Abstract
The proteoliposome (PL) of Neisseria meningitidis serogroup B has been reported as a safe and potent vaccine adjuvant, inducing a TH1-skewed response. The present study describes a pre-clinical safety evaluation of an allergy therapeutic vaccine candidate based on purified allergens from Dermatophagoides siboney house dust mite and PL as adjuvant, both components adsorbed onto aluminum hydroxide gel. Two separate studies of acute toxicity evaluation were performed in mice and rabbits, and two repeat-dose studies were conducted in non-sensitized and allergen-sensitized Balb/c mice, respectively. The study in sensitized mice intends to model a therapeutic setting. Aerosolized allergen challenge was used in both settings to model natural respiratory exposure. In the therapeutic setting, mice were administered with three doses containing 2 μg allergen at weekly intervals [subcutaneous route] and subsequently challenged with aerosolized allergen for 6 consecutive days. Parameters of general toxicity effects were assessed via measures of behavior, body weight, food and water consumption, and macroscopic evaluation of organs. Histological examination of organs and the injection site was performed. Potential immunotoxicity effects at the systemic level were assessed by blood eosinophil counting and serum allergen specific IgE by ELISA The vaccine did not produce general or functional toxic effects of significance, at a dose up to 100 μg allergen per kg body weight. An expected local reaction at the injection site was observed, which could be attributed mostly to the immunological effect of aluminum hydroxide. The models implemented here suggest an acceptable safety profile of this vaccine for testing in clinical trials of allergy immunotherapy.
Introduction
Therapeutic allergy vaccination, also known as allergen-specific immunotherapy, is recognized as an effective treatment strategy, although burdened by the risk of anaphylactic reactions. Such vaccination is believed to exert its beneficial effects on the immune system, at least in part, by modifying the T-helper (TH)-2 lymphocyte response to subsequent natural allergen exposure. The use of TH1 promoting adjuvants in allergen-specific vaccines seems to be beneficial in allergic humans and animal models of allergic diseases (Pfaar et al. Citation2012; Barboza et al. Citation2013; Kun and Li Citation2016; Mohammadi-Shahrokhi et al. Citation2017), and it is coherent with the anti-allergic effect of natural exposure to TH1 microbial stimuli, promoting the so-called TH1 deviation (Debarry et al. Citation2007; Romagnani Citation2014). In addition, the use of immunopotentiating adjuvants has a potential to reduce the allergen content in the vaccine and ameliorate its safety.
Proteoliposomes (PL) from Neisseria meningitidis serogroup B have been reported as a nontoxic and potent vaccine adjuvant for an anti-meningococcal vaccine, inducing a TH1-skewed response (Pérez and Lastre Citation2013; Pérez et al. Citation2013; Tamargo et al. Citation2013). An anti-allergic vaccine based on purified allergens from Dermatophagoides siboney house dust mite and PL as adjuvant, both components adsorbed onto aluminum hydroxide gel is currently in testing in a Phase I clinical trial (http://registroclinico.sld.cu/, RPCEC00000139). A major potential benefit provided by this adjuvanted vaccine would be an enhancement of the allergen immunotherapy efficacy, resulting in a reduction in the number of injections required for that treatment and the amount of allergen (Pérez et al. Citation2013). The present study performed a pre-clinical toxicological evaluation of this vaccine candidate.
Non-clinical toxicity evaluations are a safety requirement for pharmaceutical products entering into clinical trials (ICH Citation2011). Methods of toxicity assessment of allergenic products are scarcely reported (EMEA Citation1997, Citation2008). A clear and updated regulatory guidance for safety testing of allergenic vaccines is lacking (EMEA Citation2000, Citation2008). Owing to the nature of allergen vaccines, we propose a specific safety testing strategy to assess possible adverse reactions in different immunological scenarios. For this purpose, the models implemented here incorporated an allergen challenge as an indicator of functionality of the immune response and assessment of a potential toxicity. Aerosolized allergen exposure intends to reproduce natural physiological exposure. The strategy described here is based on the use of two different models: administration of the product in naive or allergen sensitized mice, respectively.
