Abstract
This study examined immune/inflammatory parameters following an acute tobacco smoking episode in smokers with varying smoking histories. Twenty-eight male habitual smokers were categorized according to smoking history, e.g. younger smoker (YSM) or middle-aged smoker (MSM). Participants were matched for fitness and smoking habits and following baseline testing, undertook a smoking protocol involving consumption of two cigarettes within 15 min. Venous blood was collected pre- and immediately, 1 h, and 4 h post-protocol to permit analyses of circulating levels of interleukin (IL)-6, IL-1β, IL-1ra, monocyte chemoattractant protein-1 (MCP-1), C-reactive protein (CRP), and leukocytes. No baseline differences were observed between groups for IL-1ra, IL-1β, or leukocytes. MCP-1 and IL-6 levels were significantly (p < 0.05) elevated at baseline in YSM. Both groups showed an increase in MCP-1 levels from pre- to immediately post-cigarette consumption. The MSM also displayed an increase in IL-6 post-smoking, followed by a decline over the period from 1 to 4 h thereafter. A significant decline in circulating lymphocyte and eosinophil levels from immediately post-cigarette consumption to 1 h later was observed in both groups. Monocyte levels in the YSM followed a similar profile but no significant effects on this cell type were evident in the MSM. From these results, a 10-year difference in smoking history induces mild leukopenia. Altered responses due to smoking were also evident with respect to levels of circulating biomarkers, which may be indicative of an effect of differences in cumulative smoking history.
Introduction
Long-term tobacco smoking constitutes a significant risk factor for the development of pulmonary and systemic diseases, including cardiovascular disease (CVD) and diabetes (Ambrose & Barua Citation2004; Domagala-Kulawik Citation2008). While the unfavorable effects of tobacco smoke on immune/inflammatory processes have been identified in epidemiological studies (Tracy et al. Citation1997; Frohlich et al. Citation2003), precise mechanisms responsible for such changes remain to be defined. Inhalation of tobacco smoke is reported to augment production of immune and inflammatory cells, in part by inducing changes in expression of interleukins (IL)-6, IL-4, IL-1β, IL-1 receptor agonist (ra), tumor necrosis factor (TNF)-α, as well as chemokines such as monocyte chemoattractant protein (MCP)-1 and macrophage inflammatory protein (MIP)-2 (Kuschner et al. Citation1996; Tracy et al. Citation1997; Blann et al. Citation1998; Frohlich et al. Citation2003). Consequently, these altered responses result in a heightened systemic inflammatory state and may be related to the immunosuppression observed in long-term smokers (Tracy et al. Citation1997; Sopori Citation2002; Frohlich et al. Citation2003).
Tobacco smoke is a strong pro-inflammatory stimulus, capable of promoting an acute influx of immune and inflammatory cells (Domagala-Kulawik Citation2008). Acute exposure to tobacco smoke induces a marked influx of inflammatory cytokines, activated monocytes and neutrophils to the lung tissue (Blann et al. Citation1998; Thatcher Citation2005), although, the acute systemic inflammatory responses remain less defined. Despite the scarcity in the literature, data from secondhand smoke (SHS) exposure suggests that even brief SHS exposure causes significant alterations in the immune system, resulting in the up-regulation of pro-inflammatory cytokines such as TNFα, IL-4, IL-5, IL-6, and interferon (IFN)-γ (Flouris et al. Citation2009, Citation2010). Such effects may be attributed to the diverse constituents in tobacco, as either active or passive exposure to tobacco smoke results in delivery of thousands of toxic constituents. Among these noxious stimuli, constituents like nicotine also exert anti-inflammatory actions via α7-nicotinic acetylcholine receptors (Park et al. Citation2007). The presence of compounds like hydroquinone and carbon monoxide may also be responsible for some immunosuppressive effects of tobacco smoke (Arnson et al. Citation2010). Thus, immunomodulating effects of tobacco smoke can be contradictory (Stampfli & Anderson Citation2009) and may explain the broad diversity of tobacco-induced health conditions.
