Abstract
Some organophosphate insecticides have immunomodulating capacities, but it is unknown whether different compounds within this class affect the immune system to the same extent. In this in vitro study, human immortalized T-lymphocytes or bronchial epithelial cells were treated with diazinon or chlorpyrifos in the absence or presence of cellular stress factors, thereby mimicking a stimulated immune system. Cytotoxicity was determined and cytokine release or cytokine-promoter studies were performed to study immunomodulatory effects of these chemicals, whereby the same concentrations of chlorpyrifos and diazinon were used. Results showed that chlor- pyrifos was cytotoxic at concentrations ≥ 250 μM, whereas diazinon was not toxic at concentrations up to 1 mM. The immunomodulatory effects of these two compounds were similar for most cytokine promoters tested and induction of cellular stress enhanced these effects. The results were compared to data obtained with blood mononuclear cells, which confirmed the results of stably transfected cell lines, but refer to a higher sensitivity of primary cells. In conclusion, these two pesticides act in a different manner on cell viability and on some immune parameters, but cell viability was not linked to immunomodulation. The results also imply that healthy and diseased individuals are differentially affected by these pollutants.
Introduction
Pesticides are ubiquitous in the environment as a result of their widespread domestic and agricultural use. Organophosphate pesticides (OPs) constitute 70% of the insecticides used in the United States, and chlorpyrifos was the most used OP prior to 2001 when EPA restricted its use. Although the toxicity of chlorpyrifos and, to a minor degree, diazinon have been studied in animals, including rodents, the human data are limited to case reports and observational studies. Several controlled epidemiological studies have examined chronic neurological effects following acute poisoning with OPs. Other epidemiological studies examined occupational exposure either during the manufacture and formulation or the use of OPs in farming (Albers et al., Citation2004; Burns et al., Citation2006). In the Agricultural Health Study cohort, chlorpyrifos and diazinon were associated with a significant increased lung cancer risk (Alavanja et al., Citation2004) and chlorpyrifos was associated with prostate cancer (Blair et al., Citation2005). The exposure to OPs is not limited to occupational exposure but also includes exposure via dust and aerosols (Fenske et al., Citation2002; Hore et al., Citation2005) or ingestion of contaminated food.
Almost all detailed studies of OPs immunomodulation, both in vivo and in vitro, were performed in rodents. These studies have shown that oxidative stress caused by diazinon and its metabolites can contribute towards organ pathologies such as necrotic degeneration of trabaeculea (spleen and thymus), hyperplasia of cortex and medulla (lymph nodes, thymus) and hyperplasia of white and red pulp of the spleen (Handy et al., Citation2002). Chlorpyrifos induces lipid peroxidation and DNA single strand breaks (Bagchi et al., Citation1995) leading to immune effects that are comparable to those observed with diazinon, with clear effects on monocytes and T- and B-lymphocytes. In general, most experimental models have identified decreased immune functions following acute exposure. Increased lymphocyte counts paralleled by reduced responsiveness to immune stimulating factors were observed following chlorpyrifos (Blakley et al., Citation1999) or diazinon exposure (Handy et al., Citation2002). Contrary to such observations, enhanced immune activity was detected following exposure to lower non-cholinergic doses (Pruett, Citation1992). Finally, exposure to organophosphate pesticides has also been associated with respiratory symptoms and may be related to asthma and other health effects (Albers et al., Citation2004).
Taken together, the epidemiological data, when combined with the results obtained in the different in vivo and in vitro experiments described above, indicate that the OPs chlorpyrifos and diazinon could affect the immune system and thereby induce or stimulate the development of a number of diseases.
Since inhalation of OPs might affect bronchial epithelial cells, we analyzed the induction patterns of several cytokine promoters after exposure to chlorpyrifos or diazinon in the absence and presence of the pro-inflammatory cytokine tumor necrosis factor (TNF)-α. Asthma and allergic disorders are characterized by elevated levels of the TH2 cytokines interleukin-4 (IL-4) and IL-13, whereas, for example, a chronic inflammatory response in asthmatic airways is maintained by the TH1 cytokine interferon-γ (IFNγ). Therefore, IL-4 and IFNγ promoter induction in immortalized T-lymphocytes as a result of OP exposure was determined. Moreover, the results obtained with the immortal cell lines were compared with those obtained using human peripheral blood mononuclear cells, whereby the levels of secreted cytokines was determined after exposure to chlorpyrifos or diazinon. The goal of this in vitro study was to examine the concentration-dependent effects of chlorpyrifos and diazinon in the absence and presence of immune stimuli on cytokine regulation, to compare the two chemicals with each other and to determine the relation between the immunomodulatory effects and the cytotoxic effects of these chemicals.
