Abstract
Diabetes mellitus (DM), one of the commonest metabolic disorders, can impair the function of cells involved in cellular and/or humoral immunity. This study sought to define potential effects upon cell-mediated immune cells due to an acute hyperglycemic state (in vitro) for comparison against those that might be attributable to a diabetic phenotype itself. Peripheral blood mononuclear cells (PBMC) were isolated from ten diabetic patients (5 with Type I disease and 5 with Type II) and 10 healthy controls. The cells were then challenged with 1 of 3 different mitogens (concanavalin A, phytohemagglutinin, pokeweed mitogen) in the presence of differing glucose concentrations (0, 100, 200, 400, or 800 mg/dl), and proliferative responses assessed. Neutrophils (PMNC) from the blood samples, exposed to the same experimental conditions, were analyzed for respiratory burst activity using nitroblue tetrazolium. The results indicated that there was significant inhibition of the proliferative responses to mitogens among the stimulated PBMC and in respiratory burst activity among the PMNC obtained from the diabetic patients. However, these effects were not affected by either the added presence of increasing amounts of exogenous glucose, the type of diabetes the patients had, the length of time the patient had had the disease, or whether or not the patients had been receiving insulin treatments. In contrast, the PBMC from healthy individuals appeared to display dose-trend decreases in responsiveness to mitogens; interestingly, similar effects on their PMNC were not evident. It was thus concluded that in situ ongoing repeated hyperglycemic states caused changes in cells of the immune system that could have been caused by repeated “continuous” exposures to excess sugar. Further studies are needed to more clearly identify hyperglycemia (sugar)-sensitive targets on/in these cells that could contribute to the appearance of the diabetic immunodeficiency in these types of patients.
Keywords::
Introduction
Like allergy, diabetes mellitus (DM) is a very common chronic disorder. Its incidence worldwide has been increasing; this estimate is the result of either a true higher incidence of the disease (arising from the modern lifestyles) or from changes in the established minimum level of blood glucoses accepted as determinative of a positive diagnosis. In either case, DM is characterized by disordered metabolism and chronic abnormally high levels of glucose due to absolute or relative insulin deficiency. There are two major forms of the disease; Type 1 diabetes is usually due to autoimmune destruction of pancreatic β cells that produce insulin, while Type 2 diabetes is characterized by insulin resistance in target tissues. This latter scenario causes a need for very high amounts of insulin, and DM develops when β cells cannot meet this demand.
Diabetes has profound effects on many body systems. The hallmark of its effects on the body defenses and the immune system is a noted increased incidence of infections (Smitherman and Peacock, Citation1995; Joshi et al., Citation1999). Many factors contribute to this increase in infection rates beside any direct suppression on the immune system, including vascular impairment, neuropathy, tissue injury/poor wound healing, and skin damage. In this respect, worldwide, diabetes—akin to malnutrition—can be considered a major cause of secondary immunodeficiencies. A thorough review of the scientific literature concerning the relationship between diabetes and immunology clearly indicates there is great interest in determining the role of immunology in the pathogenesis of diabetes (insulin-dependent DM) (Kockum et al., Citation1993; Tuomilehto et al., Citation1995). Increasingly, rather than solely examining the role of altered immune function in the causation of the disease, studies have begun to focus on the effects of the diabetes phenotype on the immune system itself (i.e., see Ananworanich and Shearer, Citation2001; CitationSentochnick, 2006).
Several aspects of immune function have been reported to be depressed in diabetics; however, this area is still not well understood nor has yet been thoroughly investigated. In general, the immune system is not only affected by the hyperglycemic state, but also by the concomitant acidosis or ketosis that arises in these patients. It is generally accepted that there is reduction in: numbers of CD4 T-lymphocytes; NK cells cytotoxic activity; cutaneous anergy; and, late phase reactions (Jones and Peterson, Citation1981; Fontana et al., Citation1999). Although there are reports of transient IgG subclass deficiency in Type I DM, humoral immunity remains relatively intact (except for T-lymphocyte-dependent antibody production). Complement function is also affected by hyperglycemia, especially by the C3 component; this, in turn, interferes with host recognition and responses to microbes (Vergani et al., Citation1983). Many functional aspects of poly-morphonuclear cell (PMNC) function have also been reported to be affected, including chemotaxis, phagocytosis, bactericidal activity, and antioxidant production. Nevertheless, much of the research cited in the literature about diabetic immunodeficiency is rather old and does not reflect the newer knowledge and understanding of the immune system (Tan et al., Citation1975; Richard et al., Citation1987; Tater et al., Citation1987; Andersen et al., Citation1988; Balasoiu et al., Citation1997; Delamaire et al., Citation1997; Mowat and Baum, 1997).
