Abstract
A growing body of evidence suggests that epicutaneous sensitization of protein allergens induces immediate-type hypersensitivity (IHS) following induction of Type 2 immune responses in animals and humans. Thymic stromal lymphopoietin (TSLP) derived from keratinocytes is a cytokine that can activate dendritic cells and has been implicated in development of inflammatory Type 2 helper T-cells. However, there is no direct evidence that allergens directly regulate TSLP expression in keratinocytes. This study aimed to evaluate the response of TSLP to protein allergens in cultured human keratinocytes and to identify appropriate endpoints for IHS. The transcription of long-form TSLP (loTSLP) was strongly induced by ovalbumin, wheat gluten (WG), acid-hydrolyzed WG (acid-HWG), and extracts from feces of Dermatophagoides pteronyssinus and D. farina, and trypsin, but not by rare allergens, human serum albumin (HSA), or extracts of mite bodies. In acid-HWG, loTSLP mRNA was significantly augmented by acid hydrolysis of WG for 0.5 h compared to WG. However, prolonged acid hydrolysis attenuated this induction similarly to that reported in previous animal studies. These results suggested that intense loTSLP transcriptional induction was a characteristic of a high-allergenic protein. Additionally, TSLP production was induced by exposure to ovalbumin, WG, and acid-HWG in combination with a trio of cytokines, i.e. interleukin (IL)-4, IL-13, and tumor necrosis factor (TNF)-α. However, no TSLP protein was detected following exposure to HSA, even in the presence of these cytokines. With acid-HWG, TSLP protein release was consistent with loTSLP transcription. Thus, intense loTSLP transcriptional induction and TSLP protein expression are each effective indicators that can be used for in vitro screening of IHS.
Introduction
Immediate-type hypersensitivity (IHS) induced by protein allergens can be life-threatening. A well-known IHS-derived inflammatory disorder is food allergy, which is thought to affect nearly 5% of adults and 8% of children (Sicherer and Sampson Citation2014). Furthermore, IHS to hydrolyzed wheat protein caused by the use of skin moisturizers, facial cream, and facial soap in cosmetics was recently reported (Lauriere et al. Citation2006; Fukutomi et al. Citation2011). However, evaluation methods for IHS have been restricted to use in animals. Conventional indices for detecting IHS include direct induction of anaphylactic reactions/allergen-specific IgE production in animal models. On the other hand, there is an increasing need for alternative tests for in vivo methods for safety evaluations of new raw materials. Although many alternative tests for delayed hypersensitivity have been developed from the viewpoint of the mechanisms in the early stages of sensitization (Gerberick et al. Citation2004; Sakaguchi et al. Citation2009; Emter et al. Citation2010), there is presently no alternative test for IHS.
A growing body of evidence suggests that epicutaneous sensitization to protein allergens induces IHS in animal models (Dunkin et al. Citation2011; Adachi et al. Citation2012; Bartnikas et al. Citation2013; Tordesillas et al. Citation2014; Matsunaga et al. Citation2015) and that epicutaneous sensitization induces IHS symptoms in humans (Amaro and Goossens Citation2008; Fukutomi et al. Citation2011; Inomata et al. Citation2015; Katayama et al. Citation2016). Although it was previously thought that protein antigens could not penetrate into the skin because of their molecular size, human and animal data suggests that they can penetrate into the skin (Kimber et al. Citation2014). In addition, epithelium-derived cytokines, such as thymic stromal lymphopoietin (TSLP) and interleukin (IL)-33, and antigen-presenting cells, such as dendritic cells, Langerhans cells, and basophils, are involved in Type 2 immune responses induced by epicutaneous sensitization (Soumelis et al. Citation2002; Nakajima et al. Citation2012; Muto et al. Citation2014; Noti et al. Citation2014). TSLP polarizes to inflammatory T-helper Type 2 (TH2) responses through elevated OX40 ligand expression on dendritic cells (Ito et al. Citation2005; Murakami-Satsutani et al. Citation2014). These past studies proposed that TSLP has an important role in epicutaneous sensitization in IHS. However, few studies have investigated the underlying mechanisms of allergens in the directly induced expression of TSLP in keratinocytes.
Two human transcript variants of TSLP have been recently investigated (Xie et al. Citation2012; Bjerkan et al. Citation2015). The long-form TSLP (loTSLP) is reportedly responsible for pro-inflammatory responses and is induced by poly(I:C) [the Toll-like receptor (TLR) 3 ligand], the synthetic diacylated lipoprotein FSL-1 (TLR2–TLR6 ligand), and flagellin (TLR5 ligand) (Xie et al. Citation2012; Fornasa et al. Citation2015). On the other hand, the short-form of TSLP (shTSLP) exerted anti-inflammatory and antimicrobial effects and was constitutively expressed in unstimulated keratinocytes (Bjerkan et al. Citation2015; Fornasa et al. Citation2015). Since TSLP is a dual-functioning cytokine, it is important to distinguish the long from the short form in analyses (Bjerkan et al. Citation2015). However, the expression profiles of the two types of TSLP following allergen exposure remain largely unknown.
The aim of this study was to investigate the effects of allergens that induce IHS reactions by epicutaneous sensitization on the expression of the two forms of TSLP in keratinocytes. This study used a model that involved the initial process of epicutaneous sensitization of protein allergens to enable screening for IHS in vitro.
Materials and methods
Cell culture and cytokine stimulation
Primary human keratinocytes (Thermo Fisher Scientific/Life Technologies, Waltham, MA) were cultured in HuMedia KG2 (Kurabo, Osaka, Japan) and then seeded at 4.5 × 104 cells/well in flat-bottomed 24-well culture plates for real-time quantitative polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA). Cells were also seeded in 6-well culture plates for Western blotting. Once cells reached 80% confluence (2–3 days after plating), medium was replaced with HuMedia KG2 without hydrocortisone, according to the procedure of Kinoshita et al. (Citation2009). After additional culture for 24 h, the cells were stimulated with test samples and/or the cytokines human IL-4 (100 ng/ml) and IL-13 (100 ng/ml) as well as tumor necrosis factor (TNF)-α (20 ng/ml) (all from R&D Systems, Minneapolis, MN).