It is well established that long-term smoking is associated with increased risk for many non-communicable diseases (Ambrose & Barua Citation2004; Domagala-Kulawik Citation2008). As evidence, current literature reports long-term smokers to have a dose-dependent heightened basal inflammatory profile (Tracy et al. Citation1997; Frohlich et al. Citation2003). However, it is unknown if such consequences exist among smokers who present with a relatively shorter smoking history. While an influx of immune cells in the lungs of young smokers has been reported (van der Vaart et al. Citation2005), it is unknown if these effects extend to peripheral immune responses. Given the vagaries of systemic immune/inflammatory responses to tobacco smoking, it also remains unknown as to whether the acute immune and inflammatory responses to smoking differ with varying periods of tobacco smoke exposure such as length of smoking history.
In the absence of such data, the current study was undertaken to provide a cross-sectional comparison of immunologic/inflammatory responses to smoking as it relates to smoking history. Although appreciating that acute responses are not directly predictive of chronic adaptations, the overall aim of this study was to assess effects of acute tobacco smoke inhalation on inflamma-tory and leukocyte responses in smokers with a shorter and longer smoking history. It was hypothesized that smokers with a longer smoking history would present with elevated baseline markers of the inflammatory profile than smokers with a shorter smoking history, and the former would also present with suppressed acute post-smoking immune-inflammatory responses.
Methods
Participants
The subject population consisted of 14 younger smokers with a shorter smoking history (YSM) (< 25 years) and 14 middle-aged with a longer smoking history (MSM) (> 30 years) (characteristics provided in ), matched for fitness and smoking behavior (i.e. all current active smokers). Age was delimited in this study as whilst a number of middle- aged smokers were recruited, ≈ 10 were excluded as they presented with preexisting medical conditions and were thus ineligible to participate. All the participants reported as apparently healthy and free from any known metabolic, cardiovascular or pulmonary disease, immunological irregularities or other conditions (e.g. recent influenza or surgery, periodontal disease, etc.) associated with systemic inflammatory responses. Based on pre-study health questionnaire information, any participant confirmed as having these conditions, or taking anti-inflammatory or any other potentially confounding medications, was excluded. The self-reported smoking history for the YSM and MSM populations was 5.2 [± 1.7] years of smoking and 12.3 [± 6.8] cigarettes/day and 14.6 [± 6.5] years of smoking and 15.8 [± 7.3] cigarettes/day, respectively (). Prior to study commencement, all participants were required to provide written and verbal consent following an outline of all procedures and measures. This study conformed to the Declaration of Helsinki and was approved by the Research in Human Ethics Committee at Charles Sturt University.
Table 1. Mean ± SD Baseline descriptive, anthropometric, dual-energy X-ray absorptiometry (DXA), biochemistry and smoking variables within the young smoker (n = 14) and middle-aged smoker (n = 14) populations.
Baseline testing
Participants reported to the laboratory between 0530 h and 0800 h, rested and fasted for a baseline testing session. Stature (Stadiometer: Custom CSU, Bathurst, Australia), body mass (HW 150 K, A & D, Bradford, MA), and waist and hip circumferences (steel tape, EC P3 metric graduation, Australia) were obtained as measures of anthropometry based on standardized techniques. Body mass index (BMI) was calculated from mass and stature, further waist and hip circumferences provided a waist to hip ratio. In addition, a supine dual-energy X-ray absorptiometry (DXA) scan was conducted for the determination of body composition (XR800, Norland, Cooper Surgical Company, Trumbull, CT). Scanning resolution and speed were set at 6.5 × 13.0 mm and 130 mm/s, respectively. Whole-body scans were analyzed (Illuminatus DXA, ver. 4.2.0, Trumbull, CT) for total body lean mass and total body fat mass and are reported in absolute (kg) and relative (%) terms. Resting blood pressure was obtained through a commonly used indirect technique involving the use of an aneroid sphygmo-manometer and stethoscope (Welch-Allyn, Arden, NC); further, participants were fitted with an Rs800cx heart rate (HR) monitor (Vantage NV, Polar, Finland) to obtain a measure of resting heart rate. Additionally, a baseline blood sample was collected (as outlined below) to determine blood fasting glucose and total cholesterol levels.
