Abstract
Natalizumab is a monoclonal antibody to human α4 integrin indicated for treatment of multiple sclerosis and Crohn’s disease that prevents extravasation of leukocytes into surrounding tissues and their involvement in inflammation. Because α4 integrins and their receptors are involved in hematopoiesis and immune cell trafficking, natalizumab may interfere with these processes. We evaluated the effects of natalizumab on immune function in monkeys using in vitro and in vivo studies. Consistent with the pharmacologic effects of natalizumab, dose-related increases in white blood cell counts and spleen weights were observed. Administration to monkeys did not result in statistically significant alterations in the percentages of circulating B-cells, T-cells, T-cell subsets (CD4, CD8), or stem cells (CD34). A modest and highly variable delay in the primary humoral response to T-cell-dependent antigens was observed. Ex vivo studies using cells from natalizumab-treated monkeys demonstrated that treatment did not alter immune regulatory or effector cell functions in blood lymphocytes or spleen cells. A similar lack of effect on these functions was observed in vitro following treatment of PBMC and monocytes from human donors. Overall, natalizumab was well tolerated in monkeys, demonstrated the expected pharmacologic effect on cell trafficking, and showed no adverse effect on immune cell function.
Introduction
Natalizumab is a humanized monoclonal immunoglobulin (Ig) G4 antibody to human α4 integrin indicated for the treatment for multiple sclerosis (MS) and Crohn’s disease (CD) (TYSABRI, Citation2008). Natalizumab binds to the α4 subunit of α4β1 and α4β7 integrins and blocks the interaction of these integrins with their receptors, including fibronectin, vascular cell adhesion molecule-1 (VCAM-1), and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) (Danese et al., Citation2005). Endothelial adhesion molecules tend to be over-expressed on endothelial cells in inflamed tissue. Both α4 integrins are involved in the adhesion of leukocytes to endothelial cells and, together with other integrins, mediate the trafficking of lymphocytes across the endothelium and into the parenchymal tissue. Thus, natalizumab may prevent the trafficking of lymphocytes into inflamed tissue.
In addition to their role in leukocyte trafficking in inflammation, α4 integrins and their receptors are widely involved in fetal and adult lymphopoiesis and hematopoiesis as well as leukocyte activation and survival (Arroyo et al., Citation1996; Lasky, Citation1996; Rose et al., Citation2002). In a mouse model, α4 integrins appeared to be essential for maintaining normal hematopoiesis in fetal liver, spleen, and bone marrow, most likely by regulating the proliferation/differentiation balance of hematopoietic progenitors (Arroyo et al., Citation1999). Inhibition of α4 integrin by a function-blocking antibody reduces homing of fetal liver hematopoietic stem and progenitor cells to bone marrow (Qian et al., Citation2007). Adult mice deficient in α4 integrins appear to have impaired B-cell responses (Banerjee et al., Citation2008).
Although clinical trials of natalizumab showed no major differences compared with placebo in immune function with respect to general infections (Ghosh et al., Citation2003; Sandborn et al., Citation2005; Sands et al., Citation2007), three cases of progressive multifocal leukoencephalopathy (PML) were observed in natalizumab-treated patients. Two occurred in patients with MS who were receiving concomitant interferon-β and one occurred in a patient with CD who had received azathioprine and was significantly lymphopenic while treated with natalizumab (Kleinschmidt-DeMasters et al., Citation2005; Langer-Gould et al., Citation2005; van Assche et al., Citation2005; Yousry et al., Citation2006). Five cases have been reported in MS patients in the post-marketing setting in the context of use of natalizumab as a monotherapy. PML is a demyelinating brain disorder caused by reactivation of JC virus, a human polyomavirus that is widespread in the population. Reactivation of the JC virus typically occurs when immune competence is compromised, e.g., hematological malignancies, late stages of AIDS, or with immunosuppressive therapy for organ transplantation (Heilbronn et al., Citation1993).
Because natalizumab is an immune modulator, the series of studies that were undertaken as part of the product development program included the evaluation of the potential immunotoxic effects of natalizumab in monkeys (rhesus and cynomolgus). Natalizumab is not cross-reactive in species that are commonly used for immunotoxicity evaluation (e.g., mice, rats) but is in these species, which demonstrate sequence homology, target binding and pharmacologic effects that are highly similar or identical in those parameters in humans. Sequence data for α4 integrin is available for the rhesus monkey (NCBI) and demonstrates a 97% homology to the human sequence, a close degree a homology that is probably shared by the cynomolgus monkey as well, though data is not available to confirm this. Natalizumab binds to α4 integrins in rhesus and cynomolgus monkeys with an affinity similar to the binding in humans (Kd = 0.04–0.07 μg/ml) and results in the same pharmacologic effects (increases in lymphocyte counts due to blockade of α4-mediated adhesion). Tissue cross-reactivity studies of natalizumab using a panel of human tissues and cynomolgus monkey tissues demonstrate the expected specific staining of lymphocytes in multiple tissues (lymph nodes, intestinal lamina propria, spleen, thymus, and in the lamina propria or interstituim of the ureter, prostate, and salivary gland) in both species and was located at the cell periphery consistent with the membrane expression of α4 integrins.
In addition, both human and cynomolgus demonstrated limited staining of glandular epithelium of the salivary gland and basilar endometrial cells of the uterus. These data support the use of these monkey species as appropriate models of the human system.
Studies included multiple-dose toxicology studies, as well as separate in vitro and in vivo immunotoxicity assessments. In addition to standard histology of lymphoid organs included in all studies, an immunohistochemical evaluation of lymphoid organs for T- and B-cell distribution was incorporated into a 1-month study.
Materials and methods
Animals
The following studies were performed in accordance with Institutional Animal Care and Use Committee (IACUC) approved protocols. Studies 1 and 2 were conducted at IIT Research Institute (Chicago, IL) and Studies 3–6 were conducted at Charles River Laboratories Preclinical Services Nevada (Reno, NV).
Juvenile and adult cynomolgus monkeys and adult rhesus monkeys were housed individually in temperature- and humidity-controlled air-conditioned rooms. Lighting was controlled to provide 12 hours of light and 12 hours of dark. Commercial monkey chow was provided daily and water was available ad libitum.
Test articles, dose preparation, and dosing rationale
Test articles consisted of natalizumab and natalizumab vehicle which were supplied by Elan Pharmaceuticals (South San Francisco, CA). The vehicle consisted of 50 mM histidine, 150 mM sodium chloride in Study 1 and 10 mM sodium phosphate, 140 mM sodium chloride, with 0.02% polysorbate 80 in Studies 2–6. All test articles were supplied as sterile liquids in glass vials. On dosing days, appropriate mixtures of natalizumab and/or natalizumab vehicle were prepared aseptically in sterile, pyrogen-free containers to give the final concentration of the dosing solutions.
The recommended dosage of natalizumab in patients with MS or CD is 300 mg per month, which results in maximum blood concentrations of natalizumab of approximately 100 μg/ml and steady-state trough concentrations in the range of 10 to 30 μg/ml. In vitro studies using human peripheral blood mononuclear cells (PBMC) have shown that natalizumab fully saturates α4 integrins at serum concentrations as low as 5 μg/ml. Dose selection and the frequency of dosing (every other day or weekly) in the repeat-dose toxicity studies conducted in monkeys were based on the need to provide adequate safety margins (i.e., expected concentration in animals/expected concentration in humans), while attempting to avoid the development of antibodies to the humanized IgG construct of natalizumab.