Materials and methods
Vaccine formulation
The vaccine candidate was obtained from Centro Nacional de Biopreparados, (Bejucal, Cuba). The preparation contained 8 μg/ml of Dermatophagoides siboney major allergen Der s 1, 100 μg/ml of proteoliposome (outer membrane vesicle) of Neisseria meningitidis serogroup B, and 2 mg/ml of aluminum hydroxide. The vaccine was formulated in phosphate-buffered saline (PBS, pH 7.2); Tiomersal (0.1% [w/v]) had been added as a preservative. The studies were carried out with three pilot-scale batches of the vaccine candidate. The vaccine was prepared in accordance to Good Manufacturing Practices (GMP).
All other chemicals/reagents used throughout these studies were obtained from Sigma (St. Louis, MO), unless otherwise noted.
Animals
Clinically healthy specific pathogen-free mice, rats, and rabbits were purchased from CENPALAB (Havana, Cuba). Mice of the non-consanguineous Cenp: NMRI strain and rabbits of the non-consanguineous F1 (NZB x SGB) strain were used to assess the acute toxicity of the vaccine candidate. Balb/C mice were used to assess repeat dose toxicity in non-allergic and allergen-sensitized subjects. Groups of equal number of male and nulliparous non-pregnant females were used for all experiments. All animals were housed individually under controlled environmental conditions (i.e. 21 ± 3 °C; 40–70% relative humidity, 12-h light/dark cycle). All animals had ad libitum access to water and rodent/rabbit chow throughout the studies. The Ethics Committee on Animal Care of the Centro Nacional de Biopreparados approved all protocols used herein.
Single dose toxicity
Single-dose toxicity evaluation was performed in mice and rabbits following a standard design acute toxicity study. In brief, groups of five mice were injected in the dorsal region with one subcutaneous (SC) 0.25 ml dose of vaccine equivalent to 100 μg Der s1/kg BW. For rabbits, groups of three were given a single SC dose (in total of two 1-ml injections/rabbit) equivalent to 20 μg Der s 1/kg in the dorsal region. Control groups of two animals/each sex were administered placebo. Symptoms of potential toxicity (e.g. altered general behavior, changes in skin/mucous membranes, appearance of secretions, dyspnea, depilation, piloerection, diarrhea, tremors, convulsions, excitement, lethargy, and/or death) were followed for 14 days. At that point, net body weight gain was measured and animals were euthanized by an intra-peritoneal (IP) injection of pentobarbital [200 mg/kg]. Once death was confirmed, necropsy was performed and macroscopic evaluation of organs undertaken.
Repeat-dose toxicity in non-allergic mice
Balb/c mice were given three doses each – at 7-day intervals – of 0.25 ml of the vaccine candidate in different sites of the dorsal region by SC injection. Three consecutive GMP batches of the experimental vaccine were tested (Batch1, Batch2, Batch3) in three different groups of mice. Control mice were administered a formulation of allergen adsorbed onto aluminum hydroxide (alum) lacking PL (100 μg allergen + 25 mg alum/kg) known to induce a typical TH2 allergic response (TH2 control). Two other non-allergen control groups were administered PBS or alum (25 mg/kg). The size of some of the groups varied to accommodate other experimental outputs outlined below; however, at a minimum the number of mice/group was 10 in each study.
Body weight as well as water/food consumption was assessed. For the latter, mice were kept in individual cages and water/food consumption was measured as the difference between volume/weight added and amount remaining (not consumed) over each 7-day period. Some animals in each group were euthanized at Day 21 to provide baseline data for organ weights, body weights, and tissue histology (see below). To assess potential toxicities associated with immune responses induced by the vaccine upon host re-exposure to the test antigen, 1 week after their last dose, mice underwent an allergen challenge test via repeated exposure (30 min/day, 1 week) to allergen aerosol in a whole-body chamber. For this, an MPC Aerosol Medication Nebulizer (Braintree Scientific, Braintree, MA) was connected to a plastic chamber where the mice were kept for the challenge (Schroeder et al. Citation2009). The allergen solution used for nebulization was 500 μg/ml (measured via specific ELISA kit; Indoor Biotechnologies, Charlottesville, VA). After taking into account both starting concentration and chamber volume, actual allergen concentration in the air was calculated as ≈ 105 μg/L.