To ensure comparable aerobic fitness between groups, participants performed a Graded Exercise Test (GXT) on a LODE Excalibur Sport electronically-braked cycle ergometer (LODE BV, Groningen, the Netherlands) for determination of peak oxygen consumption (VO2 peak). The younger population began incremental GXT at 100 W and increased by 25 W every minute until volitional exhaustion; the middle-aged population began GXT at 25 W and increased 25 W every minute until exhaustion, with HR obtained every minute until GXT completion. Pulmonary gas exchange was measured by determining O2 and CO2 concentrations and ventilation to calculate VO2 using a True2400 metabolic gas analysis system (Parvo-Medics, East Sandy, UT). The system was calibrated according to manufacturer instructions. This involved pneumotachometer calibration using a 3 L syringe. Gas analyzers were calibrated using a two-point fully-automated process involving room air and gas calibration for fractional gas concentration with a gravimetric gas mixture of known concentrations (CO2, 4.1 (0.1)%; O2, 15.7 (0.2)%).
Experimental protocol: cigarette consumption
Within 7 days following the baseline session, fasted\rested participants reported to the laboratory at a similar time (6 AM–9 AM) for completion of the smoking protocol. Participants were instructed to smoke two filtered cigarettes (Winfield Blue, 12 mg tar and 1 mg nicotine) within 15 min in a private but open area near the laboratory. During the protocol, participants were instructed to smoke as per their normal smoking behavior, with adequacy of smoking ensured by visual observation by the research team. The smoking protocol was chosen based on previous research published by van der Vaart et al. (Citation2005) who reported two cigarettes of the same brand within 30 min, with encouragement to inhale deeply. Given the lack of active smoking research, this was the guideline for selection of the smoking protocol used in the current study. Further, selection of the brand of cigarette was based on research by van der Vaart et al. (Citation2005), involving two cigarettes of 12 mg tar/1 mg nicotine. The selected brand in the present study is considered average in terms of nicotine dose. Prior questioning regarding smoking habits deemed this brand an appropriate brand and nicotine content among the test group.
Blood collection and analysis
During and following the smoking protocol, venous blood was collected pre- and post-(0 min, 1 h, 4 h) cigarette consumption via a 21-G catheter inserted into the medial antecubital vein. In each case, ≈ 40 ml blood was collected and aliquoted into serum separator tubes (SST) for analysis of blood lipid profile and C-reactive protein (CRP), ethylene diamine tetraacetic acid (EDTA) tubes for analysis of MCP-1, IL-6, IL-1ra, IL-1β, glucose, lactate, and both total and sub-population leukocyte counts.
EDTA tubes were centrifuged immediately post-aliquot (3500 rpm, 15 min, 4 °C); SST tubes were left to clot at room temperature for 20 min prior to centrifugation. Supernatants were immediately stored at −80 °C or −20 °C for EDTA and SST, respectively. Total cholesterol was analyzed using an enzymatic method and polychromatic endpoint technique measurement (Dimension Xpand Plus, Siemens Healthcare Diagnostics, Sydney, Australia). HDL cholesterol was measured using accelerator selective detergent methodology. Triglycerides were assessed using an enzymatic method and biochromatic endpoint technique measurement (Dimension Xpand Plus, Siemens Healthcare Diagnostics, Sydney, Australia). Glucose and lactate concentrations were analyzed pre- and post-cigarette consumption in an ABL825 Flex Analyzer (Radiometer Medical ApS, Bronshoj, Denmark). Further, HbA1c levels were measured using automated high-performance liquid chromatography methodology (BioRad Variant, BioRad Laboratories, Sydney). Leukocyte counts were determined using an XT-1800i cell counter (Sysmex, Mundelein, IL). Concentrations of CRP were determined using a commercial solid-phase chemiluminescent immunometric assay and those of IL-6, IL-1ra, IL-1β, MCP-1 were analyzed using commercially-available ELISA kits, according to manufacturer instructions (ELISAkit, Melbourne, Australia and Merk Millipore, Billerica, MA). Intra- and inter-assay coefficient of variance was < 10% for the ELISA kit and < 5% intra-assay and < 20% inter-assay for the Millipore kit. All samples were analyzed in duplicate.