Interestingly, many of these reported effects have seemed to be independent of the type of diabetes, patient age, diseases duration, and/or level of HbA1c (measure of amount of glycosylated hemoglobin in blood that provides reliable estimate of how well DM is being managed over the previous 2–3-mo period). All proteins, exemplified by hemoglobin, can be affected by glycosylation via enzymatic and non-enzymatic modification (Brownlee, Citation1994). This process is time- and glucose level-dependent and can change the structure/function of proteins, including those involved in immune responses.
The main objective of the study here was to determine the effects on select immune cells from an acute artificial (in vitro) hyperglycemic state as opposed to that from the prolonged (in vivo) hyperglycemic states near-continuously expressed in diabetics. The study also sought to examine the potential effects from treatment with insulin vs. oral hypoglycemic agents, and whether effects on immune system cells might differ as a function of the type of DM present.
Materials and methods
Study subjects
The project was evaluated by research committees of the Allied Medical Technologies and Higher Education at the Jordan University of Science and Technology. All studies took place in the Princess Basma Hospital (PBH) and Jordan University of Science and Technology (JUST) laboratories in Irbid, Jordan during the period of January–July 2001, and were approved by the JUST Human Research Committee. Written informed consent forms were obtained from patients attending the Diabetes Clinic of the PBH. Inclusion criteria were the presence of a poorly-controlled diabetes mellitus (DM; fasting blood sugar >200 mg/dl and high HbA1c >8.2%), but no concurrent active infection or ketoacidosis at that time. Five patients with Type I and five with Type II DM were ultimately selected for study. Ten age- and sex-matched non-diabetic controls were also sampled. Clinical data regarding the demographics of these patients, disease details, and treatment were obtained from patients and their clinical records.
Blood preparation for analyses
All procedures were performed under strict aseptic conditions. Whole blood (20 ml) from patients and controls was collected in heparin tubes (40 μl heparin/10 ml blood) and then transported to the laboratory in a cooled unit for immediate processing. Each sample was first divided into four fractions (5 ml each), then overlaid atop 5 ml of Histopaque-1077 (ρ = 1.077 g/ml) and centrifuged at 400 × g for 30 min at room temperature. The resulting supernatant was discarded and the opaque interface collected for its peripheral blood mononuclear cell (PBMC) content; the pellets were collected for the polymorphonuclear cells (PBMC).
Mitogen responsiveness
The PBMC isolated from each subject’s blood sample was diluted up in glucose-free RPMI-1640 with 5% FBS (pH 7.4) and washed by centrifugation at 400 × g for 10 min; this process was repeated three times. The final PBMC pellet was resuspended in 1 ml of glucose-free RPMI with 10% FBS. Viability of the PBMC was determined via trypan blue exclusion; cell numbers were then adjusted to 2 × 106 cell/ml using glucose-free RPMI with 10% FBS to assess their responsiveness (i.e., ability to proliferate) to mitogens. Lectins, like superantigens, stimulate lymphocytes irrespective of any antigenic specificity. Concanavalin A and phytohemagglutinin are selective T-lymphocyte mitogens as compared to pokeweed mitogen that stimulates both T- and B-lymphocytes. These lectins are among the most widely used to assess the functional status of cell- mediated immunity (more precisely, its cellular components) ex vivo or in vitro (Weiss and Samuelson, Citation2003).
Proliferation after challenge with mitogens was performed as follows. Aliquots (100 μl) of the PBMC suspension were distributed (in triplicate) into wells of a 96-well round-bottom microtiter plate that contained the various different glucose concentrations. The glucose levels initially present were higher than the desired final ones (e.g., 200, 400, 800, or 1600 mg/dl) in order to account for the diluting effect from the addition of mitogen solution. Thereafter, 100 μl mitogen (concanavalin-A [ConA; 160 μg/ml], pokeweed mitogen [PWM; 1 μg/ml]), or phytohemagglutinin-P [PHA; 2.5 μg/ml]) solution was added to dedicated wells. Each mitogen was prepared in glucose-free phosphate-buffered saline (PBS, pH 7.4). The plate was then incubated 72 hr at 37°C in a 5% CO2-bearing atmosphere and at 95% relative humidity (Harbeck and Giclas, Citation1991).