Preparation of test samples
Ovalbumin (OVA, A5503, grade V), wheat gluten (WG, G5004), trypsin (T1426), and human serum albumin (HSA, A5843) were obtained from Sigma (St. Louis, MO). Glupearl® 19 S (GP19S), a partially acid-hydrolyzed WG (HWG), was supplied by Katayama Chemical, Inc. (Osaka). House dust mite-related products [extracts of mite body (MB) and feces (Fe) of Dermatophagoides pteronyssinus (Der p) and Dermatophagoides farinae (Der f)] were provided by LSL Co., Ltd. (Tokyo, Japan). Polyinosinic-polycytidylic acid [poly(I:C)], a positive control for TSLP induction, was purchased from Bio-Techne/TOCRIS Bioscience (Bristol, UK).
WG, GP19S, and acid-HWG were prepared in our laboratories, as previously described (Matsunaga et al. Citation2015). In brief, WG and GP19S were suspended in a 1 M Tris (hydroxyl-methyl)-amino-methane buffer (pH 11.4). After standing at room temperature for 24 h, samples were neutralized with 1 N HCl. Acid-HWG was prepared by hydrolyzing gluten in Tris solution with 0.1 N HCl at 100 °C for the indicated time, and then the solution was neutralized with 1.5 M NaOH. A control sample was prepared by adding WG in Tris to HCl and NaOH simultaneously without heating (0 h). A control vehicle sample (Tris-HCl) was prepared by neutralized Tris with HCl. OVA, trypsin, HSA, house dust mite-related products, and poly(I:C) were dissolved in phosphate-buffered saline (PBS, pH 7.2; Wako Chemical, Osaka).
The maximum concentration of test sample used in the studies was determined by the cytotoxicity, solubility, and stock concentration of the test sample. Some protein samples were contaminated with lipopolysaccharide (LPS; Supplementary Table 1). However, the maximum dose of contaminated LPS in assays (i.e. < 30 μg/ml) did not affect loTSLP transcription (Supplementary Figure 1).
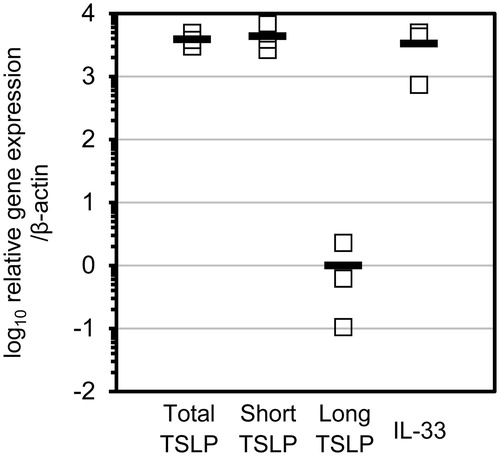
Figure 1. ShTSLP, IL-33, and loTSLP transcription in unstimulated keratinocytes. Total RNA samples were obtained from cultured primary human keratinocytes with no stimulation. Relative gene expression represents ratio of total and two isoforms of TSLP mRNA and IL-33 mRNA compared to average of β-actin mRNA levels. Each open square represents data from individual keratinocyte cultures; horizontal bar represents average from n = 3.
Exposure to test samples
For real-time quantitative PCR and ELISA assays, cells in 400 μl culture medium (in 24-well culture plates [in triplicate]) were treated with 100 μl prepared sample. After the indicated incubation periods, culture supernatants were collected and placed at −80 °C until analysis. Remaining adherent cells were checked for viability using an LDH Cytotoxicity detection kit (Takara, Tokyo). For the Western blot studies, cells in 1600 μl culture medium (in 6-well culture plates [in triplicate]) were treated with 400 μl prepared sample. After 72-h incubation, culture supernatants were collected and placed at −80 °C until analysis.
Real-time quantitative PCR analysis
After removal of the medium/test sample, washed adherent cells were processed for PCR analyses by direct addition of 350 μl RLT buffer (Qiagen, Hilden, Germany) bearing 3.5 μl 2-mercapto-ethanol (2-ME; Thermo Fisher Scientific) to each well. Total RNA from the lysed cells was extracted using an RNeasy Micro Kit in combination with QIAshredder (Qiagen), according to manufacturer instructions. After confirming RNA concentration and purity using a NanoDrop 2000 system (Thermo Fisher Scientific), first-strand cDNA was then synthesized using a High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Thermo Fisher Scientific). Real-time PCR analysis was then performed using TaqMan® Universal PCR Master Mix and an Applied Biosystems® 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). Transcription levels of total-TSLP (toTSLP), IL-33, and β-actin were analyzed using PCR primer sets recommended by Thermo Fisher Scientific (i.e. Hs00263639_m1, Hs00369211_ml, and Hs01060665_g1). Analysis of loTSLP transcription was performed using a specific long form probe set (Hs01572933_m1). The primer and probe sets for shTSLP were designed and synthesized by TaqMan MGB probe service (Applied Biosystems) from the shTSLP-specific region of human TSLP, transcript variant 2, mRNA (NM_138551.4): forward-5′-CGCCTATG-AGCAGCCACATT and reverse-5′- GGCGGTGGGATTGAAGGT (amplicon length of 61 bp, Supplementary Figure 2). Relative gene expression levels were normalized to β-actin. Relative differences in gene expression levels compared with control CT values were calculated using the 2–ΔΔCt method (Livak and Schmittgen Citation2001).
Figure 2. OVA and GP19S-induced shTSLP and loTSLP transcription. (A) Total RNA samples were obtained from cultured primary human keratinocytes after treatment with OVA or GP19S for 3 h [in triplicate]. Relative gene expression represents ratio of each mRNA in sample-exposed cells to corresponding mRNA in control cells. (B) Time-dependent changes in keratinocyte TSLP transcription after treatment with poly(I:C) (100 μg/ml, positive control), OVA (68 mg/ml), or GP19S (1 mg/ml) [in triplicate]. Relative gene expression represents ratio of each mRNA in sample-exposed keratinocyte to each mRNA in control. (C) Dose-related changes in keratinocyte TSLP transcriptions after treatment with poly(I:C) (positive control), OVA, or GP19S for 3 h [in triplicate]. Relative gene expression represents ratio of each mRNA in exposed cells to corresponding mRNA in controls. *p < 0.05, **p < 0.01 vs. PBS control.