At 48 h after the final aerosol challenge (i.e. Day 30), all remaining mice/group were euthanized by cervical dislocation and blood collected for analyses; organs were also collected at necropsy for analyses. Peripheral blood eosinophil levels (EOS) were analyzed using eosin stain 1% (Quimefa, Cuba) and counting in a Newbauer chamber; results were expressed as EOS/ml blood. The remaining blood sample was processed to yield serum. For quantification of serum allergen-specific IgE levels, indirect ELISA microtiter plates (Maxisorp Nunc, Copenhagen, Denmark) were coated overnight at 4 °C with 2000 BU/ml of D. siboney allergenic extract in carbonate buffer [pH 9.6]. Non-specific binding was blocked with 1% PBS-T (PBS + 0.05% Tween 20)-BSA (bovine serum albumin [10 mg/ml]) for 1 h at 37 °C. After incubating with test sera (1:2) for 2 h, plates were incubated with horseradish peroxidase-labeled rat anti-mouse IgE monoclonal antibody (1:1000; Southern Biotechnology, Birmingham, AL) for 1 h at 37 °C. Presence of bound secondary antibody was then detected by addition of 100 μl kit-provided 3,3´,5,5´-tetra-methylbenzidine (TMB) solution. Optical density (OD) in each well was then measured at 450 nm in a PR 521 plate-reader (SUMA, Havana). All results were expressed in arbitrary units based on OD values.
At necropsy, lungs, liver, kidneys, heart, and spleen of each host were removed, weighed, and then subjected to macroscopic evaluation. Organs with clearly-evident macroscopic lesions were subjected to further histological assessment. These samples were fixed in 10% neutral formalin, embedded in paraffin, cut to 3-μm sections, and stained with hematoxylin and eosin.
Local tolerance at the injection site was assessed by evaluating skin and subcutaneous tissues macroscopically and, postmortem, histologically. An extra subset of mice in the Batch3 group was maintained for an additional 1 week to assess any reversibility of possible lesions.
Repeat-dose toxicity in allergen-sensitized mice
Mice were sensitized by IP injection of the allergen absorbed onto alum (at Der s 1/Der s 2 [250 μg/kg] + alum [25 mg/kg]) and received an identical booster immunization 10 days later; subsequently, mice underwent the aerosolized allergen exposure using the doses and schedules described above for allergen challenge. One week later, the now-sensitized mice were given three SC doses of the active treatment (0.25 ml dose of vaccine, equivalent to 100 μg allergen + 1.25 mg PL + 25 mg alum/kg BW), whereas placebo (PBS) was administered to a sensitized control group. Both treatments were administered at 7-day intervals in different sites of the dorsal region. One week after the final dose, mice were re-challenged with aerosolized allergen to assess the functional toxicity of the established immune reaction. Body weight was assessed and potential toxicity symptoms were followed.
Blood samples were taken at Days 0 (Pre-sensitization), 19 (48 h after sensitization), 45 (1 week after final immunization), and 53 (48 h after final aerosol challenge); mice were then euthanized as described above. Blood smears were prepared and stained with modified Giemsa (Quimefa, Havana); slides were then evaluated using a light microscope (200 cells/slide, 10 slides/host) and the proportion of peripheral blood eosinophils (EOS) among total white blood cells (WBC) was determined.
From the blood, serum was isolated using standard protocols and samples stored at −80 °C until analyzed. The quantification of serum allergen-specific IgE levels was assessed by indirect ELISA in microtiter plates as described above.
Statistical analysis
Comparisons between groups were performed by 1-way ANOVA (analysis of variance) supplemented by a Tukey test and a 2-way-ANOVA supplemented by a Bonferroni test in the case of pre/post allergen challenge tests. All evaluations were done using Prism v.4.0 software (GraphPad Inc., San Diego, CA).
Results
Single-dose toxicity
There were no deaths or signs of systemic toxicity noted in association with a single SC dose of the vaccine in mice (equivalent to 100 μg Der s1/kg BW) or in rabbits (equivalent to 20 μg Der s1/kg). No significant differences (p > 0.05) between treated and control groups were noted regarding body weight gain at the end of the study ().
Table 1. Survival and body weight gain in single dose toxicity study of vaccine candidate.