Assessments of cell proliferation were done using a colorimetric MTT (tetrazolium) assay. Specifically, MTT solution was added to all wells at 10 μl/100 μl medium, and then the plates were re-incubated 4 hr at 37°C. After this period, 100 μl acidified isopropanol was added to each well to thoroughly dissolve any dark blue formazan crystals that had formed in the cells. After a few minutes at room temperature, the plates were read on an ELISA plate reader (Dynatech MR 5000, Alexandria, VA) at 580 nm. The MTT method rather than the more common [3H]-thymidine incorporation assay was selected for use here based upon several considerations, such as equipment availability, technical ease, less biohazard risk, and a good correlation between the methods to assess lymphocyte stimulation—despite their different principles. The proliferation index (PI) was calculated as follows:
PI = Optical density of stimulated cells/Optical density of unstimulated cells
Nitroblue tetrazolium assay
Respiratory burst activity of PMNC was assessed by nitroblue tetrazolium (NBT) reduction, a phenomenon associated with reactive oxygen formation, inflammation, and nascent killing mechanisms employed by PMNC and other leukocytes (Wikstrom et al., Citation1996). To assess the effects of the ongoing DM or acute exposure to hyperglycemic states on this activity in PMNC, PMNC in the post-Histopaque pellets generated from each patient’s blood were re-suspended in glucose-free RPMI with 10% FBS and centrifuged at 1500 × g for 8 min. The resulting supernatant was discarded and the cells in the buffy coat region transferred to a test tube. Viable cells were counted and then adjusted to 3 × 106 cells/ml.
NBT reduction by the cells was then performed as follows. Aliquots (100 μl) of the PMNC suspension were distributed (in triplicate) into wells of a 96-well round-bottom microtiter plate that contained the various different glucose concentrations. As before, glucose levels initially present were higher than the desired final ones (e.g., 200, 400, 800, or 1600 mg/dl) in order to account for the diluting effect from the addition of NBT solution. An equal volume of glucose-free PBS containing NBT (1 mg/ml) and phorbal myristate acetate (PMA; 1 mg/ml) reagents was then added to each well. The well contents were gently mixed and the plate was incubated first for 30 min at 37°C in a waterbath and then for 15 min at room temperature. Plates were then centrifuged at 1000 rpm for 10 min and the well supernatants then removed. The wells were then washed with methanol (100 μl/well) and allowed to dry. The insoluble blue formazan dye present in the cells was then solubilized by adding 0.12 ml of 2 KOH followed by 0.14 ml DMSO, and the optical density at 630 nm of the solution in each well was read using an ELISA plate reader.
Statistical analysis
Microsoft Office 2003 EXCEL software and SSPS software package Version 12 were used to compute the data and perform all statistical analyses. In collaboration with the statistician in the Department of Public Health at JUST, the model used for analysis of the results was a two-way ANOVA test with Bonferroni correction for multiple comparisons. A p-value < 0.05 was considered significant.
Results
summarizes the clinical data of both groups of patients with DM. The patients with Type II DM tended to comprise an older age group with average ages of 58.6 yr vs. 30.2 for the Type I patients. Control subjects in these studies had an average age of 52.2 yr. There was no significant difference in average disease duration among the patients who had Type I DM vs. those with Type II DM (i.e., 12.3 vs. 13.2 yr). The other major difference between the patients was that all five of those subjects with Type I DM were receiving insulin (Humulin, Eli Lilly and Co, Indianapolis, IN) prior to the study, while only one of the five with Type II disease received this medication. Regardless of disease type, several of the patients analyzed in these studies had vascular complications of diabetes and associated systemic/organ-specific pathologies. More of the study patients with Type II DM had conditions like high blood pressure and hyperlipidemia than did counterparts with Type I DM.
Table 1. Clinical characteristics of both Type I and II diabetic patients and the total group.
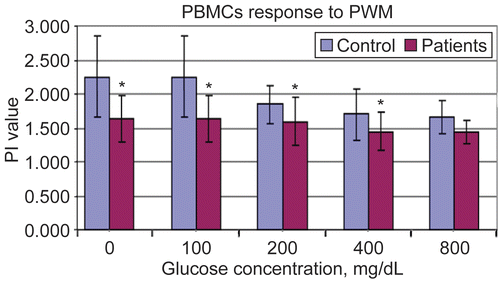
, , and summarize the results of studies of the stimulation of peripheral blood mononuclear cells (PBMC) by the ConA, PHA, or PWM mitogens. There was a statistically-significant difference (using Bonferroni correction) between all the diabetics (pooled) and the controls (p-values provided in ). Values using cells from the diabetics averaged 20.4–21.4% below that of the control subjects, across the types of mitogens tested. As a function of the exogenous glucose concentration tested, there were no significant differences in proliferation indexes (PI) among the PBMC from the diabetics. However, there was a dose-related trend toward suppressed proliferation indices by the cells from control subjects.