![Figure 2. OVA and GP19S-induced shTSLP and loTSLP transcription. (A) Total RNA samples were obtained from cultured primary human keratinocytes after treatment with OVA or GP19S for 3 h [in triplicate]. Relative gene expression represents ratio of each mRNA in sample-exposed cells to corresponding mRNA in control cells. (B) Time-dependent changes in keratinocyte TSLP transcription after treatment with poly(I:C) (100 μg/ml, positive control), OVA (68 mg/ml), or GP19S (1 mg/ml) [in triplicate]. Relative gene expression represents ratio of each mRNA in sample-exposed keratinocyte to each mRNA in control. (C) Dose-related changes in keratinocyte TSLP transcriptions after treatment with poly(I:C) (positive control), OVA, or GP19S for 3 h [in triplicate]. Relative gene expression represents ratio of each mRNA in exposed cells to corresponding mRNA in controls. *p < 0.05, **p < 0.01 vs. PBS control.](/cms/asset/df782f9c-f959-4277-9007-94f3c572e6f6/iimt_a_1349220_f0002_b.jpg)
Detection of intracellular and secreted proteins via ELISA or Western blot
Secreted total TSLP was measured using a human TSLP Quantikine ELISA Kit (R&D Systems) according to manufacturer protocols. Kit limit of detection was 1.94 pg TSLP/ml.
For detecting intracellular proteins, after removal of the medium/test sample, washed cells in the wells were lysed with a solution containing 0.2 M Tris buffer (pH 7.4), 2% sodium dodecyl sulfate (SDS), protease inhibitor cocktail (cOmplete Mini; Roche Diagnostics, Indiana-polis, IN), and phosphatase inhibitor cocktail (PhosSTOP; Roche). Lysates were mixed at a ratio of 4:1 (v/v) with 5 × loading buffer [containing 20% 2-ME], sonicated 5 min, and then boiled for 1 min. For analyses of secreted proteins, the isolated supernatants from the keratino-cyte cultures were concentrated 300→100 μl [for ] or 1900→100 μl for Western blot analyses using Amicon Ultracel 3 K columns (Merck Millipore, Darmstadt, Germany). As above, the samples were mixed with 5 × loading buffer containing 20% 2-ME [4:1, v/v] and then boiled for 1 min. Total protein in each sample was not quantified due to presence of SDS and 2-ME.
In each case, proteins were then resolved by SDS-polyacrylamide gel electrophoresis using 8–16% Mini-PROTEAN® TGX™ Pre-cast Protein Gels (Bio-Rad Laboratories, Hercules, CA). The separated proteins were then transferred onto a polyvinylidene fluoride membrane (Immun-Blot® PVDF Membrane, Bio-Rad), and then incubated in a solution of PBS containing 0.5% Tween-20 (PBST) supplemented with 5% skim milk and containing anti-human TSLP polyclonal antibody (10 μg/ml, clone ab47943, Abcam, Cambridge, UK) or anti-β-actin monoclonal antibody (1:1000, clone 13E5; Cell Signalling Technology, Danvers, MA) at 4 °C overnight. After undergoing washing with PBST four times, the membrane was incubated with PBST/5% skim milk containing horseradish peroxidase-conjugated anti-rabbit IgG antibody (1:5000, Jackson Immuno-Research Labs, West Grove, PA) at 37 °C for 3 h. In some cases, recombinant human TSLP (0.2 ng/ml, Bio-Techne/R&D systems) was mixed with primary antibody. After a further round of washings, any signals on the membrane were visualized using SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific) and an Image-Quant LAS 4000 detection system (GE Healthcare, Piscataway, NJ). Each signal intensity for TSLP in each sample was normalized to β-actin expression levels present in each sample.
Statistical analyses
Data are presented as the means ± SD for three wells. An unpaired Student’s t-test (2-tailed) or one-way analysis of variance (ANOVA) with a Tukey multiple comparison test was used for statistical analyses. A p values < 0.05 indicated statistical significance. All the analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (Kanda Citation2013). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.
Results
LoTSLP and shTSLP were significantly induced by OVA and GP19S
In unstimulated keratinocytes, loTSLP mRNA expression was ≈ 4000-fold lower than shTSLP, toTSLP, and IL-33 mRNA expression levels (). To evaluate which epithelial cell-derived cytokine was a suitable marker for detecting allergens, transcriptional induction of toTSLP and IL-33 by OVA and GP19S exposure was evaluated. Results showed that ToTSLP mRNA levels were significantly enhanced by OVA and by GP19S (). However, IL-33 mRNA expression was marginally enhanced by GP19S and not enhanced by OVA. Therefore, TSLP was chosen as the target molecule for detecting IHS in vitro.
In a time-course experiment, toTSLP, shTSLP, and loTSLP transcript levels were dramatically augmented within the first 3 h of treatment, and subsequently decreased – following exposure to poly(I:C), OVA, or GP19S (). Therefore, the exposure time for subsequent studies was set at 3 h. In a dose-response experiment, all TSLP mRNA expression levels were significantly up-regulated by OVA and GP19S in a dose-related manner ().
LoTSLP was strongly induced by high- but not bylow-allergenic proteins
To study levels of TSLP induction by allergens, toTSLP, shTSLP, and loTSLP mRNA expression levels were evaluated by exposure to four high-allergenic proteins that frequently cause allergy in humans (e.g. WG, extracts of Der p and Der f feces, trypsin) and three low-allergenic proteins that contained low major allergen components (extracts of house dust mite bodies [Der p and Der f]) (Tovey et al. Citation1981), or rare allergens in humans (HSA). Each reference relating to the allergenicity of the test material is noted in Supplementary Table 2. In a dose-response experiment, toTSLP, shTSLP and loTSLP mRNA expression levels were significantly induced by WG, Der p and Der f fecal extract, trypsin, and HSA each in dose-related manners (). Interestingly, as compared with controls, loTSLP mRNA expression was highly (>100-fold) potentiated by all high-allergenic proteins. In contrast, this expression was only marginally induced by Der p MB (3.6-fold), Der f MB (25-fold), or HSA (17-fold).