Repeat-dose toxicity in non-allergic mice
The repeat-dose schedule of the vaccine induced no apparent signs of general toxicity in non-sensitized mice. Overall, no significant differences were noted between the results of different vaccine batches, confirming the homogeneity of the test substance regardless of the batch. Thus, for purposes of simplicity, data from the vaccine groups were pooled. Regardless of sex, there were no significant differences between vaccinated and control groups regarding body weight () or food/water consumption (). In general, males tended to eat/drink more than female counterparts during the 3-week period. Regarding organ indices (index = organ weight/total BW), no significant differences were detected in the indices for lungs, heart, kidneys, or liver between vaccine treated mice and either control (). This was true either before or after the host challenge with allergen/antigen post-vaccine treatment period. There were no sex-related effects noted in these organs as well.
Figure 1. Parameters of general toxicity of repeat-dose administration of vaccine to mice. (A) Body weight behavior in male (left) and female (right) mice. (B) Average total food (left) and water (right) consumption for each week. As data within each of the Alum and Placebo groups did not differ at any timepoint, for the purpose of simplicity in (B) these values were pooled and showed as Placebo.
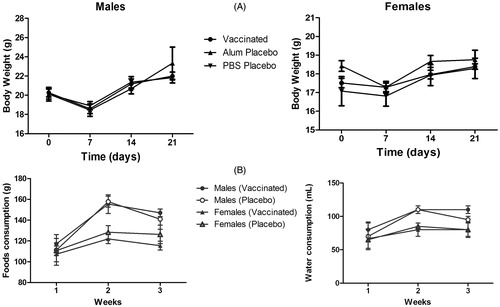
Figure 2. Organ toxicity of repeat-dose administration of vaccine to mice. (A) Relative organ weight in male (left) and female (right) mice. (B) Splenic indices of male (left) and female (right) mice. (C) Representative H&E-stained spleen. Reactive hyperplasia with lymphoid follicles was seen in vaccinated mice (left) and TH2 control mice (right). Magnification for group: 2.5X (left), 10X (right).
Some increase (44% vs. both controls) in splenic index was noted in vaccinated mice prior to antigen re-exposure (, Day 21). Splenic indices then diminished slightly 3 week after the final administration of the vaccine (, Day 35); this was assessed in the group that was intentionally left for evaluating possible lesion reversion. Normal histology was observed in almost all the organs evaluated. The exception was an appearance of some modifications in the spleens of vaccinated mice and the TH2 control hosts including localized hyperplasia in the secondary lymphoid follicles (). These types of changes would be congruent with an induced immunomodulation.
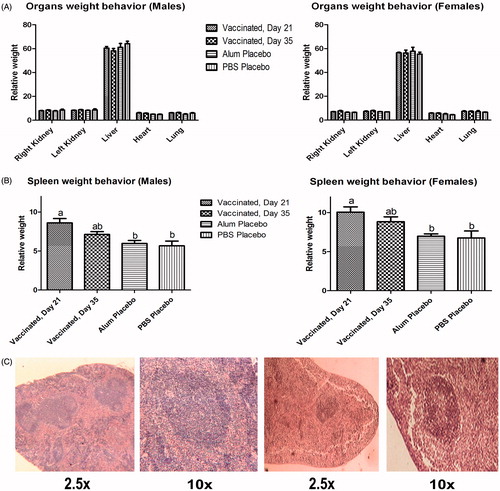
In the absence of allergen challenge, EOS levels in mice that had received the vaccine did not differ from those in either of the two control groups (data not shown). After allergen exposure, mice that had received vaccine treatment showed a significant 125-fold decrease in levels of peripheral blood eosinophils (in terms of cells/ml) as compared to the TH2 control mice ().
Figure 3. Immunotoxic impact of repeat-dose administration of vaccine to mice. (A) Blood eosinophils levels after allergen re-challenge. (B) Allergen-specific IgE levels in serum. Values shown are means ± SD from n = 10/group. Values significantly different at p < 0.01.
With regard to allergen-specific IgE in serum, prior to challenge (post-vaccination) and after aerosol exposure for 1 week to the antigen, the TH2 control mice were significantly (p < 0.001) elevated compared to vaccinated mice and placebo (PBS)-treated hosts (), suggesting a diminished allergic response in vaccinated mice.