Serum natalizumab and anti-natalizumab antibody levels
Serum samples were tested for natalizumab concentrations using an ELISA method developed for this purpose. Briefly, a mouse monoclonal anti-natalizumab antibody (Elan Pharmaceuticals) was coated using 100 μl/well at 2.0 μg/ml in 0.1 M sodium bicarbonate (pH 8.3) and incubated at room temperature overnight. Plates were washed with Tris-Buffed Saline (pH 7.5) with 0.05% Tween-20 (TBST), and then blocked with 0.1 M Tris with 20% sucrose for 1 hour at room temperature. Plates were then washed once with TBST. Natalizumab standards and serial dilutions of samples in Assay Buffer (PBS, 0.25% casein, 0.05% Tween-20) were added to the wells and incubated for 90 minutes at room temperature, followed by 100 μl/well of alkaline phosphatase-labeled mouse anti-human IgG4 (Southern Biotech, Birmingham, AL) for 1 hour. Plates were washed three times with TBST between each cycle of additions. pNPP served as the colorimetric substrate and was added at 100 μl/well for 30 minutes, followed by 100 μl/well 1 N NaOH to stop the color reaction. Plates were read at 405 nm in a SpectraMax 250 plate reader (Molecular Devices, Sunnyvale, CA). The lower limit of quantitation (LLOQ) for natalizumab in serum was 0.25 μg/ml in this assay.
A similar ELISA method starting with 96-well microtiter plates coated with natalizumab was used to measure anti-natalizumab antibody concentrations in serum samples. Natalizumab was coated using 100 μl/well at 0.25 μg/ml in sodium phosphate buffer (pH 8.5) containing 0.02% BSA and incubated at room temperature overnight. Plates were washed with Tris-Buffed Saline/Tween (TBST) and then blocked with 0.1 M Tris with 20% sucrose for 1 hour at room temperature. Plates were then washed once with TBST. Anti-natalizumab standards (mouse monoclonal, Elan Pharmaceuticals) and serial dilutions of samples in Assay Buffer (PBS, 0.5% BSA, 0.05% Tween-20) were added to the wells and incubated for 2 hours at room temperature, followed by 100 μl/well of biotinylated natalizumab for 1 hour, the 100 μl/well streptavidin-alkaline phosphatase conjugate (Boehringer Mannheim GmbH, Mannheim, Germany) for 30 minutes. Plates were washed 4 times with TBST between each cycle of additions. pNPP served as the colorimetric substrate and was added at 100 μl/well for 45 minutes, followed by 100 μl/well 1N NaOH to stop the color reaction. Plates were read at 405 nm in a SpectraMax 250 plate reader; the LLOQ for anti-natalizumab antibodies in serum was 0.5 μg/ml in this assay.
Study 1: Human in vitro PBMC and monocyte immune function
This study was conducted to assess the potential of natalizumab to modulate cellular immune functions in PBMC and enriched monocyte preparations in vitro. PBMC were isolated from five hepatitis B- and C/HIV-negative blood samples by standard density centrifugation. Blood samples were diluted with Dulbecco’s PBS (DPBS), layered over a gradient of Lymphocyte Separation Medium (Organon Teknika, Boxtel, the Netherlands) and isolated by density centrifugation. Cells were washed three times with DPBS and re-suspended in phenol red-free RPMI-1640 medium (Biowhittaker, Walkersville, MD) supplemented with L-glutamine, gentamicin, and 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT).
Enriched monocyte samples were prepared from the PBMC preparations by collecting cells adherent to plastic using a cell scraper following a 1-hour incubation and re-suspended as for the PBMC preparations. Studies were conducted in 96-well microplates using 2 × 105 – 2 × 106 cells/well to assess lymphoproliferation (basic activational capacity), production of immunoregulatory cytokines (T-helper cell function), polyclonal/re-directed cytotoxic T-cell activity (T-cell effector function), basal and cytokine-stimulated natural killer (NK) cell activity (natural immunity), and production of pro-inflammatory cytokines by monocytes (functional status and regulatory capacity). Vehicle or natalizumab at log dilutions from 0.01–100 μg/ml were used in all assays—a natalizumab concentration of ≈1.0 μg/ml is required to fully saturate all α4 receptors on PBMC or monocytes in the assays, so the range used covers partially to fully saturating concentrations.
Lymphoproliferation in the presence of natalizumab was assessed following a 3-day culture of PBMC in the presence or absence of the cellular activators phytohemagglutinin (PHA; Sigma, St. Louis, MO; 0–5 μg/ml) or monoclonal anti-CD3 antibody (Pharmingen, San Diego, CA; 0-1000 ng/ml). Relative proliferation was assessed colorimetrically at 450 nm by the addition of 1 mg/ml XXT/25 μM phenazine methosulfate and following the metabolic reduction of the tetrazolium salt XXT to the formazan end product using a UVMax microplate reader (Molecular Devices).
Cytokine production by T-helper cells was evaluated after stimulation with PHA or anti-CD3 at an optimal stimulatory concentration for 48 hours at 37ºC. Cytokine production by monocytes was similarly evaluated after co-culture with an optimal stimulatory concentration of bacterial lipopolysaccharide (LPS, Sigma, MO). Optimal stimulatory concentrations for PHA, anti-CD3, and LPS were determined experimentally for each lot prior to use in the assays. Commercially-available ELISA kits were used to assess production levels of interleukin (IL)-1β (R&D Systems, Minneapolis, MN), IL-2 (R&D Systems), IL-6 (Biosource International, Carlsbad, CA), IL-10 (Cytoscreen, [Invitrogen, Carlsbad, CA]), and TNFβ (Bender MedSystems, Burlingame, CA) by T-helper cells and production levels of IL-1α (R&D Systems) and TNFα (Cytoscreen) by monocytes.
The effect of natalizumab on NK cell immune function was assessed after overnight incubation of PBMC in the presence of natalizumab at log dilutions with or without human recombinant IL-2. Following the overnight incubation, PBMC were incubated at 37ºC for 4 hours in the presence of log-phase [51Cr]-labeled K562 human erythromyeloblastoid leukemia target cells at effector:target cell ratios of 33:1 and 11:1. The amount of [51Cr] released into the culture medium was measured using a gamma counter (Wallac Wizard, PerkinElmer, Waltham, MA). The percent NK cell activity (% NKA) was corrected for total releasability (TR, determined by detergent lysis of cells) and spontaneous release (SR) of [51Cr] by target cells incubated in the absence of NK cells using the formula % NKA = 100 × (ER – SR)/(TR – SR), where ER equals the amount of radioactivity released by the target tumor cells in the presence of effector NK cells.
The cytolytic effector function of T-cells in the presence of natalizumab and anti-human CD3 was assessed in a similar fashion by measuring the release of [51Cr] from OKT3 cells (ATCC #CRL 8001). The OKT3 line is a murine hybridoma that produces anti-human CD3 antibody. Binding of the anti-CD3 antibody to T-cells arms them for cytolytic function against tumour cells, in this assay the OKT3 cells themselves; i.e. OKT3 serves both as the source of the anti-CD3 and the cytolytic target. PBMC were isolated from whole blood as described above, but re-suspended in RPMI-1640/L-glutamine, gentamicin containing 10% Human AB Serum (HS) at a concentration of 1 × 106 cells/ml. Three 2-fold dilutions were made from this cell stock in the same medium. Log-phase OKT3 cells were harvested by centrifugation, cell culture medium removed and the cells suspended in 100–200 μl of FBS, to which was added 200 μCi Na251CrO4. Labelling was done for 1 hour in a 37°C water bath with gentle agitation. Following completion of the labelling time, the OKT3 cells were washed 3 times with DPBS/5% FBS, with an incubation for 30 minutes at room temperature prior to the final wash to allow for release of unincorporated label. OKT3 cells were re-suspended at 5 × 104 cells/ml in RPMI-1640/10% HS. One hundred μl were plated per well into 96-well microtiter plated followed by 100 μl of the PBMC dilutions to provide final effector:target ratios of 100:1, 50:1, and 25:1. Plates were centrifuged at 25 × g for 2 minutes, incubated for 4 hours at 37°C/5% CO2, centrifuged for 5 minutes at 200 × g, and supernatants harvested. The amount of [51Cr] released into the culture medium was measured using a gamma counter. The percent specific cytolytic activity (% SCA) was calculated in the same manner as for the % NKA described above.