Materials and methods
Chemicals
The two organophosphate pesticides chlorpyrifos (O,O-diethyl-O-(3,5,6-trichlor-2-pyridyl) thiophosphate, CAS 2921-88-2, MW = 350.59 g/mol) and diazinon (O,O-diethyl-O-(2-isopropyl-6-methyl-pyrimidin-4-yl) phosphorothioate, CAS 333-41-5, MW = 304.4 g/mol) were purchased in the highest purity available (PESTANAL®) from Riedel-de Haën (Sigma-Aldrich, Steinheim, Germany). Chlorpyrifos and diazinon were stored at 4°C and freshly dissolved (immediately before performing the cellular assays) in dimethyl sulfoxide (DMSO; SERVA, Heidelberg, Germany). Nine twofold dilutions of the OPs were used, whereby the highest concentration in cell culture was 1000 μM chlorpyrifos or diazinon. The final concentration of DMSO in the cell culture was 0.05%, independent of the concentration of OP used; this concentration of DMSO was not toxic to the cells (data not shown).
Reporter gene-based luciferase assay
The reporter gene cell lines used in this study were recently extensively described (Oostingh et al., Citation2008). In brief, the host cells used for the reporter cell lines were Jurkat and A549. The Jurkat cell line (ATCC, LGC Promochem, Wesel, Germany) is a T-cell lymphoma line where the T-cell receptor (TCR) signal transduction is intact, which indicates that original T-lymphocyte characteristics are conserved. A549 cells (ATCC, LGC Promochem) are derived from a lung epithelial carcinoma and these cells produce a range of cytokines and chemokines that are typical for epithelial cells upon suitable stimulation.
Jurkat cells were cultured in RPMI 1640, supplemented with L-glutamine, penicillin-streptomycin, glucose, HEPES, sodium pyruvate, and 10% heat-inactivated fetal calf serum (FCS). A549 cells were cultured in RPMI 1640, supplemented with L-glutamine, penicillin-streptomycin, and 10% FCS. All these cell culture reagents were obtained from PAA Laboratories (Pasching, Austria).
A nuclear factor (NF)-κB-binding sequence-transfected A549 cell line was purchased from Panomics (Fremont, CA) and used for our studies as described by the distributor. The human cell lines, Jurkat and A549, were transfected with an expression vector encoding luciferase and an insert that encodes for the promoter region of IL-4 (NCBI NM 000589), IL-6 (NCBI NM 000600), IL-8 (NCBI NM 000584), IFNγ (NCBI NM 000619), or TNFα (NCBI NM 000594), or for the binding domain of nuclear factor NF-κB as previously described (Oostingh et al., Citation2008).
Stable clones were cultured in the presence of G418 (0.5 μg/ml final concentration). The A549 cells were plated out on Day 1 in tissue culture treated 96-well plates at a density of 5 × 103 cells/well and left overnight to adhere and obtain their normal morphology. On Day 2, Jurkat cells were plated out in 96-well plates at a density of 5 × 104 cells/well. The A549 cells were treated on Day 2 with three different recombinant human tumor necrosis factor-α (rhTNFα) concentrations (1, 20, and 300 ng/ml) or left untreated. The IL-4 and IFNγ promoter-transfected Jurkat cell lines were treated with 10 μg/ml phytohemagglutinin (PHA) or left untreated. In addition, the OPs were added to the cell culture systems as indicated above.
Two separate tests were performed on these cultures; the luciferase assay and a cell viability test. The different cell lines were incubated for 24 hr in the presence of rhTNFα or PHA and/or OP, whereby two separate plates were used for each condition. After this incubation period, the luciferase assay was performed on the lysed cells from the first plate and the cell viability assay was performed on the second plate using the CellTiterBlue® assay (Promega, Mannheim, Germany). All combinations were analysed in triplicate and experiments were repeated at least three times.
Treatment of human pbmc with ops
Human peripheral blood mononuclear cells (PBMC) were isolated from buffy coats of healthy blood donors by density gradient centrifugation using Ficoll-Paque (Amersham Pharmacia Biotech, Freiburg, Germany). The cell number was adjusted to 1 × 106 PBMC/ml in RPMI 1640 supplemented with 5% FCS, 1% L-glutamine (200 mM N-acetyl-L-alanyl-L-glutamine), and 1% essential amino acids (BME-amino acids 100X; all obtained from Biochrom, Berlin, Germany). The PBMC were cultured in the absence (untreated) or presence (stimulated) of PHA (10 μg/ml final concentration) to stimulate the T-lymphocytes.
For each OP concentration, the cell culture was performed in triplicate in 96-well flat bottom microtiter plates (Greiner, Nürtingen, Germany) containing a total volume of 250 μl per well. The cultures were incubated for 20 hr in humidified atmosphere at 5% CO2 and 37°C. After incubation, the supernatants were harvested and the cytokine production analysed using enzyme linked immunosorbant assay (ELISA) as described below. The remaining cells were used for the MTT bioassay to assess the cell viability.
ELISA
The influence of chlorpyrifos and diazinon on the cytokine secretion by PBMC was analysed using ELISA. The cytokines IFNγ, IL-4, and IL-13 in supernatants of PHA-stimulated and unstimulated PBMC were measured using an indirect sandwich ELISA (OptEIA Kits; BD Biosciences, Heidelberg, Germany) following the manufacturers’ instructions. The optical density of each well was determined using a plate reader with a 450 nm detection filter and a 620 nm reference filter (Spectra Image; Tecan, Crailsheim, Germany). The calibration curves were calculated using the software supplied with the ELISA-plate reader (EasyWin Kinetics, Tecan). The lower limit of detection for all cytokines was 4 pg/ml.