Table 2. Effect of in vitro exposure to glucose on the mitogen-induced proliferation indexes (PI) and NBT reduction activities of PMNCa.
Figure 1. Proliferation indexes of PBMC to Concanavalin A at different in vitro glucose concentrations using MTT assay. Results are shown as mean ± SD of triplicate determinations. *Value significantly different from controls at p < 0.05.
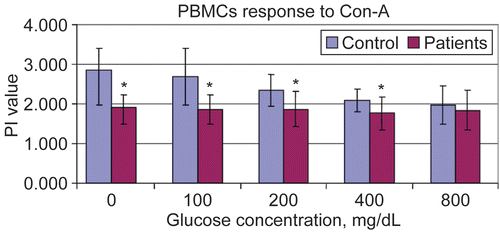
Figure 2. Proliferation indexes of PBMC to Phytohemagglutinin at different in vitro glucose concentrations using MTT assay. Results are shown as mean ± SD of triplicate determinations. *Value significantly different from controls at p < 0.05.
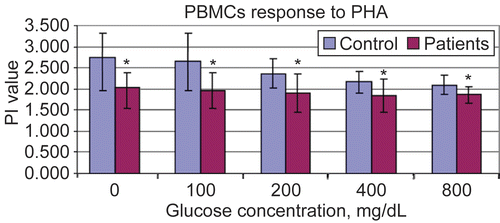
Figure 3. Proliferation indexes of PBMC to Pokeweed Mitogen at different in vitro glucose concentrations using MTT assay. Results are shown as mean ± SD of triplicate determinations. *Value significantly different from controls at p < 0.05.
The analyses of the results associated with the various subgroups among the total population of diabetics studied here revealed that there were no significant differences between responses of diabetics that had been using insulin or not (). In general, however, the responses to mitogens by the PMBC from the insulin using patients were 80–83% of control values, while those of the non-insulin users were 75–77% of control levels; each of these range of changes from control PI values were statistically significant. In assessing any potential differences between the Type I and II diabetics’ results, again it was noted that there were no significant differences between responses of these different classes of patients () and that the PI values of the cells from the patients with the individual disease types were significantly different from those of corresponding control subject cells. In general, the responses to mitogens by the PMBC from the Type I diabetics were 81–83% of control values, while those of the Type II patients were 76–78% of control levels. Last, there did not appear to be any correlation between the PI values and patient duration of disease prior to their participation in this study (data not shown).
Table 3. Effect of in vitro exposure to glucose on proliferation indexes of PMNC and on NBT from patients with insulin-treated and no insulin-treated diabetesa.
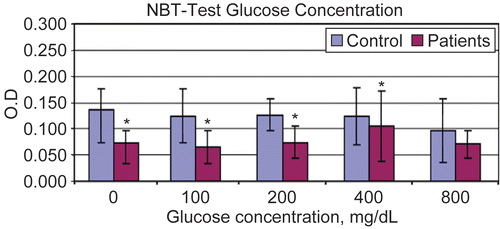
and – summarize the results of the NBT assays using PMNC isolated from the diabetic patients and control subjects. The appeared to be significant differences in the NBT-reducing responses of the cells from the diabetics as compared to those from the controls. On average, the cells from the diabetics displayed 25–35% decrements in their NBT-reducing activities/capacities. In neither test group did there seem to be any effect that could be related to any ex vivo acute exposure to increasing amounts of extracellular glucose.
Table 4. Effect of in vitro exposure to glucose on proliferation indexes of PMNC and on NBT from patients with Type I or Type II diabetesa.