Figure 3. High-allergenic proteins effects on potent loTSLP transcription in keratinocytes. Dose-related changes in TSLP transcriptions in cultured primary human keratinocytes after treatment with the indicated concentrations of high- (WG, Der p Fe, Der f Fe, and trypsin) and low- (Der p MB, Der f MB, and HSA) allergenic proteins for 3 h [in triplicate]. Relative gene expression represents ratio of each mRNA in sample-exposed keratinocytes to corresponding mRNA in control cells. *p < 0.05, **p < 0.01 vs. PBS control.
![Figure 3. High-allergenic proteins effects on potent loTSLP transcription in keratinocytes. Dose-related changes in TSLP transcriptions in cultured primary human keratinocytes after treatment with the indicated concentrations of high- (WG, Der p Fe, Der f Fe, and trypsin) and low- (Der p MB, Der f MB, and HSA) allergenic proteins for 3 h [in triplicate]. Relative gene expression represents ratio of each mRNA in sample-exposed keratinocytes to corresponding mRNA in control cells. *p < 0.05, **p < 0.01 vs. PBS control.](/cms/asset/1cce282f-aa3f-4e99-8145-ed3399bdbbbd/iimt_a_1349220_f0003_b.jpg)
On the other hand, toTSLP and shTSLP mRNA expression levels were equally enhanced by HSA and other high-allergenic proteins. Therefore, the gene expression patterns of loTSLP and shTSLP were different. Only high-allergenic proteins induced potent (>100-fold) loTSLP mRNA induction. In addition, loTSLP and shTSLP mRNA expression levels were significantly higher in GP19S (higher allergenic protein than WG)-exposed keratinocytes as compared with in WG-exposed keratinocytes ().
Synergy of loTSLP mRNA enhancement between allergen and Type 2 and pro-inflammatory cytokines
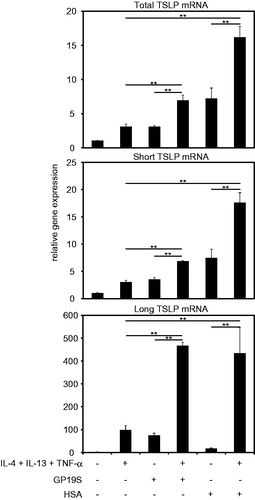
Type 2 cytokines IL-4 and IL-13 and pro-inflammatory TNFα [atopic dermatitis (AD)-related cytokines] have been reported to potentiate TSLP expression (Kinoshita et al. Citation2009). The results of the present study revealed that loTSLP was synergistically up-regulated by AD-related cytokines and proteins (GP19S or HSA) (). On the other hand, shTSLP appeared to be additively up-regulated by these cytokines and GP19S; conversely, shTSLP was seemingly synergistically up-regulated by AD-related cytokines and HSA.
Figure 4. TSLP transcriptions induced by allergen ± AD-related cytokines. TSLP transcriptions in cultured keratinocytes were modulated by 3-h incubation with 3 mg/ml GP19S or 68 mg/ml HSA in absence/presence of IL-4 (100 ng/ml), IL-13 (100 ng/ml), and TNFα (40 ng/ml) (in triplicate). Relative gene expression represents ratio of each mRNA in sample-exposed cells to corresponding mRNA in control cells. **p < 0.01: cytokines alone vs. cytokines + GP19S; GP19S vs. cytokines + GP19S; cytokines alone vs. cytokines + HSA; HSA vs. cytokines + HSA.
TSLP protein was released by allergens with allergen and Type 2 and pro-inflammatory cytokines
Results from the ELISA analyses showed that TSLP protein was released by keratinocyte exposure to any of the three levels of OVA or to the higher (i.e. 1 mg/ml) level of GP19S even without cytokines present (). TSLP protein was not detected following cell exposure to WG or HSA in the absence of cytokines. TSLP protein release due to OVA or GP19S – and even by WG – was augmented by the combination treatment of cells with IL-4, IL-13, and TNFα. The significance of the effect was uniform with WG and GP19S, but not with OVA. No TSLP protein was truly detected among cells given HSA and the cytokines.
Figure 5. High allergenic proteins specifically induced large TSLP protein with AD-related cytokines in cultured keratinocytes. (A) ELISA was used to measure released total TSLP protein in the culture media after 72 h stimulation with indicated concentrations of allergens in absence or presence of IL-4 (100 ng/ml), IL-13 (100 ng/ml), and TNFα (40 ng/ml) [in triplicate]. *p < 0.05, **p < 0.01: without vs. with cytokines. (B, C) Keratinocytes stimulated with 100 μg/ml poly(I:C) or 0.5 mg/ml GP19S in absence/presence of IL-4 (100 ng/ml), IL-13 (100 ng/ml), and TNFα (40 ng/ml) for 72 h. TSLP [and β-actin] protein levels in (B) concentrated culture media and (C) lysates of cultured keratinocytes assessed by Western blot. Numbers indicate positions of molecular weight markers. To verify specificity of the polyclonal anti-TSLP antibody used, TSLP protein that was detected (left panel) was no longer detected after pre-absorbtion with recombinant loTSLP (right panel).