Statistical analysis
All data are reported as mean ± SEM. Normal distribution was determined using a Shapiro–Wilk’s test and non-normally distributed data was logarithmically-transformed prior to analysis. A one-way analysis of variance (ANOVA) was performed on descriptive baseline variables. Further, a two-way repeated measure (grouped by time) ANOVA was used to determine within- and between-group differences. Where a main effect was noted, a one-way ANOVA test was applied to determine the source of significance. Significance was set at p < 0.05. All analyses were performed using Predictive Analytic Software (PASW) (Statistical Package for the Social Sciences for Windows, v.18.0, Chicago, IL).
Results
Baseline variables for body composition, blood lipid profile, anthropometric and smoking variables are reported in . There were no differences between the groups for VO2peak. The MSM group demonstrated significantly greater absolute and relative fat mass than their younger counterparts. The MSM group also had significantly a greater smoking history in terms of years of smoking and pack-years than the YSM; however, the groups did not differ with regard to volume of cigarettes smoked or level of dependence based upon a Fagerstrom Test for Nicotine Dependence.
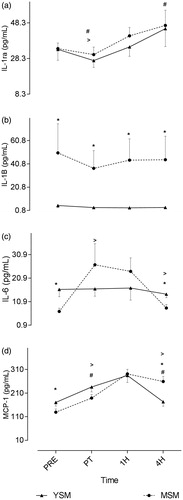
Effects on inflammatory states in the context of levels of IL-1ra, IL-1β, IL-6, and MCP-1 are presented in . A significant interaction effect (p < 0.05) for group by time was observed for IL-6 and MCP-1, though not for IL-1ra or IL-1β. Specifically, there were no differences between groups at baseline or immediately following the smoking protocol for IL-1ra. However, blood IL-1β levels were significantly elevated in the MSM compared to the YSM at baseline and across all timepoints, though no within-group changes were observed following the smoking protocol. Significantly higher baseline MCP-1 and IL-6 were evident in the YSM compared to in the MSM). In terms of within-group changes, both YSM and MSM displayed an increase in MCP blood levels from pre- to immediately post-cigarette consumption. While this effect was still evident until 1 h post-consumption, there was a significant decline thereafter out to 4-h post-consumption in the YSM. However, MCP-1 values for MSM remained significantly elevated above pre-values at the 4-h timepoint. There were no between-group differences in IL-6; while no within-group differences existed for YSM, MSM evinced a significant increase in blood IL-6 levels from pre- to post-cigarette consumption. Lastly, immediately following smoking, both the YSM and MSM displayed via a significant decrease in blood IL-1ra levels; however, in YSM (however, but not in the MSM) an increase was observed thereafter and levels remained elevated above pre-smoking values at 4 h post-consumption.
Figure 1. Pre- and post-cigarette consumption blood levels of IL-6, IL-1 receptor antagonist (ra), IL-1β, and monocyte chemoattractant protein (MCP)-1 for smokers with a shorter (YSM) and longer (MSM) smoking history. Values shown are means ± SEM. *Value significantly different between YSM and MSM (p < 0.05); #value significantly different within condition for YSM (p < 0.05); >value significantly different within condition for MSM (p < 0.05).