Discussion
Diabetic immunodeficiency research is lagging behind our understanding of the immune system. Even the most recent and comprehensive specialized reviews, like the one listed in Joslin’s Diabetes Mellitus (CitationSentochnick, 2006), rely on research published during 1980–1990. The intent of this current study was to analyze the separate effects of the diabetic phenotype, type of diabetes, and insulin treatment on select functions of cells critical to the immune system. An additional factor to be studied was the effect of an in vitro hyperglycemic state, i.e., to compare short-term exposure to elevated local glucose levels rather than the long-term repetitive in vivo exposures that occur in diabetics. This complex design necessitated use of a complex statistical model of Bonferroni correction for multiple comparisons to study the effects of each factor separately. Although the number of cases examined was only 10 per group, this study is comparable to older ones and acceptable for this type of analyses (i.e., see Casey et al., Citation1977; Gregory et al., Citation1993).
The most important factor in all of tests done was the suppression of function noted in the case of diabetics compared to controls. This did not depend on the diabetes type or being treated with insulin (or not), or the duration of the disease. It was noted for ConA, PHA (specific for T-lymphocytes), and PWM for T- and B-lymphocytes, as well as for NBT stimulation of neutrophils.
These phenomena of functional suppression was started in vivo and maintained in vitro due to the effect of prolonged hyperglycemia exposure rather than due to an acute exposure to the in vitro hyperglycemia only. However there was a trend toward suppression with very high glucose concentrations, especially for the controls, but it did not reach statistical significance with the Bonferroni correction. When direct t- and chi-square tests without the correction were done, there was significant suppression. This effect of in vitro hyperglycemia might show with larger sample size or more prolonged incubation in high glucose levels.
This study did not look for the precise mechanisms by which diabetes or hyperglycemia affected cellular immune responses and activity. Different models and explanations have been proposed, including cell surface protein glycosylation (especially surface receptors), effects of glycation end-products, and increased glycosylation of proteins and/or enzymes that leads to cellular inhibition (Sensi et al., Citation1991; Singh et al., Citation2001). These areas of still-to-be discovered information could help shed light on the mechanisms of pathogenesis of a very common disease, especially if investigators take advantage of the rapid explosion of new information about the immune system.
In addition, circulating cells—including monocytes and lymphocytes—express GLUT1, a facilitative glucose transporter on their surface that provides the cells a means to obtain their basal glucose requirements (Maraton et al., Citation2007). GLUT1 exists in the blood-brain barrier with GLUT3 to provide the constant high levels of glucose required by the brain. While B-lymphocytes and monocytes increase their abundance of GLUT4 (insulin-sensitive), T-lymphocytes, and polymorphonuclear neutrophils have GLUT1 (insulin-resistant) on their membrane. The substrate specificities, kinetic properties, and tissue distributions that dictate their functional relationship with the immune system should be further investigated. Furthermore, the effect of hyperglycemia on signal transduction in the immune cells, and on cytokine synthesis and secretion, needs further study.
Conclusions
PBMC and neutrophils of diabetic patients have suppressed stimulation responses compared to those from healthy controls. These effects are related to the long-term in vivo exposure to hyperglycemia rather than due to acute in vitro exposure to hyperglycemia only. There were no differences in this suppression due to Diabetic Type or treatment with insulin or not.
Acknowledgment
Declaration of interest: The authors report no financial conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Ananworanich, J., and Shearer, W. 2001. Immune deficiencies in congenital and metabolic diseases. In: Clinical Immunology, 2nd Edition (Rich, R., Fleisher, T.A., Shearer, W.T., Kotzin, B.L., and Schroeder, H.W Eds), London, Mosby, pp. 42.8–42.13.
- Andersen, B., Goldsmith, G.H., and Spagnuolo, P.J. 1988. Neutrophil adhesive dysfunction in diabetes mellitus: The role of cellular and plasma factors. J. Lab. Clin. Med. 111:275–285.
- Balasoiu, D., van Kessel, K.C., van Kats-Renaud, H.J., Colet, T.J., and Hoepelman, A.I. 1997. Granulocyte function in women with diabetes and asymptomatic bacteriuria. Diabetes Care, 20:392–395.
- Boyum, A. 1969. Separation of leukocytes from blood and bone marrow. Scand. J. Clin. Lab. Invest. 21(S97):77.
- Brownlee, M. 1994. Glycation and diabetic complications. Diabetes 43:836–841.
- Casey, J.I., Heeter, B.J., and Klyshevich, K.A. 1977. Impaired response of lymphocytes of diabetic subjects to antigen of Staphylococcus aureus. J. Infect. Dis. 136:495–501.
- Delamaire, M., Maugndre, D., Moreno, M., Le Goff, M., Allannic, H., and Genetet, B. 1997. Impaired leukocyte function in diabetic patients. Diabetic Med. 14:29–34.