Study 2: Adult cynomolgus monkey in vivo toxicity (4-week)
Study 2 was a 4-week toxicity study conducted in 50 adult cynomolgus monkeys. Animals approximately 3-years-of- age (5/sex/group) received saline or vehicle control or natalizumab at concentrations of 0.3, 3, or 30 mg/kg by intravenous (IV) infusion at a dose volume of 10 ml/kg every other day for 28 days. Potential immunotoxicity was evaluated by immune function assays on PBMC collected at various times and spleen cells harvested at necropsy. Assessments included total cell counts, PHA- and LPS-stimulated lymphocyte blastogenesis, NK cell activity, assessed by methods similar to those used in Study 1. Cell surface marker analysis (immunophenotyping) of T-cells (CD2), T-cell subsets (CD4 and CD8), monocytes (CD14), and B-cells (CD20) was done by double-color, fluorescent-activated cell sorter (FACS) using an EPICS 753 flow cytometer (Coulter Electronics, Luton, Bedfordshire, UK). Antibody conjugates included CD2-fluoroisothiocyanate (FITC), CD4-phycoerythrin (PE), CD8-PE, and CD20-PE from Becton Dickinson (San Jose, CA) and anti-CD14-PE from Immunotech, Inc. (Westbrook, ME). Clinical, physical, and laboratory examinations were conducted throughout the study. Histopathologic and immunochemistry evaluations of tissues were conducted at necropsy, as were determinations of organ weights.
Study 3: Adult cynomolgus monkey in vivo toxicity (6-month)
A 6-month toxicity study in cynomolgus monkeys from 2- to 5-years-of-age (referred to as “adult” going forward in this report) with a 6-week recovery period evaluated the chronic toxicity of repeated doses of natalizumab and the reversibility of test article-related effects, including end points of potential immunotoxicity. Animals (3/sex/group plus 2/sex/group in the vehicle and the two highest dose groups for the recovery arm of the study) received vehicle or natalizumab at doses of 3, 10, 30, or 60 mg/kg weekly for 6 months by IV infusion (26 doses total) at a dose volume of 10 ml/kg over a 30–60 minute period. Thirty animals were necropsied on Day 177, 24 hours after the last dose, and the remaining eight animals ( recovery groups) were necropsied on Day 219. Clinical, physical, and laboratory exams were conducted throughout the study, including hematology evaluations prior to the start of treatment, ≈ 24 hours post-dosing on Days 23, 51, 86, within 1 hour pre-dose and at 30 minutes post-dose on Day 176, Day 177, and during the recovery period on Days 179, 183, 190, 196, 203, 211, and 219, and determination of CD34+ stem cells in circulation on Days 177 and 183. Histopathologic evaluations of tissues were conducted at necropsy, as were determinations of organ weights. Stem cell analysis was accomplished using the antibody conjugate CD34-PE (detects stem cells) with a Becton Dickinson FACSCalibur Flow Cytometer (San Jose, CA).
Study 4: Juvenile cynomolgus monkey in vivo toxicity (6-month)
A similar 6-month toxicity study in juvenile cynomolgus monkeys with a 17-week recovery period evaluated the chronic toxicity of repeated doses of natalizumab and the reversibility of test article-related effects, including end points of potential immunotoxicity. A monkey was considered to be of juvenile status if it was: 1) between 1.5 and 2.5 years of age; 2) sexually immature; and, 3) had open epiphyseal growth plates at the initiation of the study (determined by X-ray). Animals (3/sex/group plus 2/sex/group in the vehicle and high dose for the recovery arm of the study) received vehicle or natalizumab at doses of 10, 30, or 60 mg/kg weekly for 6 months by IV infusion (26 doses total) at a dose volume of 10 ml/kg over a 30-minute period. Six animals in each test group were necropsied on Day 177, 24 hours after the last dose, and the remaining animals (recovery groups) were necropsied 17 weeks later on Day 291. Clinical, physical, and laboratory exams were conducted throughout the study, including hematology evaluations prior to the start of treatment, approximately 24 hours from the previous day’s dosing on Days 23 and 86, and prior to necropsy on Day 177 or on Day 291, and immunophenotyping of T-cell (CD3) (Becton Dickinson), T-helper cell (CD4) (Becton Dickinson), cytotoxic T-cell (CD8) (Becton Dickinson), B-cell (CD20) (Beckman Coulter, Fullerton, CA) and stem cell (CD34) (Becton Dickinson) populations in circulation at Day 135. Histopathologic evaluations of tissues were conducted at necropsy, as were determinations of organ weights. Hematology assessments were performed using an Advia 120 hematology analyzer (Siemens, Deerfield, IL). Flow cytometric analyses were conducted using FACSCalibur analyzer (Becton Dickinson) equipped with an argon laser capable of acquiring 4-color fluorescence and two scatter parameters via CellQuest acquisition software.
Study 5: Adult cynomolgus monkey in vivo immunotoxicity (6-week)
A 6-week immunotoxicity study in adult cynomolgus monkeys between the ages of 3.5- and 5.1-years with an 8-week treatment-free recovery period evaluated primary and secondary humoral responses (IgG and IgM antibodies) to T-cell dependent antigens in animals receiving natalizumab. Animals (3/sex/group) received vehicle control or natalizumab at doses of 3 or 30 mg/kg by IV infusion once weekly at a dose volume of 10 ml/kg for 6 weeks (i.e., Days 1, 8, 15, 22, 29, and 36). Blood samples for hematology were taken pre-study and on Days 28, 56, and 84. Animals were immunized with 1 mg of keyhole limpet hemocyanin (KLH; Pierce Biotechnologies, Rockford, IL) in Freund’s incomplete adjuvant (Imject, Pierce) on Day 7 by subcutaneous injection and on Days 35, and 63 by intramuscular (IM) injection at 2 sites. Additionally, animals were immunized at a single site with 0.5 ml tetanus toxoid (TT; Intervet, Chicago, IL) on Days 56 and 70 during the treatment-free recovery period. Blood samples for determination of IgG and IgM anti-KLH antibodies were collected at baseline before dosing with natalizumab and on Days 7 (pre-immunization), 17, 21, 28, 35 (pre-immunization), 45, 49, 56, 63 (pre-immunization), 73, 77, 84, and 91. Blood samples for determination of IgG and IgM anti-TT antibodies were collected on Days 56 (pre-immunization), 66, 70 (pre-immunization), 80, 84, and 91.
For the anti-KLH assay, KLH (13.5 μg/ml) was coated using 100 μl/well in SuperBlock™ Blocking Buffer in Tris-buffered saline (SB) (Thermo Fisher Scientific, Rockville, IL) and incubated at 2-8°C overnight. Plates were washed with PBS with 0.25% Tween-20 (PBST) and then blocked with SB for 1–6 hours at room temperature. Plates were washed three times with PBST. Anti-KLH standards and samples were diluted in SB, added to the wells and incubated for 25-35 minutes at room temperature. Sample dilutions covered a range from 250-fold to 15,000-fold. Plates were then washed four times with PBST. Goat anti-monkey IgM-HRP or IgG-HRP (Rockland Immunochemicals, Gilbertsville, PA) was added at 100 μl/well at 0.04 μg/ml (IgM-HRP) or 0.02 μg/ml (IgG-HRP) for 25-35 minutes at room temperature, followed by six washes with PBST.