While main effects by time were evident, no “group × time” interaction effects were noted in levels of leukocyte sub-populations. Baseline values for total leukocytes, as well as specific-ally for neutrophils, lymphocytes, monocytes, or eosinophils, did not differ between groups and there were no between-group differences in response to the acute smoke exposure (). Total leukocyte counts decreased in YSM immediately and on to 1 h post-cigarette consumption but increased thereafter; similar changes were evident in the MSM and values even at 1–4 h post-cigarette remained significant elevated above pre-smoking levels.
Figure 2. Pre- and post-cigarette consumption blood total and sub-population leukocyte counts for smokers with a shorter (YSM) and longer (MSM) smoking history. Values shown are means ± SEM. *Value significantly different between YSM and MSM (p < 0.05); #value significantly different within condition for YSM (p < 0.05); >value significantly different within condition for MSM (p < 0.05).
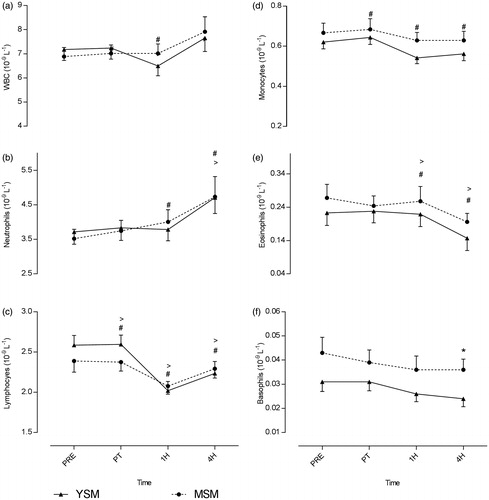
An increase in neutrophils levels from pre-smoke to immediately after smoking was noted in the MSM but not in the YSM; in the latter, that was a significant increase in the period from 1–4 h post-exposure. Levels of neutrophils in both groups were significantly elevated (relative to pre-smoking values) at 4 h post-exposure. Both groups observed a within-group increase in platelet levels in the period from 1–4 h post-cigarette consumption; in addition, values for the YSM were significantly greater than those in the MSM at the 4 h point. Acute smoke exposure induced a significant decline in lymphocytes from immediately after up to 1 h post-smoking; levels of these cells remained significantly below pre-smoking levels even at 4 h in both groups. Further, monocyte levels in the YSM followed the same trend (but this was not observed in the MSM). Similarly, levels of eosinophils declined in the period from 1–4 h post-smoking in both groups. Finally, higher basophil levels were noted in MSM at 4 h compared to YSM, however, no significance was found regarding changes in basophil levels within the respective groups.
Discussion
Smokers with a shorter smoking history (YSM) had higher circulating baseline IL-6 and MCP-1 levels than those with longer smoking histories. However, these same subjects displayed differing acute responses to smoking (in the context of IL-6 and MCP-1 s), suggestive that a more prolonged smoking history impacts the time-course response of specific biomarkers following smoking. The findings here also suggested to us that acute smoking induced mild leukopenia in some leukocyte sub-populations that may be indicative of early stage immuno-suppression – an outcome previous documented in as chronic cigarette smokers (Sopori Citation2002; Stampfli & Anderson Citation2009).
Chronic tobacco smoking results in a heightened inflammatory state that usually parallels smoking history (Tracy et al. Citation1997; Frohlich et al. Citation2003). Previous studies reported that habitual smokers had elevated inflammatory profiles compared to in non-smoker counterparts (Frohlich et al. Citation2003). However, there is no literature to date comparing inflammatory states between smokers of younger and older smoking histories. Kuschner et al. (Citation1996) reported that circulating levels of IL-1β, MCP-1, IL-6, IL-8, and TNFα were elevated in bronchoalveolar lavage fluid recovered from smokers compared to that from non-smokers. Similarly, the current study showed blood IL-1β levels were elevated among MSM compared to YSM. Findings similar to those from Kuschner et al. (Citation1996) were observed in murine models; the latter suggested that cigarette smoke could up-regulate expression of MCP-1 (Churg et al. Citation2003). However, the current study revealed an elevated MCP-1 in YSM at baseline. Though unexpected, this finding may provide some indications about physiological changes associated with prolonged smoking. Elevated MCP-1 concentrations might underlie a local inflammatory response in the lungs indicative of tissue damage (Martinovic et al. Citation2005; Deshmane et al. Citation2009). Further investigation is required to determine the precise mechanisms responsible.
