Abstract
While monoclonal antibodies are efficient therapeutics for cancer treatment, nanobodies or variable heavy domain – due to their small size, high stability, and solubility – have many advantages in comparison. Oligoclonal nanobodies are a mixture of nanobodies against different epitopes of an antigen. Specific nanobodies against vascular endothelial growth factor (VEGF, which has an important role in cancer angiogenesis) were selected from an immune camel library using biopanning. Specific binding of the nanobodies to VEGF antigen was assessed by periplasmic extract enzyme-linked immunosorbent assay (ELISA). Bioinformatics analysis and molecular docking were performed on selected nanobodies against VEGF. The in vitro inhibitory effects of each single nanobody, as well as a pool of selected nanobodies (oligoclonal nanobodies), on proliferation and tube formation by/in human umbilical vein endothelial cells (HUVEC) cells was evaluated using MTT and Tube formation assays, respectively. Four nanobodies showed the highest signal intensity in the periplasmic extract ELISA. Sequencing revealed that four unique nanobodies with different CDR3 rejoin were selected. Oligoclonal nanobodies inhibited proliferation and tube formation of the HUVEC cells more potently than did each individual nanobody. Taken together, this data from this study suggests that in vitro use of nanobodies (in an oligoclonal mode) that target distinct epitopes on VEGF could be promising as a novel therapy to treat VEGF-dependent pathologies. However, this needs to be further tested in in vivo studies.
Keywords:
Introduction
There are six important biological mechanisms in the development of cancer. These include proliferation signaling, disabling of growth suppressors, blockage of cell death, immortal replication, angiogenesis, and metastasis (Hanahan and Weinberg Citation2011). Angiogenesis is an important factor in the cancer process, with many proteins playing key roles in the angiogenic process (Otrock et al. Citation2007). Some proteins act as regulators, such as fibroblast growth factor (FGF), angiogenin, transforming growth factor (TGF), angiopoietin, interleukin (IL)-8, and tumor necrosis factor (TNF)-α. Other proteins have functional roles, like platelet-derived growth factor (PDGF), IL-1β, stromal cell-derived factor one (SDF-1/CXCL12), cyclooxygenase (COX)-2, vascular endothelial growth factor (VEGF), and VEGF receptor (VEGF-R) (Ceradini et al. Citation2004).
The VEGF family consists of five members, VEGF-A, -B, -C, and -D, and placental growth factor (PlGF). Of these, VEGF-A seems to have a more critical role than others in angiogenesis (Ferrara et al. Citation2003). VEGF-A has two receptors, including VEGF-R1 (Flt-1) and VEGF-R2 (Flk-1/KDR), to which all its activity is dependent upon. VEGF-A is a secretion glycoprotein belonging to the platelet-derived growth factor (PDGF) family (Shibuya Citation2001). Through 2015, more than 350 intact monoclonal antibodies (mAb) or fragments were evaluated in a clinical trial against a variety of pathologies; of these, more than 70 were approved for use by the United States Food and Drug Administration (FDA) (Ecker and Jones Citation2015). Among these mAb drugs, cetuximab, panitumumab, necitumumab, and nimotuzumab are used as anti-epidermal growth factor receptor (EGFR) agents; trastuzumab, pertuzumab, and ado-trastuzumab emtansine are employed against human epidermal growth factor receptor-2 (HER-2); bevacizumab and ranibizumab are used against VEGF-A, and ramucirumab is useful against VEGF-R2. These antibodies have several mechanisms of actions (MoA) for affecting tumor cells. The most common MoA is to block the antigens/receptors directly or indirectly and in turn suppress or induce cell signaling (Carter and Lazar Citation2018). One VEGF inhibitor recombinant antibody, i.e. bevacizumab, is able to deactivate all human VEGF isoforms (Hurwitz et al. Citation2004; Ferrara et al. Citation2005).
Monoclonal antibodies (mAb) are commonly using extensively; however, considerations about production costs/expensive processes have diminished the willingness of manufacturers to produce them. Further, the routinely large sizes of mAb impact on/restrict abilities to penetrate deeply into tumors/tumor cells (Kolkman and Law Citation2010). Thus, efforts to find new antibodies with both a smaller size and the effectiveness of mAb have led to increases in the production of single domain antibody fragments (sdAb; 12–14 kDa), single chain variable Fragment (scFv; 26–30 kDa), and nano-antibodies (nanobodies or Nb; 11–15 kDa) (Kolkman and Law Citation2010; Scott et al. Citation2012; Spadiut et al. Citation2014).