Repeat-dose toxicity in allergen-sensitized mice
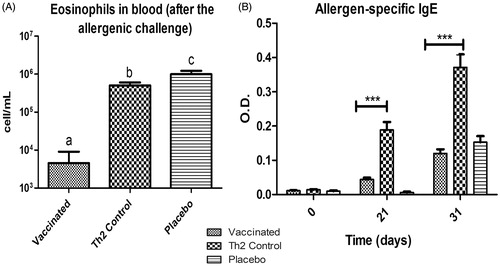
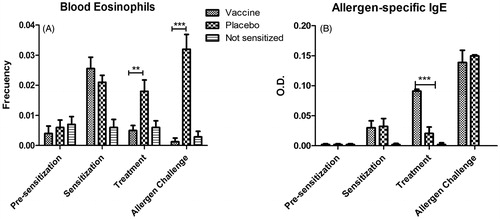
The repeat-dose administration of the vaccine candidate to sensitized mice produced no apparent signs of general toxicity. Body weight gain at all timepoints preceding the allergen challenge was similar in sensitized mice as compared to among the controls (). However, sensitized mice gained less weight than non-sensitized mice after allergen challenge.
Figure 4. Body weights of mice subjected to repeat-dose administration of vaccine candidate after allergen challenge. Values shown are mean ± SD, n = 10/group.
Regarding immunological endpoints indicating allergic reactivity, there was a significant reduction of blood eosinophil levels in vaccinated mice, both after vaccine administration and allergen challenge, as compared to non-treated mice. In spite of that, serum IgE levels were significantly (p < 0.001) increased by the vaccine, as compared to the sensitized group that received placebo. Importantly, this difference was abrogated after the allergen challenge ().
Figure 5. Immunotoxic effect of repeat-dose administration of vaccine candidate to allergen-sensitized mice. (A) Blood eosinophil levels. (B) Allergen-specific IgE levels in serum. Levels of IgE in non-sensitized mice were undetectable at all timepoints. Values shown as mean ± SD from n = 10/group. In (A), significant differences were noted between the vaccine and placebo groups, both, after treatment (p < 0.01) and allergen challenge (p < 0.001, 2-way ANOVA, Bonferroni test). Whereas in (B) the difference between the vaccine and placebo groups was significant only after treatment (p < 0.001), but not after challenge.
Local tolerance at the injection site
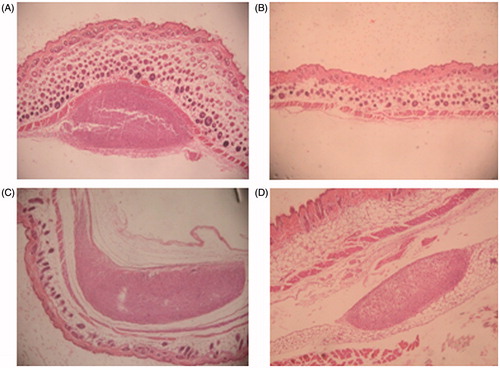
Microscopic findings at injection sites observed in repeat-dose toxicity study in non-allergic mice revealed local inflammatory responses. Primarily, these were evidenced as foreign body granulomas [characterized by aggregates of macrophages together with minor levels of polymorphs] and necrotic debris, sometimes surrounding cellulite/fibrosis, characterized by a mixed inflammatory cell infiltration of the subcutis. This local inflammatory response was also noted to approximately the same degree, in mice injected with the TH2 Control (). These local alterations were not fully reversed 14 days after ().
Figure 6. Local tolerance at injection site observed in non-sensitized mice in repeat-dose toxicity study. HE staining of administration site (representative images of four analyzed samples are shown). Magnification = 40X. (A) Vaccinated group, Day 21. (B) Saline control group. (C) TH2 Control. (D) Vaccinated group, Day 35.
Discussion
Alterations in body weight and/or food-water consumption are regarded as highly sensitive indicators for detecting alterations induced by products of low toxicity (OECD Citation2000). No obvious signs of general toxicity of the PL-adjuvant vaccine candidate were found here, both in the single and repeat-dose studies. This was an expected outcome as both components of the vaccine candidate (allergens of D. siboney house dust mite and PL from N. meningitidis) have been individually evaluated in previous settings of pre-clinical and clinical studies (Sierra and Campa Citation1990; Sierra et al. Citation1991; Aldana et al. Citation2000; Fuentes et al. Citation2003). Actually, PL is a major component of the anti-meningococcal BC vaccine (VAMENGOC-BC, Finlay Institute, Havana) in clinical use in several countries (Sierra et al. Citation1991; Pérez and Lastre Citation2013).