MTT Bioassay
After removing 200 μl cell culture supernatant for ELISA, 25 μl/well MTT solution (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, 2 g/l in phosphate-buffered saline; Sigma) was added to the remaining PBMC suspension and the cells were incubated in the dark at 37°C. After 4 hr, the metabolic conversion of MTT into purple formazan crystals by active cells was terminated by adding 100 μl/well stop solution (10% [w/v] sodium dodecyl sulfate in 50% [v/v] N,N’-dimethylformamide, SERVA). Following overnight incubation, MTT assay results were read on a plate reader (Spectra Image; Tecan) using a 570 nm filter.
Statistical analysis
Even though many toxicity studies make use of EC50 values to allow comparison of different parameters, we knowingly refrained from the use of this value. Cytokine responses, independent of the analysis mode, can be increased and decreased as a result of exposure to the same chemical depending on the concentration used, resulting in multiphasic responses. Complex responses are shown in the current study as well as in previously performed studies (Oostingh et al., Citation2008), and such response patterns cannot appropriately be described by EC50 values. For data analysis, the values, measured in the absence of chlorpyrifos and diazinon, were set to 100% for stimulated as well as untreated samples. This enabled the comparison between different data sets and also allowed the comparison of stimulated samples with untreated samples. The data were analysed using the software package Statgraphics™ Centurion version XV. One-way analysis of variance (ANOVA) was performed to determine whether overall treatment effects occurred within an experiment using a 95% confidence level. ANOVA testing was followed by post hoc testing using a multiple range test to determine which exact treatment contributed to the significant overall effects; this analysis was also performed at a 95% confidence level.
The experiments performed with the cell lines were repeated at least twice using triplicates for each experiment, the means (± standard deviations [SD]) were calculated for these assays for graphic display. For the PBMC data, all experiments were performed in triplicate, the results of at least six donors were summarized and means (± standard error of means [SEM]) were calculated and used in the graphs. In the latter case the SEM was used since the results of assays where human samples are used, often have a large variation due to inter-individual differences in age, gender, health status and genetic background.
Results
Viability of A549 and Jurkat cells after treatment with chlorpyrifos or diazinon
Cell viability was determined for A549 and Jurkat cells after a 24-hr incubation time of the cells with chlorpyrifos or diazinon in the absence or presence of cell stimulatory agents (rhTNFα or PHA) using the CellTiterBlue® test (). The results obtained with the A549 cells showed that treatment with chlorpyrifos in the absence or in the presence of a low level of rhTNFα (1 μg/l) reduced the cell viability to maximally 32% in a dose-dependent manner. In the presence of higher concentrations of rhTNFα, the cell viability was reduced to maximally 40% and 45% living cells for, respectively, 300 and 20 μg rhTNFα/l. At the highest concentrations of chlorpyrifos (≥ 500 μM), the cell viability of A549 cells treated with 20 or 300 μg rhTNFα/l was significantly higher compared to the cell viability of untreated or low dose-treated cells (p < 0.05). These results showed that chlorpyrifos is cytotoxic to A549 cells in a concentration-dependent manner and that the presence of medium to high concentrations of TNFα has a modest protective effect on the cells.
Figure 1. Cell viability of A549 cells and Jurkat cells. The viability of A549 cells (top panels) and Jurkat cells (lower panels) was determined after treatment with chlorpyrifos (CP, left panels) or diazinon (DA, right panels) in the absence and presence of rhTNFα or PHA. The percent (%) viable cells are shown in relation to untreated cells (=100%). The mean (± SD) of triplicate values are shown for a representative experiment, whereby experiments were performed at least three times. Significant increases or decreases (p < 0.05) compared to the op-free control values, as calculated by an ANOVA based multiple range test, are indicated in the figure by the presence of an asterisk.
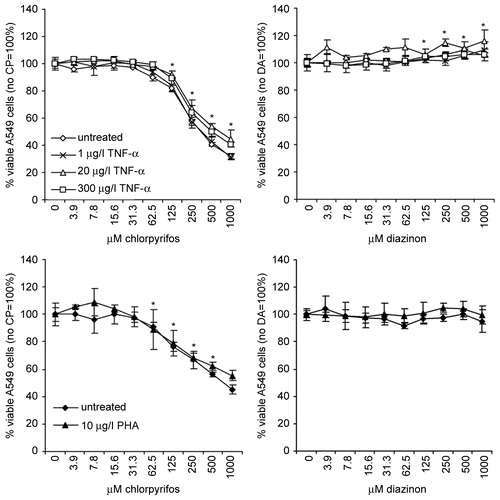
In contrast to the results obtained with chlorpyrifos, the addition of diazinon resulted in a small, but significant increase in the metabolizing capacity of the cells, which is the read-out parameter used for measuring their viability, up to a maximum of 115% with the highest concentration, whereas no cytotoxic effects of diazinon on A549 cells could be observed, independent on the addition of rhTNFα. The increase in cell viability might be caused by a stimulatory effect of diazinon, which could result in a slightly enhanced cell proliferation but might also be an adverse effect reflecting the need for higher metabolic activity of the cells to compensate for the stress exerted by diazinon.