![Figure 5. High allergenic proteins specifically induced large TSLP protein with AD-related cytokines in cultured keratinocytes. (A) ELISA was used to measure released total TSLP protein in the culture media after 72 h stimulation with indicated concentrations of allergens in absence or presence of IL-4 (100 ng/ml), IL-13 (100 ng/ml), and TNFα (40 ng/ml) [in triplicate]. *p < 0.05, **p < 0.01: without vs. with cytokines. (B, C) Keratinocytes stimulated with 100 μg/ml poly(I:C) or 0.5 mg/ml GP19S in absence/presence of IL-4 (100 ng/ml), IL-13 (100 ng/ml), and TNFα (40 ng/ml) for 72 h. TSLP [and β-actin] protein levels in (B) concentrated culture media and (C) lysates of cultured keratinocytes assessed by Western blot. Numbers indicate positions of molecular weight markers. To verify specificity of the polyclonal anti-TSLP antibody used, TSLP protein that was detected (left panel) was no longer detected after pre-absorbtion with recombinant loTSLP (right panel).](/cms/asset/7ac1fd29-094c-4362-b006-32a1f175bade/iimt_a_1349220_f0005_b.jpg)
Western blot analyses revealed that a band of TSLP protein [≈ 35 kDa] was seemingly present in culture supernatants after exposure of the cells to poly(I:C), GP19S, alone or in combination with the AD-related cytokines (, left). A TSLP band [also ≈ 35 kDa] was also found in cell lysates of cells exposed to poly(I:C) or GP19S (, left). Co-presence of the AD-related cytokines did little to affect this expression. The antibody specificity for TSLP was confirmed by an inhibition assay using recombinant TSLP protein (, right). Interestingly, within the lysate samples, an ≈ 12-kDa band specific for TSLP was present (, left); this band absent in culture supernatants.
Acid hydrolysis of WG augmented loTSLP induction potential
In studies to confirm TSLP induction potentials of various acid-HWG that had augmented or reduced epicutaneous sensitizing potential relative to that of WG in guinea pigs (Matsunaga et al. Citation2015), it was seen that loTSLP mRNA induction was augmented by WG that was acid hydrolyzed for 0.5 h (); on the other hand, induction by HWG acid hydrolyzed for 6 h was decreased to that of untreated WG (and then even less so if had been hydrolyzed for 48 h). In comparison, shTSLP mRNA levels only slightly changed in keratinocytes exposed to the various acid-HWG. Therefore, more intense loTSLP – but not shTSLP – mRNA induction in cultured keratinocytes was observable in response to proteins that were easier to sensitize by the epicutaneous route.
Figure 6. Acid hydrolysis potentiated WG-induced loTSLP expression in keratinocytes. (A) TSLP transcriptions in cultured keratinocytes after 3-h treatment with 0.5 mg/ml of WG hydrolyzed for 0, 0.5, 6, and 48 h [in triplicate]. Relative gene expression represents ratio of each mRNA in sample-exposed keratinocytes to corresponding mRNA in control cells. **p < 0.01, 0 h vs. 0.5 h (except for shTSLP mRNA); 0.5 h vs. 6 h; 0.5 h vs. 48 h (B) Released TSLP proteins in culture media after 72 h of cell stimulation with 0.5 mg/ml of WG [hydrolyzed for 0, 0.5, 1, 3, 6, 24, and 48 h] in the presence/absence of IL-4 (100 ng/ml), IL-13 (100 ng/ml), and TNFα (40 ng/ml) [in triplicate]. ELISA. **p < 0.01: 0 h vs. 0.5 h; 0.5 h vs. 1 h; 0.5 h vs. 3 h.
![Figure 6. Acid hydrolysis potentiated WG-induced loTSLP expression in keratinocytes. (A) TSLP transcriptions in cultured keratinocytes after 3-h treatment with 0.5 mg/ml of WG hydrolyzed for 0, 0.5, 6, and 48 h [in triplicate]. Relative gene expression represents ratio of each mRNA in sample-exposed keratinocytes to corresponding mRNA in control cells. **p < 0.01, 0 h vs. 0.5 h (except for shTSLP mRNA); 0.5 h vs. 6 h; 0.5 h vs. 48 h (B) Released TSLP proteins in culture media after 72 h of cell stimulation with 0.5 mg/ml of WG [hydrolyzed for 0, 0.5, 1, 3, 6, 24, and 48 h] in the presence/absence of IL-4 (100 ng/ml), IL-13 (100 ng/ml), and TNFα (40 ng/ml) [in triplicate]. ELISA. **p < 0.01: 0 h vs. 0.5 h; 0.5 h vs. 1 h; 0.5 h vs. 3 h.](/cms/asset/738b6c5a-049c-4443-ac88-d79b43cf41f7/iimt_a_1349220_f0006_b.jpg)
Total TSLP protein release from the keratinocytes was significantly augmented by acid-hydrolyzing the WG for 0.5–1.0 h (). On the other hand, TSLP protein induction by WG was drastically decreased (to undetectable levels) had the protein been hydrolyzed for 3, 6, 24, or 48 h.
Discussion
There were three major findings in this study. First, the mRNA expression of loTSLP in human primary keratinocytes was greatly elevated by treatment of cells with OVA, WG, acid-HWG, extracts from the feces of Der p and Der f, or trypsin; however, shTSLP was elevated by any test protein. Second, release of TSLP protein was augmented by high-allergenic proteins combined with Type 2 cytokines IL-4 and IL-13 and pro-inflammatory TNFα. Third, acid-hydrolysis of WG (at least, for 0.5 h) enhanced TSLP production, an outcome consistent with in vivo allergenicity observed in guinea pigs (Matsunaga et al. Citation2015).
Consistent with our findings in human epidermal keratinocytes, loTSLP is reportedly not constitutively expressed, although shTSLP is constitutively expressed in healthy skin tissue and oral keratinocytes (Bjerkan et al. Citation2015; Fornasa et al. Citation2015). The two isoforms of TSLP are not splice variants but rather are transcript variants that can be separated by two putative promoters (Fornasa et al. Citation2015). The findings of the present study revealed a difference in the mRNA expression patterns of the two TSLP isoforms. Interestingly, loTSLP was markedly induced by only high-allergenic proteins (OVA, WG, GP19S, trypsin, Der p Fe, and Der f Fe). On the other hand, shTSLP was augmented by all proteins. LoTSLP induction by the protein allergens was consistent with the allergen-induced TH2 response in vivo (Soumelis et al. Citation2002). The results of the ELISA and western blotting experiments showed that a large TSLP protein (∼35 kDa) was detected in culture medium and cell lysates after allergen exposure, whereas a small TSLP (∼12 kDa) was detected only in cell lysates, suggesting the shTSLP protein was not secreted outside of the cell. The molecular weight of loTSLP is reportedly 25 kDa in human lung fibroblasts (Hung et al. Citation2013) and 23 kDa in oral keratinocytes (Bjerkan et al. Citation2015). The current data suggest that the molecular weight of loTSLP in epidermal keratinocytes was ≈ 35 kDa. The difference from the previously-reported molecular weight may be because of post-translational modifications of the cell type or variations in experimental conditions (e.g. differences in polyacrylamide gel concentrations, use of reducing reagents, anti-TSLP antibody, etc.). To our knowledge, these results are the first to show that allergenic proteins have a high potential to induce loTSLP in epidermal keratinocytes.