As a result of discovering the structures of immunoglobulins (Ig) from Camelidae host like camels and llamas, a promising novel tool was provided for the development of antibodies with a wide range of applications. Camel Ig has two separate heavy chain antibodies (HCAb) that each consist of a variable heavy domain (VHH) and two constant domain (CH2 and CH3). However, unlike human Ig, camel Ig has neither a light (L) chain nor a CH1 domain (Hamers-Casterman et al. Citation1993; Ebrahimizadeh et al. Citation2015). A similar type of antibody lacking an L chain (V-NAR) was found in sharks and ratfishes, but these had structural differences from camel HCAb (Flajnik et al. Citation2011). Though VHH and various V-NAR have similar molecular dimensions, only VHH is termed as nanobody due to its size (2.5–4.0 nm)
Nanobodies have ideal biophysical and pharmacological properties due to both their small size and single domain entity. Furthermore, their high stability, as well as high specificity and affinity (in nM range) for cognate targets make them good candidates in “drug” development (Cortez-Retamozo et al. Citation2004; Hassanzadeh-Ghassabeh et al. Citation2013). In this vein, nanobodies can potentially be important diagnostic tools. Because a rapid diagnosis of early stages of cancer and an ability to readily evaluate efficacy of other cancer therapeutics is important, nanobodies would fit this role in that they have high penetrating potentials into tumors/cancer cells, are able to detect very small targets, and they are cleared quickly from the body (Oliveira et al. Citation2010).
As “anti-tumor” drugs, nanobodies can be divided into three classes. The first is “naked nanobody”, i.e. one without an Fc domain. The second is a recombinant fusion form attached to an activator domain. The third is a nanobody used to direct a drug to a target tissue (Bannas et al. Citation2017). Recently, a nanobody-based product was developed to inhibit VEGF and angiopoietin-2 (Könning et al. Citation2017). Another anti-VEGF nanobody is VA12, a product that showed stability at high temperatures and low pHs (Ebrahimizadeh et al. Citation2015). At this point, a few nanobody-based therapeutics have entered into clinical trials. For example, ALX-0761, ALX-0061, and ozoralizumab are anti-inflammatory nanobodies that have been tested on >700 patients. In no case were there any reported side-effects (Rissiek et al. Citation2014). ALX-0171 nanobody was tested in clinical trials against respiratory syncytial virus (RSV) infections (Detalle et al. Citation2017); its affinity was in the sub-nanomolar range and it significantly inhibited viral replication in the early phase.
Some studies have argued that some antigens bind to receptors on cells through at least two binding sites; this hypothesis highlights the importance of using antibodies as poly- or oligoclonal forms (Nowakowski et al. Citation2002). Oligoclonal nanobodies are mixtures of nanobodies against different epitopes of a given antigen (Carter and Lazar Citation2018). Until 2016, only ≈12 oligoclonal nanobodies had been medically tested (Corti and Kearns Citation2016). In the primary studies, oligoclonal nanobodies were used to inhibit many targets, including HER-2 or tumor-associated glycoprotein (TAG)-72 (Jamnani et al. Citation2012, Citation2014; Sharifzadeh et al. Citation2013). The results of those studies showed there was more potency of the antibodies against their own targets when applied in an oligoclonal rather than a monoclonal mode. Recent studies used two antibodies as an oligoclonal drug against breast cancer and melanoma (Larkin et al. Citation2015; Swain et al. Citation2015). In the first, a combination of trastuzumab and pertuzumab (anti-HER2 antibodies) was employed; in the second study, a mixture of ipilimumab (anti CTLA4) and nivolumab (anti PD1) was used.
Considering the major role of VEGF in angiogenesis and the potent effect of nanobodies, the present study sought to identify a set of oligoclonal nanobodies targeting various epitopes on VEGF (selected from immune camel VHH library) and examine these agents for potential effects on in vitro human endothelial cell proliferation and tube formation (to reflect impact on VEGF availability/functional use to the cells).