The effect of chlorpyrifos or diazinon on the viability of Jurkat cells was largely similar to that observed for the A549 cells (). The addition of chlorpyrifos reduced the viability of the Jurkat cells to maximally 45% in a dose-dependent manner. The addition of PHA led to a significantly lower suppressive effect with a maximal reduction of 55% when using 1000 μM chlorpyrifos. Diazinon had no significant effect on the viability of Jurkat cells.
Effect of chlorpyrifos and diazinon on cytokine promoter induction in Jurkat cells
In order to determine the immunomodulatory effects of chlorpyrifos and diazinon, reporter gene studies were performed using stably-transfected cell lines that have been extensively described and validated in a previously-published study (Oostingh et al., Citation2008). For the current study, two Jurkat cell lines that had been transfected with either the IFNγ or IL-4 promoter sequence were used. These two lines could give an indication for the discrimination between potential specific effects of chemicals on TH1 (IFNγ)- or TH2 (IL-4)-mediated responses.
In general, the results showed that chlorpyrifos and diazinon affected the induction of the IFNγ and IL-4 promoter in a very similar fashion (), which is in strong contrast to the cell viability results (). Moreover, addition of PHA, a specific T-lymphocyte stimulus, decreased the cytotoxicity of chlorpyrifos and diazinon, but the addition of the same cellular stimulus did enhance the effect on the cytokine promoter induction. The addition of PHA to the Jurkat cells resulted in a significant up-regulation of the cytokine promoter induction, independent of the cytokine promoter tested (data not shown [Oostingh et al., Citation2008]).
Figure 2. Effect of the two OPs on cytokine promoter induction in Jurkat cells. The induction of the IFNγ promoter (top panels) or the IL-4 promoter (lower panels) in stably-transfected Jurkat cells was determined using different concentrations of chlorpyrifos (left panels) or diazinon (right panels). Cells were incubated with PHA in order to stimulate the T-lymphocytes, which resulted in an increase of the cytokine promoter induction for both IFNγ and IL-4 promoter transfected Jurkat cells. Measured values were normalized to the samples in the absence of chlorpyrifos or diazinon (=100%), in order to allow for comparison between cell lines and between stimulated and non-stimulated samples. The mean (± SD) of triplicate values are shown for a representative experiment, whereby experiments were performed at least three times. Significant increases or decreases (p < 0.05) compared to the op-free control values, as calculated by an ANOVA based multiple range test, are indicated in the figure by the presence of an asterisk.
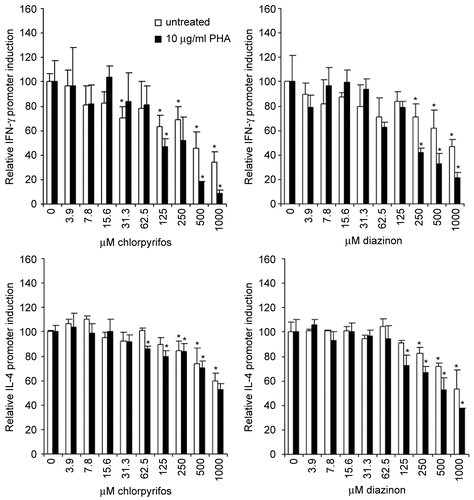
In the absence of the T-lymphocyte stimulus PHA, the induction of IFNγ was dose-dependently reduced by maximally 66% when cells were treated with 1000 μM chlorpyrifos. In the presence of PHA, the reduction of the IFNγ promoter induction was enhanced to 91% when cells were treated with 1000 μM chlorpyrifos. The difference between untreated and PHA-treated cells was significant at ≥ 250 μM chlorpyrifos (p < 0.05). Treatment of IFNγ promoter-transfected Jurkat cells with diazinon resulted in a decrease of the promoter induction by maximally 53% in the absence and 79% in the presence of PHA. The presence of PHA significantly increased the inhibiting effects of diazinon at a concentration of 250 μM diazinon and more (p < 0.05).
Results in the IL-4 promoter-transfected Jurkat cells were less pronounced compared to those found for IFNγ promoter-transfected cells. The addition of chlorpyrifos resulted in maximally decreased IL-4 promoter induction by 40% in the absence and 47% in the presence of PHA. The small difference that was observed between PHA-treated and untreated cells did not reach statistical significance. Diazinon treatment resulted in a maximal reduction of the IL-4 promoter induction by 47% in the absence, and 63% in the presence, of PHA. A trend was observed for an additional decrease in IL-4 promoter induction in PHA-treated cells.
In summary, these results showed that both organophosphates can inhibit IFNγ as well as IL-4, this effect is most likely not specific for a certain T-lymphocyte subset and points to a more general down-regulation of cytokine induction in human T-lymphocytes. Interestingly, diazinon, which did not show any cytotoxicity, did have immunomodulatory effects.