In contrast, differences in acute timeline response to cigarette smoking on MCP-1 may indicate an effect from smoking history. Furukawa et al. (Citation2004) noted that reactive oxygen species, major by-products of burning tobacco, were able to cause increases in expression of MCP-1 in non-diabetic humans. Here, while both groups observed a post-smoking elevation in blood MCP-1 levels, the YSM showed significant declines in MCP-1 after 4-h whereas MSM levels remained elevated above pre-smoking values. Such outcomes are in line with earlier studies showing that MCP-1 levels may remain elevated in smokers with prolonged smoking histories (Traves et al. Citation2002). Elevations in MCP-1 without a presence of signaling cytokines like TNFα is probably indicative of a likely direct effect of smoke on chemokine production (Churg et al. Citation2003). Such a finding would be in keeping with literature reporting MCP-1 to be elevated in defined pulmonary microenvironments, such as during airway inflammation and active recruitment of inflammatory cells (Traves et al. Citation2002).
In addition, the studies here noted that while blood IL-1β levels were elevated in the test MSM, acute smoke exposure induced no further significant change in level. These findings are in line with those of van der Vaart et al. (Citation2005) who reported no effect of acute consumption of two cigarettes on systemic concentrations of IL-1β among healthy young intermittent smokers. On the other hand, in the current study, YSM evinced significantly lower levels of IL-1β, implying an effect of long-term smoking on IL-1β accumulation.
Smoke-induced inflammatory processes can be induced by many of the toxic compounds in tobacco smoke (Domagala-Kulawik Citation2008). While there are few studies which report the acute effects of smoking on inflammatory biomarkers, the present study extends previous findings that suggested acute smoke exposure augmented systemic inflammatory markers like IL-8 (van der Vaart et al. Citation2005). In the current study, YSM evinced elevated IL-6 levels at baseline compared to their MSM counterparts; this is in contrast with the notion that longer-term smokers present with higher basal systemic inflammation, particularly given previous studies reporting elevated basal blood IL-6 levels in smokers compared to in non-smokers (Tappia et al. Citation1995; van Eeden et al. Citation2005). An explanation for this finding could be attributed to a local inflammatory response in the lungs or indicate tissue damage (Xing et al. Citation1998), or may even be related to an imbalance between T-helper (TH)-1/TH2 cytokines that is induced/modified (if already existing) by tobacco smoke (Sopori & Kozak Citation1998). While these mechanisms were not measured here, they may provide insight into the mechanism responsible for the unexpected elevation in YSM.
Given the limited literature concerning acute smoking, data from second-hand smoke (SHS) exposure reveals that even brief exposures elevate levels of circulating inflammatory TNFα, IL-4, -5, and IL-6 for up to 3 h (Flouris et al. Citation2009). In a similar manner, in the current study, blood IL-6 values in the MSM remained elevated for 1 hr post-consumption then declined thereafter (which was not observed in YSM). The IL-6 response in MSM occurred without concurrent elevations in IL-1ra, suggesting a pro-inflammatory response to smoking that may contribute to a systemic inflammatory state (i.e. commonly associated with long-term tobacco smoking; Traves et al. Citation2004) and subsequent disease progression. The novel findings here may provide insight into mechanisms responsible for altered immune regulation (Sopori Citation2002) and compromised immune and inflammatory processes (Stampfli & Anderson Citation2009) associated with long-term smoking.