PL is a nanovesicle containing major outer membrane proteins, lipopolysaccharide (LPS), phospholipids, and porines. These components are well-characterized agonists of innate immunity receptors and are most likely responsible for the ability of PL to induce DC maturation (Rodríguez et al. Citation2005). Therefore, increased leukocyte stimulation and increase in spleen weight following three doses of the vaccine were not unexpected, considering that PL has potent immunostimulatory effects (Rodríguez et al. Citation2005; Bracho et al. Citation2006). Similar immune stimulating effects have been reported with other TH1-inducing adjuvants. Animal studies evaluating potential toxicity of an allergen vaccine containing a LPS-derived monophosphoryl Lipid A (MPL) have described also remarked increases in host white blood cell levels and spleen weight (Baldrick et al. Citation2002, Citation2004, Citation2007). Nevertheless, clinical trials of this MPL-containing allergen vaccine revealed a satisfactory safety profile for use in subcutaneous injection immunotherapy in patients suffering from grass pollen allergy (Rosewich et al. Citation2010, Citation2013).
The presence of local irritation and granulomas at the injection site has been previously related to the use of aluminum hydroxide as the depot adjuvant. Aluminum salts, used for many years in human vaccines, are known to produce local reactions in animals and humans and this is known to be associated with their mechanism of adjuvanticity (HogenEsch Citation2013; Jensen-Jarolim Citation2015). Novel adjuvants, such as MPL, also induce a local inflammatory response with foreign body granulomas characterized by aggregated macrophages, polymorphonuclear cells, necrotic debris, and cellulites/fibrosis (Baldrick et al. Citation2002, Citation2004). In spite of the PL immunostimulat effect, the local reactions after the administration of the PL-containing formulation were comparable to those observed in the negative control group administered with alum-adsorbed allergen lacking PL. Therefore, the local reactions could be attributed mostly to the presence and amount of aluminum, though some influence of PL cannot be ruled out.
Subcutaneous granulomas did not fully revert even 2 weeks after the final immunization in mice. Nevertheless, clinical results in humans with the vaccine VAMENGOC-BC that incorporates both PL and alum revealed that these lesions were fully reversible within few days after the final dose (Sierra and Campa Citation1990; Sierra et al. Citation1991). Similar behavior would be reasonable to expect in clinical trials with the current allergen vaccine candidate.
In contrast to common toxicity studies that were expected to render negative results due to the known low-toxicity potential of these biological components, the main toxicity hazard for this product could arise from a complex interplay with the immune system, particularly with regard to an immuno-allergic response. Allergenic products are a separate category of biologic products with specific regulations regarding clinical evaluation, standardization and quality aspects. Nevertheless, non-clinical safety assessment has not been clearly addressed in regulatory guidance (Nordic Guidelines Citation1989; EMEA Citation2008). An important issue in safety testing of products for allergen immunotherapy would be to evaluate the functional interaction of the existing immune status with environmental exposure, since allergic diseases are triggered by environmental allergens. For this purpose, the models used here incorporated an allergen challenge as an indicator of functionality of the immune response and assessment of a potential toxicity. Aerosolized allergen exposure intends to reproduce natural physiological exposure. Nebulized allergen particles can thus reach the lower respiratory tract being able to trigger an allergic reaction in this organ, which is relevant for resembling human allergic asthma.
The strategy described here is based on the use of two different models: administration of the product in naive or allergen sensitized mice, respectively. The first model, i.e. administration of the vaccine in naïve mice, in a “preventative” setting, is a common reductionist strategy that would allow for assessing the safety of the immune response exerted by the vaccine itself, without the possible interference of the allergic status of the recipient. On the other hand, the second model, i.e. administration of the vaccine in sensitized mice, i.e. in therapeutic setting, intends to mimics the scenery of allergen immunotherapy in humans, and could be useful for assessing its safety, in terms of potential exacerbations of the allergic response and induction of anaphylactic reactions, a major concern in this therapeutic approach. This second model is more in agreement with the present novel regulatory concept of Safety Pharmacology.