The substrate 3,3’,5,5’-tetramethylbenzidine (TMB) served as the colorimetric substrate and was added at 100 μl/well, followed by 100 μl/well 2N H2SO4 to stop the color reaction. Plates were read at 405 nm in the SpectraMax 190 plate reader (Molecular Devices). For each plate, the mean absorbance value for the pre-immunization sample was subtracted from the mean absorbance value for corresponding post-dose sample for the same animal and dilution level. The resulting mean absorbance values for each of the sample dilution levels were used to generate a standard curve, which was created by fitting the points to a four-parameter curve fit. The center point titer for each of the samples was determined using ½ the maximum absorbance for the dilution set. The highest absorbance value for the dilution set was divided by two and entered into the equation of the line for the respective sample. The equation was then solved to determine the dilution factor (center point titer).
For the anti-TT assay, TT (100-fold dilution of stock) was coated using 100 μl/well in 3% bovine serum albumin (BSA) and incubated at 2–8°C overnight. Plates were washed with PBS with 0.25% Tween-20 (PBST) and then blocked with StartingBlock™ Blocking Buffer (Thermo Fisher Scientific) for 10 minutes at room temperature. Plates were washed three times with PBST. Anti-TT standards and samples were diluted in 1% BSA, added to the wells and incubated for 29-35 minutes at room temperature. All samples were initially screened at a single 250-fold dilution. Positive samples from screening were then re-assayed at sample dilutions up to 5,000-fold (or greater if still positive at the highest dilution). Plates were then washed four times with PBST. Goat anti-monkey IgM-HRP or IgG-HRP (IgM-HRP, Rockland Immunochemicals; IgG-HRP, Bethyl Laboratories, Montgomery, TX) was added at 0.04 μg/ml for 25-35 minutes at room temperature, followed by six washes with PBST.
TMB served as the colorimetric substrate and was added at 100 μl/well, followed by 100 μL/well 2 N H2SO4 to stop the color reaction. Plates were read at 405 nm in the SpectraMax 190 plate reader. The experimentally-determined mean absorbance for each animal’s serum collected on Day 56 (pre-immunization with TT) was combined into a single data set and the mean absorbance and standard deviation was determined. The cut-point was established for determining if a response was positive by adding twice the standard deviation to the mean absorbance value. A separate cut-point was generated for each gender. Any mean absorbance above the cut-point was denoted as positive, and any mean absorbance below the cut-point was denoted as negative. The least concentrated dilution that resulted in a positive value was reported as the titer (cut-point titer).
Study 6: Adult rhesus monkey in vivo toxicity (4-week)
A 4-week immunotoxicity study in adult rhesus monkeys, followed by an 8-week treatment-free recovery period, evaluated the effects of natalizumab on white blood cells (WBC) and lymphocyte populations in a second monkey species. This study was originally conducted to evaluate potential effects of natalizumab in combination with Avonex™ prior to the start of human clinical trials that would use the two together. The rhesus monkey was used instead of the cynomolgus monkey because the rhesus monkey is pharmacologically responsive to Avonex, whereas the cynomolgus monkey is not and data on the appropriate dosing and effects of Avonex were known for this species. In addition to control and combination test groups, groups receiving only natalizumab or Avonex alone were included in the study to allow for appropriate interpretation of results in the combination groups. Only the effects of natalizumab alone are discussed in this paper, as no evidence for combination effects were observed.
Animals between the ages of 2 and 6 years (5/sex/group) received vehicle control or natalizumab at doses of 30 or 60 mg/kg by IV infusion at a dose volume of 10 ml/kg over a 30-minute period once weekly for 4 weeks. Three animals per sex per test group were sacrificed on Day 29 and the remaining 2 animals per sex per group were sacrificed on Day 85. Clinical, physical, and laboratory examinations were conducted throughout the study. Histopathologic and immunochemistry evaluations of tissues were conducted at necropsy, as were determinations of organ weights. Blood for clinical pathology was collected twice pre-study and on Days 2, 16, 28, 56, and 84. Hematology analysis was done with an Abbott CELL-Dyn® 3500R Multiparameter Automated Hematology Analyzer (Abbott Diagnostics, Santa Clara, CA). Whole blood for FACS analysis was taken twice pre-study, prior to dosing on Day 1, and on Days 2, 16, 28, 56, and 84 for evaluation of lymphocyte populations using CD2 (Coulter, Miami, FL), CD3, CD4 (Pharmingen), CD8, and CD20 (Immunotech, Inc.) markers. Flow cytometric analyses were conducted using FACSCalibur analyzer (Becton Dickinson) equipped with an argon laser capable of acquiring 4-color fluorescence and two scatter parameters via CellQuest acquisition software. In addition to full necropsy with histopathological evaluation of complete tissue panels, immunohistochemical evaluation of T- and B-cell populations was carried out in samples of spleen, thymus, and mesenteric lymph nodes using antibodies to CD3 and CD20, respectively (Signet, Dedham, MA). The studies are summarized in .
Table 1. Summaries of the studies.
Results
Overall physical, clinical, and laboratory findings in monkey studies
Natalizumab was well tolerated in the five monkey studies (N = 116), with no clinically relevant natalizumab-related changes observed in terms of body weight, food consumption, serum chemistries, coagulation parameters, blood pressure, heart rate, electrocardiograms, or other measures evaluated during physical and clinical examinations. With the exception of scheduled necropsies, no animals died during the studies. A total of six animals experienced hypersensitivity-type reactions to natalizumab. One female in the 10 mg/kg group in Study 3 experienced severe reactions on two occasions and was withdrawn from the study. Of the other five animals (two in Study 3, one in Study 4, and two in Study 5), four experienced one or two milder, transient hypersensitivity-type reactions that resolved rapidly without treatment and did not necessitate removing the animals from the study or discontinuing dosing, and one responded well to treatment with diphenhydramine and dexamethasone (N = 1) and was kept on study, but further dosing was discontinued. These hypersensitivity reactions were considered to be consistent with immune complex formation (natalizumab and anti-natalizumab complexes) and complement activation.
This was supported by clinical laboratory data in the two animals with serious reactions requiring the discontinuation of dosing that demonstrated significant drops in total haemolytic complement activity (CH50) of ≥ 50% and the appearance of immune complexes (measured by the CIC-Raji assay) immediately following (within 30 minutes) the triggering natalizumab dose but not following a placebo dose. All animals with reactions were anti-natalizumab antibody positive, but not all antibody positive animals had reactions, indicating individual variation in sensitivity to the development of clinically observable reactions.
Natalizumab exposure
Natalizumab demonstrated significant immunogenicity in cynomolgus and rhesus monkeys and development of an anti-natalizumab response resulted in the reduction or elimination of exposure in animals developing this response, as seen in . On a weekly dosing schedule, natalizumab dose levels of ≤ 10 mg/kg (Studies 3–5) were of limited value for evaluation of treatment-related effects because of this restriction of exposure. Weekly doses of 30 or 60 mg/kg (Studies 3–6) provided cohorts of animals that maintained continuous exposure for as long as 6 months, with 50–67% of animals at 30 mg/kg and 60–100% of animals at 60 mg/kg maintaining continuous exposure. At these dose levels, even animals that did not maintain continuous exposure experienced natalizumab exposure for the majority of the week-long dosing interval, based on immediate post-dose serum concentrations and the half-life of natalizumab in monkeys of approximately 3 days. In animals maintaining continuous exposure in any given study, pre and post-dose natalizumab serum concentrations showed greater individual variation by the last dose than that seen following the second dose (Day 8), an indication that anti-natalizumab antibodies may be present in some of these animals as well, but cannot be measured due to assay interference. However, all these animals had natalizumab levels at or significantly above the level required for full pharmacologic activity (1–5 μg/ml) even at the nadir prior to the next dose. Further, calculations based on data from the 6-month studies of the cumulative area under the concentration–time curve demonstrated that the majority of animals had exposures of at least 30-fold those of human exposure levels.