Chronic tobacco smoke inhalation results in alteration of many immune functions (Sopori Citation2002), some of which are changes to circulating leukocytes. Previous research has shown that tobacco smoke is capable of stimulating an immune response (Kawada Citation2004; van der Vaart et al. Citation2005) and, further, that habitual smokers may exhibit leukocytosis as a result of long-term smoking (Blann et al. Citation1998). However, even if leukocyte counts are elevated, function of these cells is often compromised (Arnson et al. Citation2010). Kawada (Citation2004) reported current cigarette smoking was associated with elevated leukocyte counts, whereas Blann et al. (Citation1998) reported counts increased after acute smoking. Contrary to the previous findings, the current study reported successive consumption of two cigarettes reduced levels of certain circulating leukocyte sub-populations, particularly lymphocytes and eosinophils in both test groups – for up to 1 h. Similar to as in the study here, van der Vaart et al. (Citation2005) reported smoking two cigarettes acutely suppressed lymphocytes and eosinophils in sputum and blood, respectively, in middle-aged smokers. Although smoking is reported to increase leukocyte counts, the present study suggests consumption of two cigarettes acutely suppresses leukocyte sub-populations for up to 4 h, however, these values remained within normal clinical ranges. Regardless of the underlying mechanism(s), these changes illustrate an important fact about how smoking affects host resistance, i.e. impact on lymphocytes and monocytes that recognize and destroy invading pathogens (Hughes et al. Citation1985; Koyasu & Moro Citation2012), and eosinophils, in part, that can also help modulate an immune response (Venge Citation1990).
Despite the novel findings of this study, certain limitations must be acknowledged. Firstly, with regard to the age of the MSM here, while an older population would have been desirable, many subjects would likely then have presented with preexisting medical conditions. On the upside, despite age being a limitation, the study here benefited from the fact that these MSM had comparable health status to YSM, thus eliminating any age-related constraints. Overall, it must be noted that the results in the present study should be interpreted with caution, as while these changes may be attributed to smoking, they could in some way also be a result of age-associated changes. Finally, it should be noted that the smoking protocol here would not be considered "normal" smoking behavior; thus, caution in interpreting the results is recommended.
Conclusions
The present study suggested to us that ∼10 years difference in smoking history between male smokers results in changes to immune and inflammatory responses to smoking, particularly in leukocyte subpopulations and inflammatory biomarkers over time. Specifically, acute cigarette smoking induces mild leukopenia among eosinophils and monocytes. Moreover, changes post-cigarette consumption in levels of the biomarkers IL-6 and MCP-1 might suggest an effect of smoking history on the time-course immune and inflammatory responses. These findings could provide some insight into physiological mechanisms underlying development of systemic immune/inflammatory changes associated with long-term smoking. While the aforementioned markers only represent a small portion of immune changes that might occur as a result of smoking, they also portend other physiologic changes of greater magnitude.
Acknowledgements
The authors would like to acknowledge the staff at Central West Pathology at Bathurst Base Hospital and the institutional staff at the University Exercise Physiology Laboratories Bathurst NSW for their assistance. They would also like to acknowledge the participants for their participation in the study.
Disclosure statement
The authors declare no conflict of interest. The authors alone are responsible for the content of this manuscript.
References
- Agarwal N, Joshi S, Deshpande V, Biswas D. 2013. Correlation between glycated haemoglobin and glucose testing for diabetes mellitus screening. Indian J Med Sci. 67:149–154.
- Ambrose J, Barua R. 2004. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J Am Coll Cardiol. 43:1731–1737.
- Arnson Y, Shoenfeld Y, Amital H. 2010. Effects of tobacco smoke on immunity, inflammation, and autoimmunity. J Autoimmun. 34:258–265.
- Birtcher K, Ballantyne C. 2004. Measurement of cholesterol: A patient perspective. Circulation. 110:296–297.
- Blann A, Kirkpatrick U, Devine C, Naser S, McCollum C. 1998. The influence of acute smoking on leucocytes, platelets and the endothelium. Atherosclerosis. 141:133–139.
- Churg A, Wang R, Tai H, Wang X, Xie C, Dai J, Shapiro S, Wright J. 2003. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am J Respir Crit Care Med. 167:1083–1089.