Safety Pharmacology studies are intended to measure functional signs of potential toxicity. These variables may be investigated in separate studies or incorporated in the design of toxicity studies (ICH Citation2011). Accordingly, in addition to the usual toxicological endpoints commonly used in the evaluation of vaccines and drugs, this study incorporated in both models, indicators of the allergic response as potential immunotoxicity endpoints: serum IgE and blood eosinophil levels. Both are major players of the immediate hypersensitivity and late-phase allergic reaction, respectively, allowing, therefore, a more complete assessment of the allergic response triggered by exposure to respiratory allergens. Interestingly, this work has shown a relative disconnection of both mechanisms, since sensitized mice showed an increase of IgE during vaccine administration and a parallel decrease of eosinophils as compared to sensitized mice receiving placebo, which is congruent with other reports (Hogan et al. Citation1997). However, this IgE increase did not led to an increased functional IgE response upon allergen re-exposure. These findings deserve further thorough research of the mechanisms involved in the response to the vaccine.
Overall, the study results support the lack of adverse effects of the vaccine on the previously established allergic disease; since after an allergen challenge, vaccinated mice failed to show an increase of the functional allergy response as compared to the placebo group.
Conclusions
The pre-clinical assessment of a novel mite allergen vaccine containing proteoliposomes from Neisseria meningitidis as an immunoactive adjuvant has produced no signs of direct toxicity or functional immunoallergic toxicity effects, at dose levels greatly in excess to those proposed for early phase clinical trials. The models implemented here support the safety of this vaccine for allergy immunotherapy.
Acknowledgements
This study was in part supported by collaboration agreements between the Centro Nacional de Biopreparados and Finlay Institute, Cuba.
Disclosure statement
The authors declare that they have no competing financial/conflicts of interests. The authors alone are responsible for the content of this manuscript.
Additional information
Funding
References
- Aldana L, González Y, Labrada A, García A, Amaya R. 2000. Repeat dose toxicity test for allergenic extracts Blomia tropicalis and Dermatophagoides siboney. Study of 28 days. Estudio de 28 días. Rev Cubana Farm. S34:517–518.
- Baldrick P, Richardson D, Wheeler A. 2002. Safety evaluation of a glutaraldehyde-modified tyrosine-adsorbed house dust mite extract containing monophosphoryl lipid A (MPL) adjuvant: A new allergy vaccine for dust mite allergy. Vaccine. 20:737–743.
- Baldrick P, Richardson D, Wheeler A, Woroniecki S. 2004. Safety evaluation of a new allergy vaccine containing the adjuvant monophosphoryl Lipid A (MPL) for the treatment of grass pollen allergy. J Appl Toxicol. 24:261–268.
- Baldrick P, Richardson D, Woroniecki SR, Lees B. 2007. Pollinex Quattro Ragweed: safety evaluation of a new allergy vaccine adjuvanted with monophosphoryl lipid A (MPL) for the treatment of ragweed pollen allergy. J Appl Toxicol. 27:399–409.
- Barboza R, Camara N, Gomes E, Sa S, Florsheim E, Mirotti L, Labrada A, Alcântara-Neves N, Russo M. 2013. Endotoxin exposure during sensitization to Blomia tropicalis allergens shifts TH2 immunity towards a TH17-mediated airway neutrophilic inflammation: Role of TLR4 and TLR2. PLoS One. 8:e67115.
- Bracho G, Lastre M, del Campo J, Zayas C, González D, Gil D, Acevedo R, Taboada C, Solís R, Pérez O. 2006. Proteoliposome derived cochleate as novel adjuvant. Vaccine. 24:S30–S31.
- Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blümer N, von Mutius E, Bufe A, Gatermann S, Renz H, Holst O, et al. 2007. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol. 119:1514–1521.
- [EMEA] European Agency for the Evaluation of Medicinal Products. 1997. CPMP/SWP/465/95 1997. Note for guidance on pre-clinical pharmacological and toxicological testing of vaccines. London: The European Agency for the Evaluation of Medicinal Products.
- [EMEA] European Agency for the Evaluation of Medicinal Products. 2000. CPMP/SWP/1042/99. Note for guidance on repeated dose toxicity. London: The European Agency for the Evaluation of Medicinal Products.
- [EMEA] European Agency for the Evaluation of Medicinal Products. 2008. CHMP/BWP/304831/2007. Guideline on allergen products: production and quality issues. London: The European Agency for the Evaluation of Medicinal Products.
- Fuentes D, González B, González Y, Aldana L, Arteaga M, Bada A, Labrada A, Bellido de Luna A. 2003. Pathological alterations produced by the subcutaneous inoculation of the allergenic extracts of Blomia tropicalis and Dermatophagoides siboney in rats and mice. Rev Toxicol. 20:23–26.