Table 2. Natalizumab exposure levels.
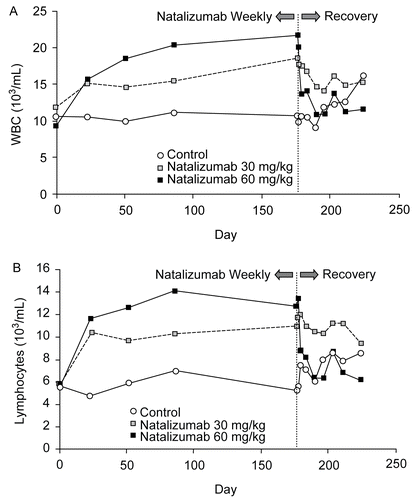
Circulating WBC counts
In all five monkey studies, persistent, dose-related increases in WBC and lymphocyte counts occurred among animals with serum levels of natalizumab equal to or greater than that required to saturate the available α4 integrin pool (i.e., levels at or above the range of 1–5 μg/ml). shows the fold increase for WBC and lymphocyte counts at various intervals in these studies. WBC and lymphocyte counts returned to normal following cessation of dosing and serum levels of natalizumab decreased below the saturation threshold (, Study 3) or when anti-natalizumab antibody responses sufficient to compromise exposure occurred (as in virtually all animals in dose levels of ≤ 10 mg/kg). As an example of this latter phenomenon, lymphocyte counts from the 10 mg/kg dose group of Study 4 show that 4/6 animals had elevated counts (1.5-fold baseline value) at Day 23 of the study, but only 1/6 had elevated counts at Day 86 (and Day 177). Animals with elevated counts had no measurable anti-natalizumab antibodies, while those without elevated counts did. The increases in WBC in all studies were primarily attributable to increases in lymphocyte counts, which were approximately 1.3- to 2.4-fold greater than baseline and vehicle/saline control levels (except for the 3 mg/kg arm of the rhesus monkey study, where no increase was shown, as seen in ). Neutrophil counts were usually within the normal range (0.61-fold to 1.29-fold that of controls; mean 0.9). Monocyte, eosinophil, and basophil counts, which are normally very low in these monkeys, were essentially unchanged or slightly higher in animals receiving natalizumab relative to the levels in control animals.
Table 3. Mean change in white blood cell (WBC) and lymphocyte counts in studies of cynomolgus and rhesus monkeys receiving natalizumab.
Peripheral blood phenotyping
Phenotyping was performed in Studies 2, 3, 4 and 6 and examined the effects of natalizumab treatment on WBC populations, with emphasis on lymphocytes. Markers evaluated included CD2 (lymphocytes, monocytes, NK cells), CD3 (T-cells), CD4 (T-helper cells), CD8 (T-suppressor cells), CD14 (monocytes), CD20 (B-cells), and CD34 (immature hematopoietic cells). The specific antibody panel used for any particular study was determined by the available reagents and the assay qualification/validation status for reagents at the time a given study was performed. Evaluation of the CD34 population was included, as these cells have been shown to be dependent on the expression of α4β1 for retention in the bone marrow (for a review see Oostendorp and Dörmer, Citation1997) and, therefore, represent a cell population whose homeostasis might be disrupted by natalizumab treatment.
A summary of phenotyping results is shown in and representative results from the juvenile cynomolgus study are shown in . The percentage of cell types was determined as the percent of all WBC for CD2, CD14, and CD34 and as the percent of all lymphocytes for CD3, CD4 and CD8. Phenotyping results were consistent between shorter duration studies (Studies 2 and 6 [28 days]) and longer duration studies (Studies 3 and 4 [6 months]) and between monkey species (Studies 2–4 [cynomolgus] versus Study 6 [rhesus]). As noted by hematology, there were significant increases in absolute WBC and lymphocyte counts by FACS from these studies. The increases in absolute T-cell counts and T-cell subset counts were generally comparable in magnitude, i.e., significant changes in the CD4/CD8 ratios were not observed. For example, in animals receiving natalizumab at a dose of 60 mg/kg weekly in the 6-month juvenile study, the levels of CD3+ T-cells, CD4+ T-helper cells, and CD8+ T-cytotoxic cells increased by approximately 2.5-, 2.3-, and 2.1-fold, respectively, over levels observed in control animals.
Figure 2. Phenotype analysis of circulating leukocytes in Study 4 by (A) percentages and (B) absolute counts of cellular subsets.
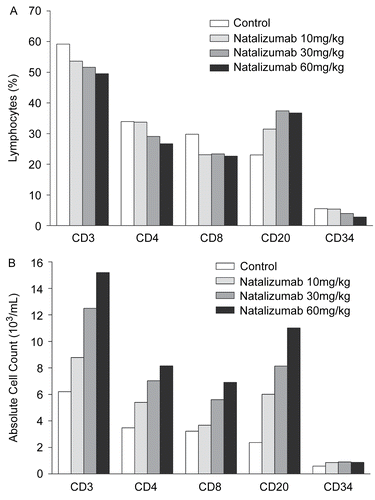
Table 4. Summary of phenotyping of WBC populations in monkeys treated with natalizumab.
There were no statistically significant changes in the percentage of T-cells (CD3), T-cell subsets (CD4, CD8), or B-cells (CD20) in animals receiving natalizumab at doses up to 60 mg/kg weekly. However, a non-significant trend toward a dose-related increase in B-cell and decrease in T-cell percentages was observed in all three studies (Studies 2, 4, and 6) where these populations were measured, with the exception of the CD4 population in Study 2. It is not clear whether the lack of effect on this cell population in this one study was real, indicating a need for longer treatment time to see the effect, or chance outcome resulting from the relatively small effect size - though the presence of the effect on the CD8 population in the same study and on both CD4 and CD8 populations in the rhesus study of the same duration make the latter explanation more likely. Treatment with natalizumab resulted in increases in the number of circulating stem cells (CD34+) in the juvenile cynomolgus study (Study 4) to slightly less than 2-fold that of controls (594 cells/μl vs. 850-910 cells/μl), though the overall percentage of the cells in the total WBC pool actually decreased due to the concomitant large increases in total cell count driven by the lymphocyte increases. Increases in CD34+ cells were not observed in the adult cynomolgus study (Study 3). However, the percentage of these cells in the adult animals is extremely low (undetectable in about half of animals), making the determination of changes difficult. The percentage of monocytes (CD14+) was increased and this increase reached statistical significance in male, but not female animals..
Organ weights, histopathology, and immunohistochemistry
Natalizumab-related changes detected post-mortem (some not evident in all studies) were restricted to increases in spleen weights (absolute and/or relative), mild-to-moderate follicular hyperplasia in the lymph node and spleen, and minimal–to-mild focal leukocyte infiltrates (characterized as mixed leukocytic) in the liver. An increase in spleen weights of 28–148% was the most consistent post-mortem finding in these studies, being observed in at least some animals in all studies at dose levels of ≥ 10 mg/kg and was statistically significant in some studies at ≥ 30 mg/kg. Lymph node hyperplasia was observed in the 28-day rhesus study and in one 60 mg/kg treatment animal in the 6-month adult cynomolgus study. Hyperplastic lymphoid follicles (spleen and lymph node) retained normal anatomic relationships and boundaries and were considered characteristic of follicles exhibiting polyclonal lymphocyte expansion and/or accumulation.