Cytokine promoter induction in A549 cells after treatment with chlorpyrifos or diazinon
In addition to the Jurkat cells, stable transfected A549 cells containing the IL-6, IL-8, or TNFα promoter sequence, and one commercially available A549 cell line stably-transfected with three copies of the NF-κB binding sequence, were used. The use of these non-professional immune cells was performed to study whether the inhibition of cytokine promoter induction was comparable between different cell types. Moreover, the potential link between lung diseases and OPs might be due to an altered activation of bronchial epithelial cells after inhalation of these compounds. A549 cells were stimulated with the pro-inflammatory cytokine TNFα to mimic an ongoing inflammatory disease. The addition of this cytokine resulted in a significant up-regulation of cytokine promoter induction, independent of the cytokine promoter tested (Oostingh et al., Citation2008).
Chlorpyrifos treatment of A549 cells resulted in a biphasic IL-6 promoter induction pattern when the cells were simultaneously treated with rhTNFα. The IL-6 promoter induction was significantly reduced by 43% in untreated cells at the highest concentrations of chlorpyrifos used (). RhTNFα strongly enhanced the IL-6 promoter induction in chlorpyrifos-treated cells, with a maximum induction of 298% when using 20 μg rhTNFα/l and 125 μM chlorpyrifos. Diazinon did not significantly affect the IL-6 promoter induction in A549 cells. In contrast, the addition of rhTNFα to diazinon-treated cells resulted in a slight trend towards an increased IL-6 promoter induction, but this increase did not reach statistical significance.
Figure 3. Induction of four different cytokine promoters in the presence of chlorpyrifos or diazinon. The induction of the IL-6, IL-8, and TNFα promoter and the NF-κB binding sequence (from top to bottom) in A549 cells was analysed after treatment with chlorpyrifos (left panels) or diazinon (right panels). These experiments were performed in the absence and presence of different concentrations of rhTNFα in order to compare a healthy immune system (no TNFα) with a primed immune system, rhTNFα increased the induction of all promoters tested. Measured values were normalized to the samples in the absence of chlorpyrifos or diazinon (=100%), in order to allow for comparison between cell lines and between stimulated and non-stimulated samples. The mean (± SD) of triplicate values are shown for a representative experiment, whereby experiments were performed at least three times. Significant increases or decreases (p < 0.05) compared to the op-free control values, as calculated by an ANOVA-based multiple range test, are indicated in the figure by the presence of an asterisk.
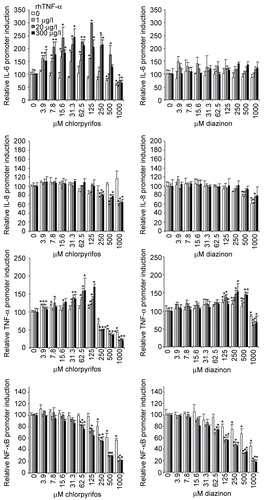
The induction of the IL-8 promoter was reduced by maximally 43% in A549 cells that had been treated with chlorpyrifos and rhTNFα simultaneously, but treatment with chlorpyrifos only did not significantly affect the IL-8 promoter induction. Diazinon treatment did reduce the IL-8 promoter induction by maximally 38% when cells were treated with high concentrations of diazinon in the absence of rhTNFα; addition of this pro-inflammatory cytokine decreased the inhibitory effect in a dose-dependent manner.
The TNFα promoter induction was affected in a similar manner by chlorpyrifos and diazinon, whereby intermediate concentrations of these substances induced an increase in cytokine promoter induction and high concentrations a reduction. Nevertheless, the effects were more pronounced for chlorpyrifos compared to those observed for diazinon (). The concentration that caused a reduction in promoter induction was lower for chlorpyrifos (250 μM) compared to diazinon (1000 μM). In the presence of chlorpyrifos, the TNFα promoter induction was significantly increased at low to medium concentrations of this compound by maximally 170% (3.9 μM to 125 μM) and reduced to maximally 20% when incubated with high concentrations (≥ 250 μM). The increase as well as the decrease was significantly more pronounced in rhTNFα-stimulated cells, whereby 300 μg rhTNFα/l showed the strongest responses. Incubation of cells with diazinon resulted in a maximum increase of the induction of 154% when the cells were treated with 250 μM diazinon and 300 μg rhTNFα/l, and the lowest induction rates of 58% were found when cells were incubated with 1000 μM diazinon and 1 μg rhTNFα/l. Addition of the pro-inflammatory cytokine did enhance the observed responses.
Finally, we found that the NF-κB binding sequence engagement was almost identical for both OPs; a reduction of the induction of this sequence could be observed and was enhanced when rhTNFα was added to the cultures. The maximum reduction in the absence of the pro-inflammatory cytokine was 57%, whereas the addition of 1 μg rhTNFα/l increased this reduction to 20%. The difference between untreated and rhTNFα-treated cells was significant at a concentration of ≥ 62.5 μM chlorpyrifos, independent of the concentration of rhTNFα used (p < 0.05). In the presence of diazinon, the NF-κB binding sequence induction was inhibited to maximally 46% in the absence, and 18% in the presence, of rhTNFα, in a dose-dependent manner.