- Hogan SP, Mould A, Kikutani H, Ramsay AJ, Foster PS. 1997. Aeroallergen-induced eosinophilic inflammation, lung damage, and airways hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J Clin Invest. 99:1329–1339.
- HogenEsch H. 2013. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 3:1–13.
- ICH. 2011. ICH Harmonized Tripartite Guideline: Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals S6 (<R1i>). [Parent Guideline dated 16 July 1997. Addendum dated 12 June 2011]. Geneva.
- Jensen-Jarolim E. 2015. Aluminum in allergies and allergen immunotherapy. World Allergy Org J. 8:1–6.
- Kun L, Li L. 2016. Therapeutic strategies towards allergic diseases. Intl J Allergy Med. 2:1–6.
- Mohammadi-Shahrokhi V, Rezaei A, Andalib A, Rahnama A, Jafarzadeh A, Eskandaril N. 2017. Improvement of TH1/TH2 and TH1/Treg imbalances by adjuvants CPG, MPLA and BCG in a model of acute asthma induced by allergen Derp2 in BALB/c mice. Iran Red Crescent Med J. 19:e41114.
- Nordic Guidelines. 1989. Registration of allergen preparations. 2nd ed. Finland, Iceland, Norway, Sweden: Nordic Council on Medicines in cooperation with the Drug Regulatory Authorities in Denmark.
- [OECD] Organisation For Economic Co-Operation And Development. 2000. Guidance document on the recognition, assessment, and use of clinical signs as humane endpoints for experimental animals used in safety evaluation. Series on testing and assessment. ENV/JM/MONO 7. Paris: Organisation For Economic Co-Operation And Development.
- Pérez O, Lastre M. 2013. Proteoliposome: VA-MENGOC-BC® core and adjuvant platform. VacciMonitor. 22:1–3.
- Pérez O, Romeu B, Cabrera O, González E, Batista-Duharte A, Labrada A, Pérez R, Reyes L, Ramírez W, Sifontes S, et al. 2013. Adjuvants are key factors for the development of future vaccines: Lessons from the Finlay adjuvant platform. Front Immunol. 4:1–12.
- Pfaar O, Cazan D, Klimek L, Larenas Linnemann D, Calderon M. 2012. Adjuvants for immunotherapy. Curr Opin Allergy Clin Immunol. 12:648–657.
- Rodríguez T, Ugrinovic S, Pérez O, Bracho G, Mastroeni P. 2005. Interactions of proteoliposomes from serogroup B Neisseria meningitidis with bone marrow-derived dendritic cells and macrophages: adjuvant effects and antigen delivery. Vaccine. 23:1312–1321.
- Romagnani S. 2014. T-Cell Sub-populations. In: History of allergy, Bergmann K, and Ring J, Editors. Basel: Karger; p. 155–164.
- Rosewich M, Lee D, Zielen S. 2013. Pollinex Quattro: An innovative 4 injection immunotherapy in allergic rhinitis. Human Vaccines Immunother. 9:1523–1531.
- Rosewich M, Schulze J, Eickmeier O, Telles T, Rose M, Schubert R, Zielen S. 2010. Tolerance induction after specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A in children. Clin Exp Immunol. 160:403–410.
- Schroeder W, Mitrescu L, Hart M, Unnithan R, Gilchrist J, Smith E, Shanley C, Benedict K, Taraba L, Volckens J, et al. 2009. Flexible low-cost system for small animal aerosol inhalation exposure to drugs, proteins, inflammatory agents, and infectious agents. Biotechniques. 46:iii–viii.
- Sierra V, Campa C. 1990. Pre-clinical and clinical studies with the anti-meningococal vaccine BC: VA-MENGOC-BC®. Rev Interferon Biotechnol. Special Volume.
- Sierra V, Campa C, Varcácel M. 1991. Vaccine against group B Neisseria meningitidis: Protection trial and mass vaccination results in Cuba. NIPH Ann. 1991:195–207.
- Tamargo B, Márquez Y, Ramírez W, Cedré B, Fresno M, Sierra G. 2013. New proteoliposome vaccine formulation from N. meningitidis serogroup B, without aluminum hydroxide, retains its anti-meningococcal protectogenic potential as well as TH1 adjuvant capacity. BMC Immunol. 14:S12.