Examination of immunohistochemically stained spleen, mesenteric lymph node, and thymus tissue sections indicated that in the control and in treated groups, the distribution of cells that stained positively for CD3 or CD20 was consistent with the expected distribution for T-lymphocytes and B-lymphocytes within these tissues. Leukocyte infiltrates were observed only at dose levels ≥ 30 mg/kg in the 28-day rhesus study and in the juvenile (but not adult) cynomolgus study. In all studies with recovery groups, these changes were found to be fully reversible following the elimination of natalizumab from circulation; nor were they observed in animals that lost exposure during the study due to the formation of anti-natalizumab antibodies. For example, the single animal in the 10 mg/kg dose group of Study 4 that maintained natalizumab exposure through to necropsy at Day 177 demonstrated these changes, but the rest of the animals in the same cohort that had lost exposure did not.
Immune function tests in monkey PBMC and spleen cells
In Study 2, administration of natalizumab at doses up to 30 mg/kg every other day for 4 weeks had no statistically significant effect on basal NK cell activity of either PBMC or spleen cells or on the blastogenic response of PBMC and spleen cells to PHA or LPS (). While not statistically significant, a small but consistent increase in proliferation to PHA was observed in the PBMC and spleen cells of natalizumab-treated animals, indicating that the T-cells in these populations were slightly more responsive to the mitogenic effects of PHA than those of untreated animals, though the mechanism for this change or its biological relevance (given the small effect size) are unknown. It should be noted that cellular responses in the LPS assay were extremely low, indicating the possibility that this assay was not optimised and/or was relatively insensitive and therefore potentially would have missed treatment-related effects.
Table 5. Results of immune function tests in lymphocytes and spleen cells obtained from cynomolgus monkeys in Study 2 that were treated with natalizumab 30 mg/kg every other day for 4 weeks.
Primary and secondary humoral responses
Cynomolgus monkeys receiving natalizumab at either 3 or 30 mg/kg weekly in Study 5 were capable of mounting IgG and IgM immunologic responses to the T-cell-dependent antigens KLH and TT that were essentially indistinguishable from those of control animals, although a slight reduction in the primary response was observed at early time points (, ). Initial anti-KLH IgM levels were significantly lower at Days 17 and 21 post-KLH administration in animals receiving natalizumab than in control animals (p < 0.05), but were not significantly different from control levels at subsequent time points. Non-detectable anti-TT IgM and anti-IgG titers were also more frequent at earlier time points in animals receiving natalizumab at 30 mg/kg weekly than in control animals or animals receiving natalizumab at 3 mg/kg weekly. At Day 66, 50% of animals in the high-dose group had non-detectable anti-TT IgM titers compared with 8% of animals in the other two groups. Similarly, 83% of animals in the high-dose group had non-detectable anti-TT IgG titers at Day 70 compared with 25% of animals in the other two groups. However, anti-TT IgM and IgG titers were similar in all three treatment groups after administration of the TT booster immunization on Day 70. While there appeared to be a slight, yet highly variable effect of natalizumab on the early immune response in this study, the biological significance of this observation is unclear in light of the considerable inter-animal variability in the magnitude of each response within dose groups () and the absence of a similar effect following subsequent boost immunizations.
Figure 3. (A) IgM and (B) IgG responses to immunization with keyhole limpet hemocyanin (KLH; Days 7, 35, and 63) and tetanus toxoid (TT; Days 56 and 70) in Study 5. Closed symbols/solid lines, anti-KLH antibodies (center-point titer); open symbols/dotted lines, anti-TT antibodies (cut-point titer); circles, control; triangles, natalizumab 3 mg/kg; squares, natalizumab 30 mg/kg; *p < 0.05 vs. control.
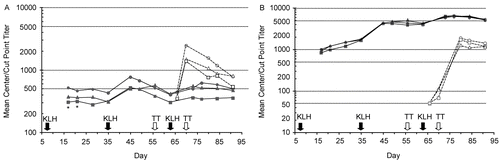
Table 6. Anti-KLH and anti-TT titers in monkeys treated with natalizumab.
Human in vitro PBMC and monocyte immune function tests
Exposure of human cells to natalizumab in vitro did not appear to significantly alter immune regulatory and effector cell functions in either PBMC or monocytes. illustrates the results of the highest dose, 100 μg/ml. Natalizumab at concentrations of 0.01–100 μg/ml had no effect on anti-CD3-mediated or T-cell mitogen (phytohemagglutinin, PHA)-mediated lymphoproliferation, except for a higher mean optical density (OD) (16%) that was statistically significant when PBMC were incubated with PHA at the lowest concentration (0.5 μg/ml) in the presence of natalizumab at 100 μg/ml. In the absence of any effect at higher PHA concentrations or at any other natalizumab concentration, it is unlikely that this is a biologically meaningful result. Additionally, natalizumab at concentrations of 0.1–100 μg/ml had no effect on anti-CD3-mediated cytokine production by PBMC or on LPS-mediated cytokine production by monocytes. Basal NK cell activity was also unaffected by exposure to natalizumab. IL-2 has been shown to stimulate the anti-tumor cytolytic potential of NK cells (Robinson and Morstyn, Citation1987; Naume and Espevik, Citation1991; Meropol et al., Citation1996; Mirandola et al., Citation2004), and this enhanced functionality appears to have been preserved in the presence of natalizumab. Anti-CD3-mediated cytotoxicity was not significantly different between T-cells exposed to natalizumab and T-cells without natalizumab.
Table 7. Results of in vitro immune function tests in PBMC and monocytes from healthy human volunteers (N=5).
Discussion and conclusions
As expected, in these animal studies, anti-natalizumab antibodies developed in a large proportion of the monkeys studied, and led to the lowering of serum concentrations of natalizumab in some animals. The formation of anti-natalizumab antibodies in monkeys is directly attributable to the fact that natalizumab, a recombinant humanized monoclonal antibody, is a foreign protein in non-human primate species because of its protein structure, which is approximately 95% human and 5% murine. In clinical trials of natalizumab in patients with MS or CD, antibodies to natalizumab were detected in only approximately 10% of the patients. Approximately half of these antibodies were transient, being detected only on a single sample at 12 weeks and not detected at later time points.
Persistent antibodies, observed in approximately 5% of patients, were associated with increased clearance of drug, loss of efficacy, and increased likelihood of hypersensitivity reactions (TYSABRI, Citation2008). Nevertheless, for the monkey studies presented here, the fact that antibodies developed in a large proportion of the animals represents a limitation to the interpretation of the study results at lower dose levels. Counterbalancing this is the fact that at higher dose levels (30– 60 mg/kg), at least a 30-fold safety margin was achieved in the majority of animals (based on cumulative AUC, 6-month studies), which suggests that the overall results reflect sufficient exposure to natalizumab and adequate safety margins for appropriate evaluation of potential natalizumab-related effects.
The results from the six nonclinical studies confirm that the pharmacologic activity of natalizumab is consistent with and essentially limited to the inhibition of leukocyte trafficking from the circulation into surrounding tissues. Natalizumab did not adversely affect lymphocyte proliferation, lymphocyte percentages, cytokine production, NK cell activity, T-cell cytolytic function, or humoral responses to T-cell-dependent antigens. This was not unexpected, as these post-lymphopoietic functions are mediated through cell-cell surface molecule interactions that are independent of α4 integrins. The increases in circulating WBC and spleen weights that were consistently observed in the monkey studies were expected, given the pharmacologic activity of natalizumab (i.e., inhibition of extravasation of lymphocytes from the circulation into parenchymal tissues). Indeed, increases in WBC counts were also observed in the human clinical studies of natalizumab.