In summary, the results of the different A549 cells lines showed that the effect of chlorpyrifos and diazinon must occur, at least in part, via different pathways, since IL-6 and IL-8 were affected in a different manner. It is likely that the effects observed at high levels of OPs are at least in part caused by the toxicity of these substances, which explains the enhanced reduction of the IFNγ promoter in Jurkat cells and the IL-6 and TNFα promoter in chlorpyrifos-treated A549 cells. Moreover, the addition of cellular stress influences the effects of chlorpyrifos stronger than those observed for diazinon, especially with respect to the IL-6 and the IL-8 promoter. It could also be observed that there is a cell type-dependent difference between Jurkat cells and A549 cells.
Effect of chlorpyrifos and diazinon on peripheral blood mononuclear cells
In order to determine whether the effects observed in the Jurkat cell line are a realistic reflection of the effects observed in primary cells obtained from healthy human blood donors, cell viability () as well as IL-4, IL-13, and IFNγ secretion () were determined for PHA-stimulated PBMC.
Figure 4. Cell viability of peripheral blood mononuclear cells (PBMC) after treatment with chlorpyrifos (filled symbols) or diazinon (open symbols). The percent (%) viable cells are shown in relation to untreated cells (=100%). The mean (± SEM) of at least six different blood donors are shown, triplicates were normalized to the individual control of each donor. Significant decreases (p < 0.05) as calculated by an ANOVA based multiple range test are indicated in the figure by the presence of an asterisk.
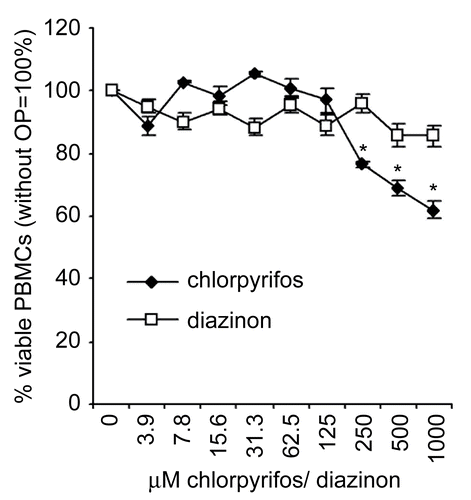
Figure 5. Alterations in cytokine secretion by PBMC due to incubation with chlorpyrifos or diazinon. The secretion of IFNγ, IL-4, and IL-13 by PHA-stimulated PBMC in cell culture supernatant after treatment with chlorpyrifos (dark columns) or diazinon (white columns). Measured values were normalized to the untreated samples (=100%), in order to allow for comparison between the different chemicals. The mean (± SEM) of at least six different blood donors are shown, triplicates were normalized to the individual control of each donor. Significant decreases (p < 0.05) compared to the op-free control values, as calculated by an ANOVA-based multiple range test, are indicated in the figure by the presence of an asterisk.
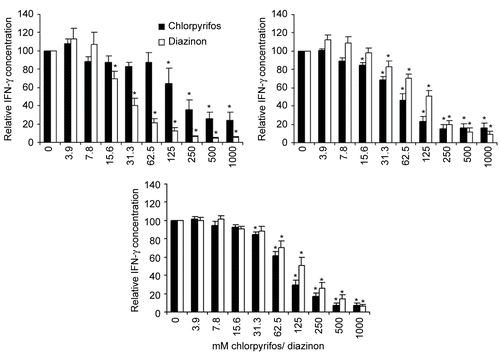
The secretion of all three cytokines was strongly increased when PBMC were treated with PHA, and no detectable levels of these cytokines were measured in untreated cultures; these data were therefore omitted from further analysis. A significant concentration-dependent decrease in IFNγ secretion by PBMC was found and reached 24.1% of control level at a concentration of 1000 μM chlorpyrifos. Diazinon had similar effects, reducing the cytokine secretion to 5.1% of the control at a concentration of 1000 μM diazinon. However, not only the secretion of the TH1-cytokine IFNγ, but also the secretion of the TH2 cytokines IL-4 and IL-13, was significantly decreased in the presence of chlorpyrifos and diazinon (). This reduction was dose-dependent in all cases, reaching a maximal reduction of 16.5% and 9.3% of the control value for IL-4 secretion in cells treated with 1000 μM chlorpyrifos or diazinon, respectively. The maximal reduction in IL-13 secretion by PHA-stimulated PBMC was 6.7% and 6.3% of chlorpyrifos- or diazinon-treated cells, respectively, in comparison to the controls.
In summary, the results obtained with PHA-stimulated PBMC correspond to those observed with the promoter study in the Jurkat cells. However, the responses observed for PBMC were in general stronger compared to those observed for the Jurkat cells. This could be due to the fact that primary cells are more sensitive compared to immortalized cell lines, as was previously shown for chemical responses tested in another cell line (Oostingh et al., Citation2008). However, it might also be that the analysis of cytokine promoter activity is less sensitive compared to analysis of cytokine secretion. Nevertheless, the use of the reporter cell line-based assays could result in an underestimation of immunotoxic effects.
In the absence of PHA, all cells that are present in the PBMC suspension will contribute to the observed responses, whereas the addition of PHA stimulated the T-lymphocytes only and the observed effects can be solely related to this cell type. All cytokines were strongly down-regulated after incubation with either chlorpyrifos or diazinon, indicating a general inhibition of T-lymphocyte-mediated responses.
