addition to inflammatory modulation, naltrexone may elicit other complementary effects, potentially mediated through blockade of a second type of opioid receptor, the opioid growth factor (OGF) receptor. [Met5]-enkephalin binding to the OGF receptor generally inhibits growth and repair. Naltrexone blockade of the receptor, which is expressed throughout the gastrointestinal tract, has been shown to promote cell proliferation and re-epithelialization in esophageal epithelium (Zagon et al., Citation1997). As evidenced by the improved histology scores, healing of the gastrointestinal mucosa observed in our studies may be the result of the simultaneous effects of naltrexone on inflammation and epithelial renewal.
Current therapies for IBD that target pro-inflammatory cytokines (i.e., anti-TNFα monoclonal antibodies) eliminate the cytokines and carry an increased risk of infection due to immune suppression (Sandborn and Targan, Citation2002; Hanauer, Citation2007). Humanized monoclonal antibodies also show diminished efficacy over time and have significant secondary complications, decreasing their suitability for long-term use. Because opioid receptor blockade down-regulates, but does not eliminate, pro-inflammatory cytokines, naltrexone therapy may have fewer undesirable side-effects than currently-used agents. Additionally, the versatility of naltrexone for oral administration presents advantages in patient compliance.
Our results also support our previous clinical report of efficacy with low-dose naltrexone. A low-dose of naltrexone (4.5 mg), was shown to decrease inflammation in human subjects with Crohn's disease (Smith et al., Citation2007). In the moderate colitis study presented here, naltrexone was effective in microgram per kilogram doses. In the severe colitis portion, the lower dose (0.1 mg/kg) exhibited a trend toward improvement, while high-dose (10 mg/kg) showed no effect, although both treatments were arguably complicated by the induction of more severe colitis than in the moderate study. This reflects current care for patients with severe colitis, in which the inflammation at times exceeds medical management, necessitating surgery. While naltrexone has been approved by the Food and Drug Administration for alcohol withdrawal syndromes (Petrakis et al., Citation2007) at a dose of 50 mg daily, it is unknown how this higher dose affects inflammatory responses and cytokines.
Precedence for greater efficacy of low-dose therapies already exists. Others have shown that micromolar concentrations of dextromethorphan, a d-isomer of the codeine analog levorphanol, induces a neuroprotective effect in the brain by suppression of proinflammatory factors superoxide, NO and TNFα (Liu et al., Citation2003, 2005). This inhibitory effect on free radical generation by ultra-low concentrations of dynorphins is shared by another opioid peptide, enkephalin (Zaitsev et al., Citation1991; Efanov et al., Citation1994), where femtomolar concentrations of an enkephalin analog inhibited the reactive burst from human neutrophils and mouse macrophages. Thus manipulating the opioid-opioid receptor axis with small doses of these compounds plays a role in modulating inflammatory mechanisms.
These data demonstrate a role of endogenous opioids in the development and progression of IBD and the effectiveness of opioid receptor blockade in reducing of colonic inflammation and damage. On the whole-animal, tissue, and molecular levels, naltrexone treatment alleviated the effects of DSS-induced colitis. Further work will more specifically define the role of endogenous opioids in IBD, the mechanisms linking opioid receptor activation and cytokine production, and the therapeutic potential of naltrexone or other opioid antagonists for the treatment of IBD.
Authors Matters and Harms contributed equally. Gail Matters is also affiliated with the Department of Medicine, Penn State, and John F. Harms is also affiliated with the Department of Biological Sciences, Messiah College, Grantham, Pennsylvania.
The authors wish to thank Dan Krissinger and Rob Brucklacker of the Functional Genomics Core Facility of the Section of Research Resources, Pennsylvania State College of Medicine for assistance with the Real Time RT-PCR, and Dr. David Mauger, Department of Public Health Sciences, Pennsylvania State College of Medicine, for statistical consultation. Lynn Budgeon, Melissa Nelson, and Evan Gilius are acknowledged for their expert technical help. Grant Support: Supported by a gift fund at Pennsylvania State University to JPS.