- Fontana, G., Lapolla, A., Sanzari, M., Piva, E., Mussap, M., De Toni, S., Plebani, M., Fusetti, F., and Fedele, D. 1999. An immunological evaluation of Type II diabetic patients with periodontal disease. J. Diabetes Compl. 13:23–30.
- Gregory, R., McElveen, J., Tattersall, R.B., and Todd, I. 1993. The effects of 3-hydroxybutyrate and glucose on human T-cell responses to Candida albicans. FEMS Immunol. Med. Microbiol. 7:315–320.
- Harbeck, R.J., and Giclas, P.C. (Eds) 1991. Lymphocyte stimulation with mitogens and antigens. In: Diagnostic Immunology Laboratory Manual, Philadelphia: Lippincott Williams and Wilkins, pp. 211–219.
- Jones, R.L., and Peterson, C.M. 1981. Hematologic alterations in diabetes mellitus. Am. J. Med. 70:339–352.
- Joshi, N., Caputo, G.M., Weitekamp, M.R., and Karchemer, A.W. 1999. Infection in patients with diabetes mellitus. New Engl. J. Med. 341:1906–1912.
- Kockum, I., Wasmuth, R., and Holmberg, E. 1993. HLA-DQ primarily confers protection and HLA-DR susceptibility in Type 1 (insulin-dependent) diabetes studied in a population-based affected families and controls. Am. J. Human Genet. 53:150–167.
- Maraton, E., Dimitriadis, G., Kollias, A., Boutati, E., Lambadiari, V., and Mitrou Band Raptis, S. 2007. Glucose transporter expression on the plasma membrane of resting and activated WBC. Eur. J. Clin. Invest. 37:282–290.
- Mowat, A.G., and Baum, J. 1971. Chemotaxis of polymorphonuclear leukocytes from patients with diabetes mellitus. New Engl. J. Med. 284:621–627.
- Richard, M.D., Elliot, G., Yoo Kim, W.L., Elizabeth, K., Thomas, T.A., Anthony, T.W., Michael, E.M., and Daniel, P.C. 1987. A computer-assisted image-analysis system for analyzing polymorphonuclear leukocyte chemotaxis in patients with diabetes mellitus. J. Infect. Dis. 155:737–741.
- Sensi, M., Pricci, F., Andreani, D., and Di Mario, U. 1991. Advanced nonenzymatic glycation endproducts (AGE): Their relevance to aging and the pathogenesis of late diabetic complications. Diabetes Res, 16:1–9.
- Sentochnick, D.E. (2006). Chapter 60, Infection and diabetes. In: Joslin’s Diabetes Mellitus, 14th Edition ( Kahn, C.R. Ed. ), Philadelphia: Lippincott, Williams and Wilkins, pp. 1018–1021.
- Singh, R., Barden, A., Mori, T., and Beilin, L. 2001. Advanced glycation end products: A review. Diabetologia 44:129–146.
- Smitherman, K.O., and Peacock, J.E. 1995. Infectious emergencies in patients with diabetes mellitus. Med. Clin. N. Amer. 79:53–77.
- Tan, J.S., Anderson, J.L., Watanakunakorn, C., and Phair, J.P. 1975. Neutrophil dysfunction in diabetes mellitus. J. Clin. Lab. Med. 85:26–33.
- Tater, D., Tepaut, B., Bercovici, J.P., and Youinou, P. 1987. Polymorphonuclear cell derangements in Type I diabetes. Hormone Metab. Res. 19:642–647.
- Tuomilehto, J., Virtala, E., and Karvonen, M. 1995. Increase in incidence of insulin-dependent diabetes mellitus among children in Finland. Int. J. Epidemiol. 24:984–992.
- Vergani, D., Johnson, C., Abdullah, N., and Barnett, A.H. 1983. Low serum C4 concentrations: An inherited predisposition to insulin dependent diabetes? Br. Med. J. 286:926–928.
- Weiss, A., and Samelson, L.E. 2003. Chapter 11. T-Lymphocyte activation. In: Fundamental Immunology, 5th Edition, (Ed. Paul, W.E.,) Philadelphia: Lippincott, Williams, and Wilkins, pp. 321–364.
- Wikstrom, T., Braide, M., Bagge, U., and Risberg, B. 1996. Spontaneous nitroblue tetrazolium (NBT) reduction related to granulocyte priming and activation. Inflammation 20:281–292.