Because TSLP transcription was augmented at 1–3 h after protein exposure, the protein allergen seemed to directly react with the keratinocytes. The molecular mechanisms underlying allergen recognition in keratinocytes are not well understood. Kouzaki et al. (Citation2009) reported that two protease allergens, trypsin and papain, induced toTSLP via proteinase activity and protease-activated receptor 2 (PAR-2) in a human airway epithelial cell line. In addition, major mite allergen components, Der p 1 and Der f 1, have cysteine protease activities, and >95% of Der p 1 has been found to be associated with mite feces rather than with the mite body and could activate PAR-2 (Tovey et al. Citation1981; Asokananthan et al. Citation2002; Takai et al. Citation2005). Some allergens have protease activities that induce barrier disruption and TSLP production. However, little is known about how non-proteolytic allergens (OVA and WG) induce TSLP production. Some TLR ligands react to cognate TLR in cultured human keratinocytes and induce TSLP (Koller et al. Citation2011; Le et al. Citation2011; Vu et al. Citation2010, Citation2011). Poly(I:C) induced production of large amounts of loTSLP mRNA, but small amounts of shTSLP mRNA via TLR3 and TSLP mRNA induction peaked at 3 h, an outcome similar to the present results for reactions to allergenic proteins (Xie et al. Citation2012). These investigations implied that protein allergens also react to TLR3 either directly or indirectly. Common transcription factors for TSLP induction include nuclear factor (NF)-κB, activator protein (AP)-1, interferon-regulatory factor (IRF)-3, and signal transducer and activator of transcription (STAT)-6 (Kato et al. Citation2007; Lee and Ziegler Citation2007; Harada et al. Citation2009). Therefore, it could be speculated that an unknown receptor for protein allergens might lead to activation of these transcription factors. However, further investigations are needed to elucidate the recognition and specific signaling pathways of protein allergens that induce production of the two TSLP isoforms.
Local expression of loTSLP has an important role in sensitization and the effecter phases of allergy (He et al. Citation2008; Leyva-Castillo et al. Citation2013; Wang et al. Citation2015). The present study showed that loTSLP was released by allergenic proteins, suggesting that production of loTSLP is characteristic of allergenicity. Although the patients sensitized by facial soap with relatively high concentrations of 0.3% GP19S induced IHS to wheat products, here, loTSLP was induced by lower doses of GP19S at the protein level (0.05% GP19S) and mRNA level (0.025% GP19S) (Fukutomi et al. Citation2014). Though dilution rates and skin absorption rate of soap must be taken into consideration, the GP19S concentration that induces TSLP production is relevant to sensitization of humans under certain conditions. The results of previous human serum experiments have shown that IgE reactivity to acid-HWG caused a peak in acidic hydrolysis for 0.5 h, and the antigenicity of acid-HWG disappeared following acidic hydrolysis for 24 h (Nakamura et al. Citation2013a). GP19S is composed of WG and de-amidated by acidic hydrolysis, and its antigenicity is higher than that of WG because of the development of a new epitope (Gourbeyre et al. Citation2012; Nakamura et al. Citation2013b,Citationc; Yokooji et al. Citation2013). The findings of the present investigation suggested that GP19S increased the expression of loTSLP due to de-amidation and/or developing new epitopes by acidic hydrolysis.
It has been reported that production of TSLP is amplified by Type 2 cytokines IL-4 and IL-13, and inflammatory TNFα (Bogiatzi et al. Citation2007; Kinoshita et al. Citation2009). In the present study, OVA and WG increased TSLP protein release via these AD-related cytokines. Patients with AD have been shown to be easily sensitized to allergens, such as GP19S (Fukutomi et al. Citation2014; Horimukai et al. Citation2014). LoTSLP protein is easily released after host exposure to some protein allergens. Therefore, it was proposed here that an allergen with high epicutaneous sensitizing potential would possess properties that induce production of TSLP. In light of epicutaneous application of protein allergen(s), this result of the current research are expected to be of use in future safety evaluations of epicutaneous immunotherapy (EPIT).
Understanding the potential of TSLP induction of protein allergens might help to advance EPIT because EPIT is applied to stripped skin, which is thought to induce TSLP, has not been found to be more effective than EPIT applied to intact skin (Oyoshi et al. Citation2010; Mondoulet et al. Citation2012). Moreover, this phenomenon was identical to that of Matsumoto and Saito (Citation2013), i.e. sensitization develops in eczematous skin while immunological tolerance is induced in healthy skin. Here, HSA exposure potentiated shTSLP transcription but did not cause a release of TSLP protein even in the co-presence of AD-related cytokines. Further, as the loTSLP gene is largely shared with the shTSLP gene, it is likely TSLP protein release was regulated by shTSLP mRNA. One reason why shTSLP mRNA was synergistically up-regulated by HSA and AD-related cytokines might be that specific pathways inducing this mRNA were more strongly activated by HSA than by GP19S. Similarly, in the current study, the extensive increases in loTSLP mRNA in the absence of AD-related cytokines could be useful as a potential marker of protein allergenicity. Oddly, while HSA treatment could potentiate loTSLP mRNA levels in the presence of the AD-related cytokines, HSA exposure did not cause an increase in release of the TSLP protein even in the co-presence of the same cytokines. This indicated to us that TSLP protein expression might be affected by not only the levels of its own mRNA but also by other factors in a host. Clearly, further mechanistic investigations are needed to confirm these hypotheses.
Conclusions
These findings offer direct evidence that allergenic proteins have high loTSLP-inducing potential in keratinocytes. Moreover, this loTSLP-inducing potential correlates with in vivo allergenicity. Thus, loTSLP was found to be an effective indicator for in vitro screening of IHS.