Discussion and conclusions
The results of this study show that chlorpyrifos and diazinon have a different effect on the viability of alveolar epithelial cells and T-lymphocytes. Chlorpyrifos decreased the cell viability significantly and all of the reductions in cytokine promoter induction levels can be explained by cell death. However, diazinon did not affect the viability of the cells, whereas treatment with this compound did reduce the induction of some cytokines, such as IFNγ and IL-4, as well as the activation of the NF-κB binding sequence, at higher concentrations. These reductions in cytokine promoter induction could therefore not be explained directly by a loss in cell viability. The explanation for these seemingly controversial results could be that diazinon does not directly affect cell viability, but does down-regulate the normal synthesis pathways in a cell at higher concentrations, thereby inhibiting cytokine production. Moreover, the analysis of cytokine promoter responses as well as cytokine secretion is based on the accumulated response of a cell population to a certain stimulus. A decreased number of viable, cytokine producing cells in culture could be overshadowed by an increased production of cytokines by the remaining cells.
The cytokine responses showed that T-lymphocytes, i.e, the cell line Jurkat as well as the PHA-stimulated primary PBMC, were not affected in a specific manner, and that treatment with either chlorpyrifos or diazinon reduced the induction of the cytokine promoter or the secretion of cytokines. These results might imply a more generally impaired T-lymphocyte response, which could for example lead to a sub-optimal clearance of pathogens or a decreased recognition of cells that are genetically changed, such as virally- infected or cancer cells. The latter could, in part, explain the link between OP exposure and cancer (Alavanja et al., Citation2004; Blair et al., Citation2005), since T-lymphocytes play an important role in the recognition of altered self. The results that were found in this study indicate that the chemical alterations in T-lymphocyte-related immune responses might not differ significantly following chlorpyrifos or diazinon exposure.
For the bronchial epithelial cells the response was more diverse; at low-to-medium concentrations of chlorpyrifos, an up-regulation of IL-6 and TNFα promoter induction could be observed which was significant in the presence of an additional stimulus, rhTNFα. A similar effect could be observed for diazinon-treated cells, but to a lesser extent. These results indicate that in the presence of an ongoing inflammation which may be caused, for example, even by a normal cold, the additional exposure to OPs could result in an increased intensity of the disease, which might increase the risk to develop chronic inflammatory diseases such as asthma and COPD (Albers et al., Citation2004). The immunomodulating effects on the bronchial epithelial cells were stronger when cells were treated with chlorpyrifos compared to diazinon, indicating that the former affected this cell type more severely.
Another conclusion that can be drawn from this study is that cell death and immunomodulation are completely different parameters, and especially that cytokine promoter induction or cytokine secretion is altered at chemical concentrations that do not affect the viability of the cells. Interestingly, some of the immunomodulatory effects observed in this study were found at concentrations of chlorpyrifos that were shown to be of relevance to realistic exposures observed in human populations (Whyatt et al., Citation2003). Maximal levels of organophosphate pesticides in the range of 10 μg/ml, corresponding to 28 μM chlorpyrifos or 36 μM diazinon, have been observed in human serum samples through occupational exposure (Pluth et al., Citation1996; Ramesh and Ravi, Citation2002). In addition, an in vivo study in chicken showed that the acute median oral lethal dose (LD50) of chlorpyrifos and diazinon in chicken was 10. 79 mg/kg and 6. 32 mg/kg, respectively, (Mohammad et al., Citation2008). In our in vitro test system, this would correspond to respectively 30 μM chlorpyrifos and 23 μM diazinon, which is within the range of the concentrations used in the presented studies and at these concentrations immunotoxic effects could be observed. Nevertheless, it is difficult to directly compare the results of an in vitro study like the one presented in this manuscript with data from an in vivo study, and it is equally complicated to directly compare the results obtained on one single cell line with those that might be observed in a human being. The problems associated with the translation of OP-induced effects between different testing systems have been carefully reviewed by Karalliedde et al. (Citation2003).
Another in vitro study, using neuronal cells, made use of similar concentrations of chlorpyrifos and diazinon as in the presented study and found these compounds to be cytotoxic at levels that were about 10-fold lower compared to the cytotoxic levels found in our study, which might be due to the cell type used or the method used for analyzing cytotoxicity (Giordano et al. Citation2007). In contrast, Tirelli et al. (Citation2006) did not see any significant cytotoxicity when using 250 μM chlorpyrifos in their in vitro test system, whereas this concentration did alter the intestinal barrier integrity. Taken together, the data from the literature is in line with the results obtained in the presented study, but parameters, such as cytotoxicity seem to be strongly dependent on the cell type and testing system used.
Further investigations are in progress to unravel the exact mechanisms behind the chemically-induced immunomodulation observed in this study.