Material and methods
Reagents
Ethylene diaminetetraacetic acid, isopropyl β-d-1-thiogalactopyranoside (IPTG), Luria–Bertani (LB) agar, and 4-choloro-1-naftol (4-CN) were each purchased from Sigma (St. Louis, MO). PstI and BstEII restriction enzymes were obtained from Fermentase (Bloomberg, Germany). Escherichia coli TG1 and WK6 cells were obtained from the Pasteur Institute of Iran (Tehran, Iran) and VCSM13 helper phage from Amersham-Pharmacia (Munich, Germany). Ni-NTA resin was obtained from Qiagen (Hilden, Germany). Triethylamine (TEA, pH 10.0), tryptone yeast extract, 3,3′,5,5′-tetramethylbenzidine (TMB), polyethylene glycol (PEG), and powdered skim milk were all purchased from Merck (Darmstadt, Germany). 3–(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), as well as 10–180 KDa protein markers were all obtained from Sinaclon Bioscience (Tehran, Iran). Horseradish peroxidase (HRP)-conjugated mouse anti-HA, -M13, and -His antibodies were each purchased from Roche (Basel, Switzerland). Fetal Bovine Serum (FBS), penicillin–streptomycin, endothelial basal growth medium (EBM-2), and Geltrex™ LDEV-Free Reduced Growth Factor Basement Membrane Matrix were all bought from Gibco (Gaithersburg, MD). Kits were also purchased for use here in DNA extraction (Vivantis DNA Extraction Kit, Cinnagen, Tehran), measures of protein content (BCA Protein Assay Kit, SMART™; Seongnam-si, Gyeonggi-do, Korea), and assaying cell viability (calcein AM Cell Viability Kit; Trevigen; Gaithersburg). PVDF membrane was bought from Thermo Fisher (Bremen, Germany).
Selection of specific nanobodies
A nanobody library was constructed in a previous study (Kazemi-Lomedasht et al. 2015). Biopanning (four rounds) was performed to enrich a nanobody library against VEGF based on methods used by Kazemi-Lomedasht et al. (2015). In brief, 10 µg VEGF/ml in NaHCO3 buffer was coated onto wells of a 96-well plate overnight at 4 °C. Control wells received only buffer. About 1012 CFU (colony-forming unit) of phage library was added to each well and the plates were incubated for 1 hr at 37 °C. Unbonded phages were removed by washing (10 times using PBS-T (phosphate-buffered saline [PBS, pH 7.4] containing 0.05% [v/v] Tween 20). Bonded phages were then eluted using 10 mM TEA and neutralized with 1 M Tris-HCl (pH 8.0). Eluted phages (output) were then amplified in logarithmic phase (OD600nm = 0.5–0.7) Escherichia coli TG1 cells. Output phages were rescued using VCSM13 helper phage. To purify released phage particles, the bacterial pellet was collected (5000 × g, 15 min) and recombinant phage particles in the pellet purified by incubation in PEG/NaCl solution (20% PEG- 2.5 M NaCl) for 1 hr at 4 °C (Lee et al. Citation2007; Kazemi-Lomedasht et al. 2015). The phage particles were then isolated by centrifugation of the PEG/NaCl solution (10000 × g, 30 min), re-suspended in PBS, and stored (as input) at −20 °C. The input phages were in turn used for the next rounds of biopanning.
Polyclonal phage enzyme-linked immunosorbent assay (ELISA) was then performed to evaluate the success of the biopanning. In brief, eluted phages from each round were added to wells coated with VEGF and detection was monitored using an anti-M13-HRP antibody (1:7000 dilution in PBS). Single colonies from the fourth round of biopanning were screened using a Periplasmic Extract ELISA (PE-ELISA). For this, ≈47 colonies were chosen and cultured at 37 °C in LB medium until they attained log phase. Expression of the nanobodies was induced by adding 1 mM IPTG and incubating the plate at 37 °C for a further 16 hr. Periplasmic-expressed nanobodies were then extracted by osmotic shock; detection of any nanobodies present was performed using an anti-HA antibody (1:2000 in PBS) (Kazemi-Lomedasht et al. 2015). After washing the wells with PBST (5 times), HRP-anti-mouse IgG (1:5000 in PBS; secondary antibody) was added. Positive wells (OD 3X control well OD) indicated which colonies’ associated nanobodies were to be sequenced.
Bioinformathics analysis
Sequencing results were analyzed using a FinchTV Chromatogram Viewer v.1.4 (Geospiza, Inc, Bloomberg, Germany), aligned using MEGA software V(0).7 (PSU, University Park, PA), and validated through an IMGT database (http://www.imgt.org/3Dstructure-DB). Nucleotide sequences were translated to amino acids using ExPASy tools (https://web.expasy. org/translate). Computation of physico-chemical parameters of four nanobodies was performed using Prot-Param (https://web.expasy.org/protparam). The 3D structures of the four nanobodies and human VEGF121 (Uniprot ID #P15692-9) were predicted using a SWISS-MODEL server (https://swiss-model.expasy.org/interactive) (Biasini et al. Citation2014). A PDB format of the 3D structure of bevacizumab (1BJ1) was retrieved from the RSCB database (http://www.rcsb.org). All results (saved as PDB files) were energy-minimized using a SWISS-PDB viewer V4.1 (Swiss Institute of Bioinformatics, Basel, Switzerland), and their 3D structures discerned using Discovery Studio software 2016 (BIOVIA Citation2017). Percent favored/outlier residues was estimated using a RAMPAGE2 online Ramachandran Plot Analysis server (http://mordred.bioc.cam.ac.uk/∼rapper/rampage.php) (Lovell et al. Citation2003). Possible binding affinity between nanobodies (as ligand) and human VEGF121 (as receptor) were estimated using HEX docking tool V 8.0 (Loria, Nancy, France) by assuming the total free energy as the template for comparison of nanobody binding affinity values (note: all parameters set in default). After each docking process, the receptor/ligand complex structure was saved, and amino acids involved in interactions identified using LIGPLOT Plus v.1.4.5 (EBI, Cambridge, UK) (Laskowski and Swindells Citation2011).