- Deshmane S, Kremlev S, Amini S, Sawaya B. 2009. Monocyte chemoattractant protein-1 (MCP-1): An overview. J Interferon Cytokine Res. 29:313–326.
- Dhingra R, Gona P, O’Donnell J. 2007. C-reactive protein, inflammatory conditions, and cardio-vascular disease risk. Am J Med. 120:1054–1062.
- Domagala-Kulawik J. 2008. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol. 59:19–34.
- Flouris A, Metsios G, Jamurtas A, Koutedakis Y. 2010. Cardiorespiratory and immune response to physical activity following exposure to a typical smoking environment. Heart. 96:860–864.
- Flouris A, Giogos S, Carrillo A, Jamurtas A, Gourgoulianis K, Kiropoulos T, Tzatzarakis M, Tsatsakis A, Koutedakis Y. 2009. Acute and short-term effects of secondhand smoke on lung function and cytokine production. Am J Respir Crit Care Med. 179:1029–1033.
- Frohlich M, Sund M, Lowel H, Imhof A, Hoffmeister A, Koenig W. 2003. Independent associ-ation of various smoking characteristics with markers of systemic inflammation in men: Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95). Eur Heart J. 24:1365–1372.
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. 2004. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 114:1752–1761.
- Hughes D, Haslam P, Townsend P, Turner-Warwick M. 1985. Numerical and functional alterations in circulatory lymphocytes in cigarette smokers. Clin Exp Immunol. 61:459–466.
- Janssen I, Katzmarzyk P, Ross R. 2004. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 79:379–384.
- Kawada T. 2004. Smoking-induced leukocytosis can persist after cessation of smoking. Arch Med Res. 35:246–250.
- Koyasu S, Moro K. 2012. Role of innate lymphocytes in infection and inflammation. Front Immunol. 3:1–11.
- Kuschner W, D'Alessandro A, Wong H, Blanc P. 1996. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J. 9:1989–1994.
- Martinovic I, Abegunewardene N, Seul M, Vosseler M, Horstick G, Buerke M, Darius H, Lindemann S. 2005. Elevated monocyte chemoattractant protein-1 serum levels in patients at risk for coronary artery disease. Circ J. 69:1484–1489.
- Park H, Lee P, Ahn Y, Choi Y, Lee G, Lee D, Chung E, Jin B. 2007. Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci. 26:79–89.
- Sopori M. 2002. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol 2:372–376.
- Sopori M, Kozak W. 1998. Immunomodulatory effects of cigarette smoke. J Neuroimmunol. 83:148–156.
- Stampfli M, Anderson G. 2009. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 9:377–384.
- Tappia P, Troughton K, Langley-Evans S, Grimble R. 1995. Cigarette smoking influences cytokine production and antioxidant defences. Clin Sci. 88:485–489.
- Thatcher T. 2005. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 289:L322–L328.
- Tracy R, Psaty B, Macy E, Bovill E, Cushman M, Cornell E, Kuller L. 1997. Life-time smoking exposure affects association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol. 17:2167–2176.
- Traves S, Culpitt S, Russell R, Barnes P, Donnelly L. 2002. Increased levels of the chemokines GRO? and MCP-1 in sputum samples from patients with COPD. Thorax. 57:590–595.
- Traves S, Smith S, Barnes P, Donnelly L. 2004. Specific CXC but not CC chemokines cause elevated monocyte migration in COPD: A role for CXCR2. J Leukocyte Biol. 76:441–450.
- van der Vaart H, Postma D, Timens W, Hylkema M, Willemse B, Boezen H, Vonk J, de Reus D, Kauffman H, ten Hacken N. 2005. Acute effects of cigarette smoking on inflammation in healthy intermittent smokers. Respir Res. 6:22.
- van Eeden S, Yeung A, Quinlam K, Hogg J. 2005. Systemic response to ambient particulate matter: Relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2:61–67.
- Venge P. 1990. What is the role of the eosinophil? Thora 45:161–163.
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei X, Achong M. 1998. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 101:311–320.