In patients with MS receiving natalizumab with or without concomitant interferon-β-1a, increases were seen in lymphocyte, monocyte, eosinophil, and basophil counts (but not neutrophil counts) that are consistent with the pharmacodynamics of natalizumab (Miller et al., Citation2003; Polman et al., Citation2006; Rudick et al., Citation2006). Similar to the results in the monkey studies, changes in WBC counts were not clinically significant and were reversible to baseline levels within 16 weeks of discontinuing treatment. In CD trials, lymphocyte counts increased up to approximately 2-fold, but median and mean counts did not exceed the upper limit of normal (Gordon et al., Citation2001; Ghosh et al., Citation2003; Sandborn et al., Citation2005; Hyams et al., Citation2007; Sands et al., Citation2007).
The increase in spleen weights observed in the monkey studies is also consistent with the mechanism of action of natalizumab, which results in increased circulating WBC due to decreased trafficking of lymphocytes across endothelial cells into tissues, as well as possible demargination of lymphocytes from tissues. In humans, it has been estimated that 10–15% of the exchangeable B-cell pool and approximately 25% of the exchangeable T-cell pool resides in the spleen (Christensen et al., Citation1978; Willfuhr et al., Citation1990). The ∼2-fold increase in monkey spleen weights is consistent with increases in the exchangeable pools secondary to the increase in circulating WBC.
Hyperplastic lymphoid follicles (spleen and lymph node) observed at necropsy retained normal anatomic relationships and boundaries and were considered characteristic of follicles exhibiting polyclonal lymphocyte expansion and/or accumulation. Latent gammaherpesviruses of humans (Epstein Barr virus – EBV) and nonhuman primates (lymphocriptovirus – LCV) are capable, under conditions of host immunosuppression, of undergoing recrudescence and inducing lymphoproliferative disease. However, lymphoproliferation that occurs under these conditions in humans and nonhuman primates is well characterized, well described and comprises three distinct diagnostic and prognostic entities (Knowles et al. Citation1995; Schmidtko et al, Citation2002) and was explicitly ruled out as the cause of this finding during the peered reviewed pathology evaluation. Rather this finding is probably secondary to the increase in the exchangeable WBC pool already noted above, with possible contributions from the potential for immunogenicity of natalizumab in these monkey species.
The incidence of leukocytic infiltrates of the liver, a normal background finding in monkeys, was increased in juvenile cynomolgus monkeys and in rhesus monkeys but not in adult cynomolgus monkeys. While the exact mechanism for this effect is not clear, its reversibility following clearance of natalizumab and the return of WBC counts to normal indicates that it may also be associated with the change in cell trafficking patterns resulting from the mechanism of action of natalizumab, and that this effect is both species-dependent as well as age-dependent within a species.
A non-significant trend toward increased B-cell:T-cell ratios was observed in the 6-month juvenile cynomolgus monkey study. However, acute and chronic administration of natalizumab to cynomolgus monkeys did not significantly alter the percentages of B-cells, T-cells, and T-cell subsets (e.g., CD4:CD8 ratios) in any of the five monkey studies, although significant increases in both absolute T-cell and B-cell counts were observed. Similarly, increases in both T-cell and B-cell counts were reported in an early clinical study in CD (Gordon et al., Citation2001). In a study of blood samples from patients with MS, Stüve and colleagues (Citation2006) reported a progressive but non-significant decrease in CD4:CD8 ratios in peripheral blood and a significant decrease in CD4:CD8 ratios in cerebrospinal fluid (CSF) in association with increasing numbers of natalizumab doses. However, CD4:CD8 ratios in CSF returned to normal within 6 months after discontinuing treatment with natalizumab.
There was no evidence that natalizumab altered primary and secondary humoral responses to T-cell dependent antigens, except for slight reductions in the primary response at early time points, or altered immune regulatory and effector cell functions in peripheral blood leukocytes from monkeys and humans, monkey spleen cells, or monocytes from humans. This suggests that natalizumab does not suppress or enhance the immune system or alter complex immune functions (e.g., interaction of macrophages, T-helper cells, B-cells).
These data are consistent with the safety profile of natalizumab with respect to the rates of infection observed in controlled clinical trials of natalizumab in patients with CD and MS. Overall rates of infection were similar between MS patients receiving natalizumab and those receiving placebo (1.5 infection per patient-year) while slightly more CD patients receiving natalizumab had infections than placebo patients (1.7 vs. 1.4) (TYSABRI, Citation2008). The rates of serious infections were comparable between treatment groups. However, opportunistic infections (OI) have been reported, particularly in the CD clinical trials ( < 1%), possibly due to the more frequent use of concomitant immune suppressive therapy in CD than in MS. There is also a suggestion of increased rate of herpes infection with natalizumab therapy.
A serious OI resulting from the reactivation of the JC virus, PML, has been reported in patients in Phase III clinical trials and the post-marketing setting who were taking natalizumab at the commercially approved dose of 300 mg/month. Concomitant use of immunomodulatory therapy could not be excluded as a confounder in the initial three cases in clinical trials involving more than 3,000 patients, but five recent cases have been reported in MS patients in the post-marketing setting with use of natalizumab as a monotherapy. As of the end of December 2008, including clinical trials and post-marketing experience, ≈ 48,300 patients have been treated with natalizumab. Of these, ≈ 20,000 have had exposure for at least 1 year, ≈ 10,700 for 18 months or more, and ≈ 4,300 have had exposure for 24 months or more. The specific link between natalizumab therapy and PML remains unclear, although reduced immune surveillance of the CNS due to blockade of lymphocyte trafficking into tissue may play a role. Natalizumab is approved for use in MS and CD (United States, only for CD), but as a monotherapy with warnings against use in immune compromised patients or those taking immune modulating medications. Extensive safety monitoring is in place to track, and hopefully reduce or mitigate, the occurrence of OIs, particularly PML.
In conclusion, administration of natalizumab to cynomolgus and rhesus monkeys resulted in increased levels of circulating WBC, consistent with the pharmacologic action of natalizumab, but did not alter the percentages of B-cells, T-cells, and T-cell subsets. Increases in WBC counts occurred at doses approximately equivalent to recommended clinical doses. Except for a modest and highly variable delay in humoral response to T-cell dependent antigens, natalizumab had no measurable effects on any of the immune functions evaluated in vitro or in vivo. Overall, a lack of measurable effects on immune function was consistently observed in both in vitro and in vivo studies.
Acknowledgments
The authors wish to thank the following for analytical and bioanalytical support: Julie Taylor, Shulah Iflah, Jessica Krakow, and James Chin, of Elan Pharmaceuticals, Inc.; Michaela Lerner, Matthew Cooper, and Meena Subramanyam of Biogen Idec, Inc.; Patricia Giclas of National Jewish Clinical Laboratories; Jerome Moore of Pacific BioDevelopment; and Caudex Medical Inc. for writing and editorial assistance.
Declaration of interest: N. Wehner is a former employee of and current consultant for Elan Pharmaceuticals. S. Parker and J. Clarke are employees of Biogen Idec. C. Gasper, G. Shopp, J. Nelson, and K. Draper, report no conflicts of interest. The authors alone are responsible for the content of the paper.
References
- Arroyo, A. G., Yang, J. T., Rayburn, H., and Hynes, R. O. 1996. Differential requirements for α4 integrins during fetal and adult hematopoiesis. Cell 85:997–1008.
- Arroyo, A. G., Yang, J. T., Rayburn, H., and Hynes, R. O. 1999. α4 integrins regulate the proliferation/differentiation balance of multi-lineage hematopoietic progenitors in vivo. Immunity. 11:555–566.
- Banerjee, E. R., Latchman, Y. E., Jiang, Y., Priestley, G. V., and Papayannopoulou, T. 2008. Distinct changes in adult lymphopoiesis in Rag2−/- mice fully reconstituted by α4-deficient adult bone marrow cells. Exp. Hematol. 36:1004–1013.
- Christensen, B. E., Jønsson, V., Matre, R., and Tønder, O. 1978. Traffic of T- and B-lymphocytes in the normal spleen. Scand. J. Haematol. 20:246–257.