Acknowledgments
This work was supported by the EU-integrated project NoMiracle (Novel Methods for Integrated Risk assessment of Cumulative Stressors in Europe http://nomiracle.jrc.it), contract No. 003956, under the EU-theme “Global Change and Ecosystems” topic “Development of risk assessment methodologies”, coordinated by Dr. Hans Løkke at NERI, DK-8600 Silkeborg, Denmark. Initial development of components of the assay was performed as part of the EU-integrated project MAAPHRI (Multidisciplinary Approach to Airborne Pollutant Health Related Issues: Modelization with combustion engine exhausts), QLK-CT-2002-02357. This work was partly funded by the priority program “Biosciences and Health” of the University of Salzburg. The authors would like to acknowledge Ulrike Tischler, University of Salzburg, for her technical assistance. The pGL3-neo vector was a kind gift from Dr. C. Owczarek, Australia.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alavanja, M. C., Dosemeci, M., Samanic, C., Lubin, J., Lynch, C. F., Knott, C., Barker, J., Hoppin, J. A., Sandler, D. P., Coble, J., Thomas, K., and Blair, A. 2004. Pesticides and lung cancer risk in the agricultural health study cohort. Am. J. Epidemiol. 160:876–885.
- Albers, J. W., Berent, S., Garabrant, D. H., Giordani, B., Schweitzer, S. J., Garrison, R. P., and Richardson, R. J. 2004. The effects of occupational exposure to chlorpyrifos on the neurologic examination of central nervous system function: A prospective cohort study. J. Occup. Environ. Med. 46:367–378.
- Bagchi, D., Bagchi, M., Hassoun, E. A., and Stohs, S. J. 1995. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology 104:129–140.
- Blair, A., Sandler, D., Thomas, K., Hoppin, J. A., Kamel, F., Coble, J., Lee, W. J., Rusiecki, J., Knott, C., Dosemeci, M., Lynch, C. F., Lubin, J., and Alavanja, M. 2005. Disease and injury among participants in the Agricultural Health Study. J. Agric. Safety Health 11:141–150.
- Blakley, B. R., Yole, M. J., Brousseau, P., Boermans, H., and Fournier, M. 1999. Effect of chlorpyrifos on immune function in rats. Vet. Hum. Toxicol. 41:140–144.
- Burns, C. J., Garabrant, D., Albers, J. W., Berent, S., Giordani, B., Haidar, S., Garrison, R., and Richardson, R. J. 2006. Chlorpyrifos exposure and biological monitoring among manufacturing workers. Occup. Environ. Med. 63:218–220.
- Fenske, R. A., Lu, C., Barr, D., and Needham, L. 2002. Children’s exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environ. Health Perspect. 110:549–553.
- Giordano, G., Afsharinejad, Z., Guizzetti, M., Vitalone, A., Kavanagh, T. J., Costa, L. G. 2007. Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol. Appl. Pharmacol. 219:181–189.
- Handy, R. D., Abd-El Samei, H. A., Bayomy, M. F., Mahran, A. M., Abdeen, A. M., and El-Elaimy, E. A. 2002. Chronic diazinon exposure: Pathologies of spleen, thymus, blood cells, and lymph nodes are modulated by dietary protein or lipid in the mouse. Toxicology 172:13–34.
- Hore, P., Robson, M., Freeman, N., Zhang, J., Wartenberg, D., Ozkaynak, H., Tulve, N., Sheldon, L., Needham, L., Barr, D., and Lioy, P. J. 2005. Chlorpyrifos accumulation patterns for child-accessible surfaces and objects and urinary metabolite excretion by children for 2 weeks after crack-and-crevice application. Environ. Health Perspect. 113:211–219.
- Karalliedde, L. D., Edwards, and P., Marrs, T. C. 2003. Variables influencing the toxic response to organophosphates in humans. Food Chem. Toxicol. 41: 1–13.
- Mohammad, F. K., Al-Badrany, Y. M., and Al-Jobory, M. M. 2008. Acute toxicity and cholinesterase inhibition in chicks dosed orally with organophosphate insecticides. Arh. Hig. Rada. Toksikol. 59: 145–151.
- Oostingh, G. J., Schmittner, M., Ehart, A. K., Tischler, U., and Duschl, A. 2008. A high-throughput screening method based on stably-transformed human cells was used to determine the immunotoxic effects of fluoranthene and other PAHs. Toxicol. In Vitro 22:1301–1310.
- Pluth, J. M., Nicklas, J. A., O’Neill, J. P., and Albertini, R. J. 1996. Increased frequency of specific genomic deletions resulting from in vitro malathion exposure. Cancer Res. 56:2393–2399.
- Pruett, S. B. 1992. Immunotoxicity of organophosphorous compounds. In: Organophosphates, Chemistry, Fate and Effects (Chambers, J. E., and Levi, P. E., Eds.), New York: Academic Press, pp. 123–149.
- Ramesh, A., and Ravi, P. E. 2002. A rapid and sensitive analytical method for the quantification of residues of endosulfan in blood. J. Environ. Monit. 4:190–193.
- Tirelli, V., Catone, T., Turco, L., Di Consiglio E., Testai, E., and De Angelis, I. 2006. Effects of the pesticide chlorpyrifos on an in vitro model of interstinal barrier. Toxicol In Vitro 21:308–313.
- Whyatt, R. M., Barr, D. B., Camann, D. E., Kinney, P. L., Barr, J. R., Andrews, H. F., Hoepner, L. A., Garfinkel, R., Hazi, Y., Reyes, A., Ramirez, J., Cosme, Y., and Perera, F. P. 2003. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ. Health Perspect. 111:749–756.