Yasutaka_Kuroda_et_al_supplemental_content.zip
Download Zip (313.1 KB)Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
References
- Adachi R, Nakamura R, Sakai S, Fukutomi Y, Teshima R. 2012. Sensitization to acid-hydrolyzed wheat protein by transdermal administration to BALB/c mice, and comparison with gluten. Allergy. 67:1392–1399.
- Amaro C, Goossens A. 2008. Immunological occupational contact urticaria and contact dermatitis from proteins: A review. Contact Dermatitis. 58:67–75.
- Asokananthan N, Graham P, Stewart D, Bakker A, Eidne K, Thompson P, Stewart G. 2002. House dust mite allergens induce pro-inflammatory cytokines from respiratory epithelial cells: The cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 16:4572–4578.
- Bartnikas L, Gurish M, Burton O, Leisten S, Janssen E, Oettgen H, Beaupre J, Lewis C, Austen K, Schulte S, et al. 2013. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol. 131:451–460.
- Bjerkan L, Schreurs O, Engen S, Jahnsen F, Baekkevold E, Blix I, Schenck K. 2015. The short form of TSLP is constitutively translated in human keratinocytes and has characteristics of an antimicrobial peptide. Mucosal Immunol. 8:49–56.
- Bogiatzi S, Fernandez I, Bichet J, Marloie-Provost M, Volpe E, Sastre X, Soumelis V. 2007. Cutting edge: Pro-inflammatory and TH2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J. Immunol. 178:3373–3377.
- Dunkin D, Berin M, Mayer L. 2011. Allergic sensitization can be induced via multiple physiologic routes in an adjuvant-dependent manner. J. Allergy Clin. Immunol. 128:1251–1258.
- Emter R, Ellis G, Natsch A. 2010. Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers in vitro. Toxicol Appl Pharmacol. 245:281–290.
- Fornasa G, Tsilingiri K, Caprioli F, Botti F, Mapelli M, Meller S, Kislat A, Homey B, Di Sabatino A, Sonzogni A, et al. 2015. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. J Allergy Clin Immunol. 136:413–422.
- Fukutomi Y, Itagaki Y, Taniguchi M, Saito A, Yasueda H, Nakazawa T, Hasegawa M, Nakamura H, Akiyama K. 2011. Rhino-conjunctival sensitization to hydrolyzed wheat protein in facial soap can induce wheat-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 127:531–533.
- Fukutomi Y, Taniguchi M, Nakamura H, Akiyama K. 2014. Epidemiological link between wheat allergy and exposure to hydrolyzed wheat protein in facial soap. Allergy 69:1405–1411.
- Gerberick G, Vassallo J, Bailey R, Chaney J, Morrall S, Lepoittevin J. 2004. Development of a peptide reactivity assay for screening contact allergens. Toxicol Sci. 81:332–343.
- Gourbeyre P, Denery-Papini S, Larre C, Gaudin J, Brossard C, Bodinier M. 2012. Wheat gliadins modified by deamidation are more efficient than native gliadins in inducing a TH2 response in Balb/c mice experimentally sensitized to wheat allergens. Mol Nutr Food Res. 56:336–344.
- Harada M, Hirota T, Jodo A, Doi S, Kameda M, Fujita K, Miyatake A, Enomoto T, Noguchi E, Yoshihara S, et al. 2009. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 40:368–374.
- He R, Oyoshi M, Garibyan L, Kumar L, Ziegler S, Geha R. 2008. TSLP acts on infiltrating effector T-cells to drive allergic skin inflammation. Proc Natl Acad Sci USA. 105:11875–11880.
- Horimukai K, Morita K, Narita M, Kondo M, Kitazawa H, Nozaki M, Shigematsu Y, Yoshida K, Niizeki H, Motomura K, et al. 2014. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol. 134:824–830.
- Hung T, Liu S, Liu G, Hsieh P, Chuang L, Guh J, Hsieh C, Yu-Ju Hung Y, Shiue Y, Yang Y. 2013. shRNA for thymic stromal lymphopoietin: A novel therapeutic approach for pulmonary fibrosis. J Cell Sci Ther. 4:144.
- Inomata N, Nagashima M, Hakuta A, Aihara M. 2015. Food allergy preceded by contact urtica-ria due to the same food: Involvement of epicutaneous sensitization in food allergy. Allergol Int. 64:73–78.
- Ito T, Wang Y, Duramad O, Hori T, Delespesse G, Watanabe N, Qin F, Yao Z, Cao W, Liu Y. 2005. TSLP-activated dendritic cells induce an inflammatory T-helper Type 2 cell response through OX40 ligand . J Exp Med. 202:1213–1223.
- Kanda Y. 2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48:452–458.
- Katayama M, Inomata N, Inagawa N, Fukuro S, Aihara M. 2016. A case of contact urticaria syndrome Stage 3 after honey ingestion, induced by epicutaneous sensitization during skin care with honey. Contact Dermatitis. 74:189–191.
- Kato A, Favoreto S, Avila P, Schleimer R. 2007. TLR3- and TH2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 179:1080–1087.
- Kimber I, Griffiths C, Basketter D, McFadden J, Dearman R. 2014. Epicutaneous exposure to proteins and skin immune function. Eur J Dermatol. 24:1014.
- Kinoshita H, Takai T, Le T, Kamijo S, Wang X, Ushio H, Hara M, Kawasaki J, Vu A, Ogawa T, et al. 2009. Cytokine milieu modulates release of thymic stromal lymphopoietin from human keratinocytes stimulated with double-stranded RNA. J Allergy Clin Immunol. 123:179–186.
- Koller B, Muller-Wiefel A, Rupec R, Korting H, Ruzicka T. 2011. Chitin modulates innate immune responses of keratinocytes. PloS One. 6:e16594.
- Kouzaki H, O'Grady S, Lawrence C, Kita H. 2009. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 183:1427–1434.
- Lauriere M, Pecquet C, Bouchez-Mahiout I, Snegaroff J, Bayrou O, Raison-Peyron N, Vigan M. 2006. Hydrolyzed wheat proteins present in cosmetics can induce immediate hypersensitivities. Contact Dermatitis. 54:283–289.