REFERENCES
- Bell S., Kamm M. A. Antibodies to tumor necrosis factor-alpha as treatment for Crohn's disease. Lancet 2000; 355: 858–860
- Cabot P. J. Immune-derived opioids and peripheral antinociception. Clin. Exp. Pharmacol. Physiol. 2001; 28: 230–232
- Chen Y. L., Law P. Y., Loh H. H. Nuclear factor-κ B signaling in opioid functions and receptor gene function. J. Neuroimmun. Pharmacol 2006; 1: 270–279
- Cominelli F. Cytokine-based therapies for Crohn's disease—new paradigms. New Engl. J. Med. 2004; 351: 2045–2048
- Efanov A. M., Koshkin A. A., Sazanov L. A., Borodulina O. I., Varfolomeev S. D., Zaitsev S. V. Inhibition of the respiratory burst in mouse macrophages by ultra-low doses of an opioid peptide is consistent with a possible adaptation mechanism. FEBS Lett. 1994; 355: 114–116
- Greeneltch K. M., Haudenschild C. C., Keegan A. D., Shi Y. The opioid antagonist naltrexone blocks acute endotoxic shock by inhibiting tumor necrosis factor-α production. Brain Behav. Immun. 2004; 18: 476–484
- Hanauer S. B. Risks and benefits of combining immunosuppressives and biological agents in inflammatory bowel disease: Is the synergy worth the risk?. Gut 2007; 56: 1181–1183
- Hanauer S. B., Present D. H. The state of the art in the management of inflammatory bowel disease. Rev. Gastroenterol. Disord. 2003; 3: 81–92
- Hook S., Camberis M., Prout M., Le Gros G. Absence of pre-proenkephalin increases the threshold for T-cell activation. J. Neuroimmunol. 2003; 140: 61–68
- House R. V., Thomas P. T., Bhargava H. N. A comparative study of immunomodu-lation produced by in vitro exposure to δ opioid receptor agonist peptides. Peptides 1996; 17: 75–81
- Hucklebridge F. H., Hudspith B. N., Lydyard P. M., Brostoff J. Stimulation of human peripheral lymphocytes by methionine enkephalin and δ -selective opioid analogues. Immunopharmacology 1990; 19: 87–91
- Jimenez N., Puig M. M., Pol O. Anti-exudative effects of opioids and expression of κ - and δ -opioid receptors during intestinal inflammation in mice: Involvement of nitric oxide. J. Pharmacol. Exp. Ther. 2006; 316: 261–270
- Kamm M. A. Review article: Biological drugs in Crohn's disease. Aliment. Pharmacol. Ther. 2006; 24(S3)80–89
- Kamphuis S., Eriksson F., Kavelaars A., Zijlstra J., van de Pol M., Kuis W., Heijnen C. J. Role of endogenous pro-enkephalin A-derived peptides in human T-cell proliferation and monocyte IL-6 production. J. Neuroimmunol. 1998; 84: 53–60
- Kandiel A., Fraser A. G., Korelitz B. I., Brensinger C., Lewis J. D. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut 2005; 54: 1121–1125
- Keane J., Gershon S., Wise R. P., Mirabile-Levens E., Kasznica J., Schwieterman W. D., Siegel J. N., Braun M. M. Tuberculosis associated with infliximab, a tumor necrosis factor-α -neutralizing agent. New Engl. J. Med. 2001; 345: 1098–1104
- Li G., Cui G., Tzeng N. S., Wei S. J., Wang T., Block M. L., Hong J. S. Femto-molar concentrations of dextromethorphan protect mesencephalic dopaminergic neurons from inflammatory damage. FASEB J. 2005; 19: 489–496
- Liu Y., Qin L., Li G., Zhang W., An L., Liu B., Hong J. S. Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. J. Pharmacol. Exp. Ther. 2003; 305: 212–218
- McCarthy L., Wetzel M., Sliker J. K., Eisenstein T. K., Rogers T. J. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001; 62: 111–123
- Mudter J., Weigmann B., Bartsch B., Kiesslich R., Strand D., Galle P. R., Lehr H. A., Schmidt J., Neurath M. F. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am. J. Gastroenterol. 2005; 100: 64–72
- Murthy S. N., Cooper H. S., Shim H., Shah R. S., Ibrahim S. A., Sedergran D. J. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig. Dis. Sci. 1993; 38: 1722–1734