- Danese, S., Semeraro, S., Marini, M., Roberto, I., Armuzzi, A., Papa, A., and Gasbarrini, A. 2005. Adhesion molecules in inflammatory bowel disease: Therapeutic implications for gut inflammation. Dig. Liver. Dis. 37:811–818.
- Ghosh, S., Goldin, E., Gordon, F. H., Malchow, H. A., Rask-Madsen, J., Rutgeerts, P., Vyhnálek, P., Zádorová, Z., Palmer, T., and Donoghue, S. 2003. Natalizumab for active Crohn’s disease. N. Engl. J. Med. 348:24–32.
- Gordon, F. H., Lai, C. W., Hamilton, M. I., Allison, M. C., Srivastava, E. D., Fouweather, M. G., Donoghue, S., Greenlees, C., Subhani, J., Amlot, P. L., and Pounder, R. E. 2001. A randomized placebo-controlled trial of a humanized monoclonal antibody to α4 integrin in active Crohn’s disease. Gastroenterology 121:268–274.
- Heilbronn, R., Albrecht, I., Stephan, S., Bürkle, A., and zur Hausen, H. 1993. Human cytomegalovirus induces JC virus DNA replication in human fibroblasts. Proc. Natl. Acad. Sci. USA 90:11406–11410.
- Hyams, J. S., Wilson, D. C., Thomas, A., Heuschkel, R., Mitton, S., Mitchell, B., Daniels, R., Libonati, M. A., Zanker, S., and Kugathasan, S. 2007. Natalizumab therapy for moderate to severe Crohn disease in adolescents. J. Pediatr. Gastroenterol. Nutr. 44:185–191.
- Kleinschmidt-DeMasters, B.K., and Tyler, K.L. 2005. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon β-1a for multiple sclerosis. N. Engl. J. Med. 353:369–374.
- Knowles, D. M., Cesarman, E., Chadburn, A., Frizzera, G., Chen, J., Rose, E. A., and Michler, R. 1995. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of post-transplantation lymphoproliferative disorders. Blood 85:552–565.
- Langer-Gould, A., Atlas, S.W., Green, A.J., Bollen, A.W., and Pelletier, D. 2005. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353:375–381.
- Lasky, L. A. 1996. Hematopoiesis: Wandering progenitor cells. Curr. Biol. 6:1238–1240.
- Meropol, N. J., Porter, M., Blumenson, L. E., Lindemann, M. J., Perez, R. P., Vaickus, L., Loewen, G. M., Creaven, P. J., Wilkes, K. A., Giedlin, M. A., and Caligiuri, M. A. 1996. Daily subcutaneous injection of low-dose interleukin-2 expands natural killer cells in vivo without significant toxicity. Clin. Cancer. Res. 2:669–677.
- Miller, D. H., Khan, O. A., Sheremata, W. A., Blumhardt, L. D., Rice, G. P., Libonati, M. A., Willmer-Hulme, A. J., Dalton, C. M., Miszkiel, K. A., and O’Connor, P. W. 2003. A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 348:15–23.
- Mirandola, P., Ponti, C., Gobbi, G., Sponzilli, I., Melloni, E., and Vitale, M. 2004. The response of human natural killer cells to interleukin-2. J. Endocrinol. Invest. 27 (S6):146–150.
- Naume, B., and Espevik, T. 1991. Effects of IL-7 and IL-2 on highly enriched CD56+ natural killer cells. A comparative study. J. Immunol. 147:2208–2214.
- National Center for Biotechnology Information (NCBI). GenBank accession number XM 001100929. http://www.ncbi.nlm.nih.gov. Accessed 26 March 2009.
- Oostendorp R. A., and Dörmer, P. 1997. VLA-4-mediated interactions between normal human hematopoietic progenitors and stromal cells. Leuk. Lymphoma 24:423–435.
- Polman, C. H., O’Connor, P. W., Havrdova, E., Hutchinson, M., Kappos, L., Miller, D. H., Phillips, J. T., Lublin, F. D., Giovannoni, G., Wajgt, A., Toal, M., Lynn, F., Panzara, M. A., and Sandrock, A. W. 2006. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354:899–910.
- Qian, H., Georges-Labouesse, E., Nyström, A., Domogatskaya, A., Tryggvason, K., Jacobsen, S. E., and Ekblom, M. 2007. Distinct roles of integrins α6 and α4 in homing of fetal liver hematopoietic stem and progenitor cells. Blood 110:2399–2407.
- Robinson, B. W., and Morstyn, G. 1987. Natural killer (NK)-resistant human lung cancer cells are lysed by recombinant interleukin-2-activated NK cells. Cell. Immunol. 106:215–222.
- Rose, D. M., Han, J., and Ginsberg, M. H. 2002. α4 integrins and the immune response. Immunol. Rev. 186:118–124.
- Rudick, R. A., Stuart, W. H., Calabresi, P. A., Confavreux, C., Galetta, S. L., Radue, E. W., Lublin, F. D., Weinstock-Guttman, B., Wynn, D. R., Lynn, F., Panzara, M. A., and Sandrock, A. W. 2006. Natalizumab plus interferon β-1a for relapsing multiple sclerosis. N. Engl. J. Med. 354:911–923.
- Sandborn, W. J., Colombel, J. F., Enns, R., Feagan, B. G., Hanauer, S. B., Lawrance, I. C., Panaccione, R., Sanders, M., Schreiber, S., Targan, S., van Deventer, S., Goldblum, R., Despain, D., Hogge, G. S., and Rutgeerts, P. 2005. Natalizumab induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 353:1912–1925.
- Sands, B. E., Kozarek, R., Spainhour, J., Barish, C. F., Becker, S., Goldberg, L., Katz, S., Goldblum, R., Harrigan, R., Hilton, D., and Hanauer, S. B. 2007. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn’s disease not in remission while receiving infliximab. Inflamm. Bowel. Dis. 13:2–11.
- Schmidtko, J., Wang, R., Wu, C., Mauiyyedi, S., Harris, N., Della Pelle, P., Brousaides, N., Zagachin, L., Ferry, J., Wang, F., Kawai, T., Sachs, D., Cosimi, B.,,and Colvin, R. 2002. Post-transplant lymphoproliferative disorder associated with an Epstein-Barr-related virus in cynomolgus monkeys. Transplantation 73:1431–1439.
- Stüve, O., Marra, C. M., Bar-Or, A., Niino, M., Cravens, P. D., Cepok, S., Frohman, E. M., Phillips, J. T., Arendt, G., Jerome, K. R., Cook, L., Grand’Maison, F., Hemmer, B., Monson, N. L., and Racke, M. K. 2006. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch. Neurol. 63:1383–1387.
- TYSABRI. 2008. [Package insert]. Elan Pharmaceuticals, Inc. South San Francisco, CA; http://www.tysabri.com.
- Van Assche, G., Van Ranst, M., Sciot, R., Dubois, B., Vermeire, S., Noman, M., Verbeeck, J., Geboes, K., Robberecht, W., and Rutgeerts, P. 2005. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N. Engl. J. Med. 353:362–368.
- Willführ, K. U., Westermann, J., and Pabst, R. 1990. Absolute numbers of lymphocytes subsets migrating through the compartments of the normal and transplanted rat spleen. Eur. J. Immunol. 20:903–911.
- Yousry, T. A., Major, E. O., Ryschkewitsch, C., Fahle, G., Fischer, S., Hou, J., Curfman, B., Miszkiel, K., Mueller-Lenke, N., Sanchez, E., Barkhof, F., Radue, E. W., Jäger, H. R., and Clifford, D. B. 2006. Evaluation patients treated with natalizumab for progressive multifocal leukoencephalopathy. N. Engl. J. Med. 354:924–933.