Sub-cloning of nanobodies into expression vectors
Plasmids were extracted from positive colonies and used as templates for amplification of nanobody genes. PCR was performed using A6E (5′-GATGTGCAGCTGCAGGAGTCTGG-RGGAGG-3′) and P38 (5′-GGACTAGTGCGGCCGCTGGAGACGGTGACCTGGGT-3′) primers (annealing: 55 °C for 40 sec). PCR products and pHEN-6C (bacterial expression vector) were then digested with BstEII and PstI restriction enzymes (using manufacturer protocols) and ligated together using T4 DNA ligase at 16 °C for 5 hr. The recombinant construct was then transferred into E. coli WK6 cells via heat-shock.
Expression and purification of nanobodies
Expression of nanobodies into the periplasmic space (due to the existence of PelB signal sequence at N-terminal of pHEN-6C plasmid) of the bacteria was induced by treatment with 1 mM IPTG for 16 hr at 28 °C. Following osmotic shock, recombinant nanobodies were purified using nickel affinity chromatography, and the nanobody concentrations determined using a BCA Protein Kit according to manufacturer instructions. The purity of the isolated nanobodies was then evaluated by Western blot analysis. In brief, after resolution over a 15% SDA-PAGE gel, protein bands were transferred to a PVDF membrane that, in turn, was blocked with 4% skim milk (in PBS) solution for 2 hr at room temperature (RT). After washing, anti-His antibody (1:500 in PBS) solution was placed on the membrane and the sample incubated for 2 hr at RT. After additional washes with PBST (3 times), signals on the blot were developed by additional of 4-CN substrate (10 mg/ml methanol) for 20 min at RT. The blots were then photographed and band intensities were quantified using Image J (https://imagej.nih.gov).
Affinity calculation
The affinity of the isolated nanobodies for VEGF was calculated using the method of Beatty et al. (Citation1987). Two concentrations of VEGF (10 and 1 µg/ml) were selected using a checkerboard assay; the VEGF solutions were coated onto dedicated wells of 96-well plates by overnight incubation at 4 °C. After washing the wells with PBST, test nanobodies (0–10 nM) were then added to dedicated wells. After a 1-hr incubation at RT, the wells received HRP-anti-His antibody (1:500 dilution in PBS). The plates were then incubated for 1 hr at RT before unbound primary antibody was removed by gentle rinsing of the wells with PBST. The wells then received 0.6 mg TMB/ml substrate and color formation was allowed to proceed for 15 min. Wells were then assessed for absorbance at 450 nm in a Epoch plate reader (BioTek, Winooski, VY). The affinity (kaff) of the ligand to its target was then calculated using a Beatty equation.
MTT assay
A HUVEC (human umbilical vein endothelial cell) primary line generated in a previous study (Kazemi-Lomedasht et al. Citation2014) was employed to evaluate inhibitory effects of the test nanobodies (in mono- and oligoclonal modes) on cell proliferation. In brief, in each well of a 96-well plate, 104 HUVEC cells were cultured in EBM-2 medium containing 2% FBS for 12 hr at 37 °C. Each of the following: PBS (negative control), H39Nb (nanobody negative control), bevacizumab (positive control), each nanobody alone, or a mixture of all nanobodies (oligoclonal mode) – all at 10 µg/ml, along with 50 ng VEGF/ml were added to dedicated wells. All materials were evaluated in triplicate wells. The plate was then incubated 72 hr at 37 °C before 50 µl of 5 mg MTT/ml was added to each wells and the plate incubated a further 4 hr. The medium in all wells were then replaced with 100 µl DMSO and the plate was incubated for 30 min. Absorbance in each well was then measured at 570 nm in the Epoch plate reader. Inhibition of proliferation was evaluated as 1 − (100 × [proliferation of treated cells/proliferation of control cells]). Data [OD570 values] for treated cells were normalized against the highest OD570 seen with the control cells, i.e., the latter taken as 100% proliferation.