- Le T, Takai T, Vu A, Kinoshita H, Chen X, Ikeda S, Ogawa H, Okumura K. 2011. Flagellin induces expression of thymic stromal lymphopoietin in human keratinocytes via toll-like receptor 5. Intl Arch Allergy Immunol. 155:31–37.
- Lee H, Ziegler S. 2007. Inducible expression of the pro-allergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NF-κB. Proc. Natl Acad Sci USA. 104:914–919.
- Leyva-Castillo J, Hener P, Jiang H, Li M. 2013. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol. 133:154–163.
- Livak K, Schmittgen T. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 25:402–408.
- Matsumoto K, Saito H. 2013. Epicutaneous immunity and onset of allergic diseases - per-”eczema”tous sensitization drives the allergy march. Allergol Intl. 62:291–296.
- Matsunaga K, Kuroda Y, Sakai S, Adachi R, Teshima R, Yagami A, Itagaki H. 2015. Anaphylactic augmentation by epicutaneous sensitization to acid-hydrolyzed wheat protein in a guinea pig model. J Toxicol Sci. 40:745–752.
- Mondoulet L, Dioszeghy V, Puteaux E, Ligouis M, Dhelft V, Letourneur F, Dupont C, Benhamou P. 2012. Intact skin and not stripped skin is crucial for the safety and efficacy of peanut epicutaneous immunotherapy (EPIT) in mice. Clin Transl Allergy. 2:22.
- Murakami-Satsutani N, Ito T, Nakanishi T, Inagaki N, Tanaka A, Vien P, Kibata K, Inaba M, Nomura S. 2014. IL-33 promotes the induction and maintenance of TH2 immune responses by enhancing the function of OX40 ligand. Allergol Intl. 63:443–455.
- Muto T, Fukuoka A, Kabashima K, Ziegler S, Nakanishi K, Matsushita K, Yoshimoto T. 2014. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int Immunol. 26:539–549.
- Nakajima S, Igyarto B, Honda T, Egawa G, Otsuka A, Hara-Chikuma M, Watanabe N, Ziegler S, Tomura M, Inaba K, et al. 2012. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signalling. J Allergy Clin Immunol. 129:1048–1055.
- Nakamura R, Nakamura R, Adachi R, Itagaki Y, Fukutomi Y, Teshima R. 2013a. Evaluation of allergenicity of acid-hydrolyzed wheat protein using an in vitro elicitation test. Int Arch Allergy Immunol. 160:259–264.
- Nakamura R, Nakamura R, Sakai S, Adachi R, Hachisuka A, Urisu A, Fukutomi Y, Teshima R. 2013b. Tissue transglutaminase generates de-amidated epitopes on gluten, increasing reactivity with hydrolyzed wheat protein-sensitized IgE. J Allergy Clin Immunol. 132:1436–1438.
- Nakamura R, Sakai S, Haishima Y, Fukui C, Suzuki T, Nakamura R, Hachisuka A, Adachi R, Teshima R. 2013c. Comprehensive analyses of hydrolyzed wheat protein using shotgun proteomics. Bull Natl Inst Health Sci. 131:50–57.
- Noti M, Kim B, Siracusa M, Rak G, Kubo M, Moghaddam A, Sattentau Q, Comeau M, Spergel J, Artis D. 2014. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immunol. 133:1390–1399.
- Oyoshi M, Larson R, Ziegler S, Geha R. 2010. Mechanical injury polarizes skin dendritic cells to elicit a TH2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 126:976–984.
- Sakaguchi H, Ashikaga T, Miyazawa M, Kosaka N, Ito Y, Yoneyama K, Sono S, Itagaki H, Toyoda H, Suzuki H. 2009. The relationship between CD86/CD54 expression and THP-1 cell viability in an in vitro skin sensitization test-human cell line activation test (h-CLAT)). Cell Biol Toxicol. 25:109–126.
- Sicherer SH, Sampson HA. 2014. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 133:291–307.
- Soumelis V, Reche P, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. 2002. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 3:673–680.
- Takai T, Kato T, Sakata Y, Yasueda H, Izuhara K, Okumura K, Ogawa H. 2005. Recombinant Der p 1 and Der f 1 exhibit cysteine protease activity but no serine protease activity. Biochem Biophys Res Commun. 328:944–952.
- Tordesillas L, Goswami R, Benede S, Grishina G, Dunkin D, Jarvinen K, Maleki S, Sampson H, Berin M. 2014. Skin exposure promotes a TH2-dependent sensitization to peanut allergens. J Clin Invest. 124:4965–4975.
- Tovey E, Chapman M, Platts-Mills T. 1981. Mite faeces are a major source of house dust allergens. Nature. 289:592–593.
- Vu A, Baba T, Chen X, Le T, Kinoshita H, Xie Y, Kamijo S, Hiramatsu K, Ikeda S, Ogawa H, et al. 2010. Staphylococcus aureus membrane and diacylated lipo-peptide induce thymic stromal lymphopoietin in keratinocytes through the TLR2-TLR2-6 pathway. J Allergy Clin Immunol. 126:985–993.
- Vu A, Chen X, Xie Y, Kamijo S, Ushio H, Kawasaki J, Hara M, Ikeda S, Okumura K, Ogawa H, et al. 2011. Extracellular double-stranded RNA induces TSLP via an endosomal acidification- and NF-κB-dependent pathway in human keratinocytes. J Invest Dermatol. 131:2205–2212.
- Wang Q, Du J, Zhu J, Yang X, Zhou B. 2015. Thymic stromal lymphopoietin signaling in CD4+ T-cells is required for TH2 memory. J Allergy Clin Immunol. 135:781–791.
- Xie Y, Takai T, Chen X, Okumura K, Ogawa H. 2012. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J Dermatol Sci. 66:233–237.
- Yokooji T, Kurihara S, Murakami T, Chinuki Y, Takahashi H, Morita E, Harada S, Ishii K, Hiragun M, Hide M, et al. 2013. Characterization of causative allergens for wheat-dependent exercise-induced anaphylaxis sensitized with hydrolyzed wheat proteins in facial soap. Allergol Intl. 62:435–445.