- Navarro F., Hanauer S. B. Treatment of inflammatory bowel disease: Safety and tolerability issues. Am. J. Gastroenterol. 2003; 98: S18–S23
- Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990; 98: 694–702
- Petrakis I., Ralevski E., Nich C., Levinson C., Carroll K., Poling J., Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and current depression. J. Clin. Psychopharmacol. 2007; 27: 160–165
- Philippe D., Chakass D., Thuru X., Zerbib P., Tsicopoulos A., Geboes K., Bulois P., Breisse M., Vorng H., Gay J., Colombel J. F., Desreumaux P., Chamaillard M. μ -Opioid receptor expression is increased in inflammatory bowel diseases: Implications for homeostatic intestinal inflammation. Gut 2006; 55: 815–823
- Philippe D., Dubuquoy L., Groux H., Brun V., Chuoi-Mariot M. T., Gaveriaux-Ruff C., Colombel J. F., Kieffer B. L., Desreumaux P. Anti-inflammatory properties of the μ opioid receptor support its use in the treatment of colon inflammation. J. Clin. Invest. 2003; 111: 1329–1338
- Pizarro T. T., Cominelli F. Cytokine therapy for Crohn's disease: Advances in translational research. Annu. Rev. Med. 2007; 58: 433–444
- Pizarro T. T., Arseneau K. O., Bamias G., Cominelli F. Mouse models for the study of Crohn's disease. Trends Mol. Med. 2003; 9: 218–222
- Sandborn W. J., Hanauer S. B. Antitumor necrosis factor therapy for inflammatory bowel disease: A review of agents, pharmacology, clinical results, and safety. Inflamm. Bowel Dis. 1999; 5: 119–133
- Sandborn W. J., Targan S. R. Biologic therapy of inflammatory bowel disease. Gastroenterology 2002; 122: 1592–1608
- Sands B. E. Inflammatory bowel disease: Past, present, and future. J. Gastroenterol. 2007; 42: 16–25
- Smith J. P., Stock H., Bingaman S., Mauger D., Rogosnitzky M., Zagon I. S. Low-dose naltrexone therapy improves active Crohn's disease. Am. J. Gastroenterol. 2007; 102: 820–828
- Strober W, Fuss I. J., Blumberg R. S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002; 20: 495–549
- Targan S. R., Hanauer S. B., van Deventer S. J., Mayer L., Present D. H., Braakman T., DeWoody K. L., Schaible T. F., Rutgeerts P. J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor-α for Crohn's disease. Crohn's Disease cA2 Study Group. New Engl. J. Med. 1997; 337: 1029–1035
- Tomassini N., Renaud F. L., Roy S., Loh H. H. Mu and delta receptors mediate morphine effects on phagocytosis by murine peritoneal macrophages. J. Neuroimmunol. 2003; 136: 9–16
- Vujic V., Stanojevic S., Dimitrijevic M. Methionine-enkephalin stimulates hydrogen peroxide and nitric oxide production in rat peritoneal macrophages: Interaction of μ, δ, and κ opioid receptors. Neuroimmunomodulation 2004; 11: 392–403
- Wang X., Seed B. A PCR primer bank for quantitative gene expression analysis. Nucl. Acids Res. 2003; 31: e154
- Williams K. L., Fuller C. R., Dieleman L. A., DaCosta C. M., Haldeman K. M., Sartor R. B., Lund P. K. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that over-express growth hormone. Gastroenterology 2001; 120: 925–937
- Zagon I. S., Wu Y., McLaughlin P. J. Opioid growth factor is present in human and mouse gastrointestinal tract and inhibits DNA synthesis. Am. J. Physiol. 1997; 272: R1094–R1104
- Zaitsev S. V., Sazanov L. A., Koshkin A. A., Sud'ina G. F., Varfolomeev S. D. Respiratory burst inhibition in human neutrophils by ultra-low doses of [D-Ala2]-methionine enkephalinamide. FEBS Lett. 1991; 291: 84–86
- Zurawski G., Benedik M., Kamb B. J., Abrams J. S., Zurawski S. M., Lee F. D. Activation of mouse T-helper cells induces abundant pre-proenkephalin mRNA synthesis. Science 1986; 232: 772–775