Tube formation assay
The inhibitory effect of the test nanobodies on formation of the tubular structures was evaluated using a tube formation assay on Matrigel. In brief, GeltrexTM LDEV was thawed on ice and 50 µl of the material then transferred into wells of 96-well plates. A total of ≈3 × 104 HUVEC cells in 100 µl EBM-2 medium was then added to each well. Each of the following: PBS (negative control), H39Nb (nanobody negative control), bevacizumab (positive control), each nanobody alone, or a mixture of all nanobodies (oligoclonal mode) – all at 10 µg/ml, along with 50 ng VEGF/ml were added to dedicated wells. All materials were evaluated for effects in triplicate wells. The plates were then incubated for 5 hr at 37 °C. After removing the medium, cells were stained using a Calcein AM Cell Viability Kit, according to manufacturer instructions. The presence of tubular structures was then detected using an INV100-FL fluorescence inverted biological microscope (BEL, Monza, Italy). The numbers of branched and tubular structures in all cells were evaluated/counted in three different fields/slide. A total of 1000 cells/slide were counted.
Statistical analysis
All data are reported as means ± SD when proper. A Students t-test was used to assess the significance of any differences between any two given test groups. All analyses were performed using Prism Software v(0).5 (GraphPad, San Diego, CA). A p-value <0.05 was accepted as statistically significant.
Results
Enrichment and screening
To qualitatively evaluate the biopanning here, titers of eluted phages from each round of biopanning were determined (). The highest enrichment was observed in the fourth round of biopanning. Polyclonal phage ELISA was done on eluted phages in each round to quantitatively evaluate the biopanning process (). Observed signal intensity was increased after each biopanning round. Forty-seven colonies (randomly selected from fourth round of biopanning) were screened for binding to VEGF in a PE-ELISA experiment. Four nanobodies, i.e. Nb25, Nb30, Nb31, and Nb34, showed the greatest binding. Sequencing showed that the four nanobodies with unique CDR3 sequence had been selected. Framework and CDR regions of the four nanobodies were identified (). The affinity of each nanobody for VEGF was calculated and found to be 1–6 nM using a Beatty analysis/ELISA-based method.
Table 1. Phage titration.
Table 2. Polyclonal phage ELISA.
Table 3. Translated sequences of four selected Nanobodies by ExPASy translate tool.
Primary analysis
Physico-chemical parameters of the four nanobodies were calculated. Each had a molecular length of ≈130 amino acids and molecular weights of ≈14 kDa (). The 3D structures of the nanobodies and the sequence of VEGF (UniProt #P15692) were predicted (), and a QMEAN score estimated for each predicted structure. The scores of all predicted structures were between −0.38 to −1.02, confirming the reliability of each prediction (, Row 5).
Figure 1. Nanobody 3-D structures predicted by Swiss Modeler. (A) Human VEGF 121. (B) Nb 25. (C) Nb 30. (D) Nb 31. (E) Nb 34.
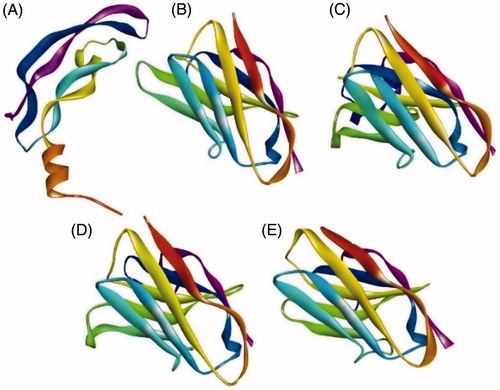
Table 4. Physico-chemical parameters predicted by ExPASy ProtParam database.
Docking results
The predicted 3D structures of Nb25, Nb30, Nb31, and Nb34 were prepared for docking analysis, i.e. water molecules were removed, force-field energy was minimized, Hydrogens were add and final structures saved in PDB format. The percentage of residues in favored/outlier regions was estimated using a RAMPAGE web server (). Total free energy from the interaction between nanobodies (or bevacizumab) as ligand and human VEGF (as receptor) was calculated. Correlation parameters were set on shape only and the final search number was 25. Lower calculated energy (more negative) meant a stronger affinity. The results showed of the tested materials, Nb34 and bevacizumab had the highest binding affinity for VEGF (). Bevacizumab, in compare with Nb34, showed a weaker binding affinity for VEGF. The amino acids involved in the interaction between VEGF and the nanobodies/bevacizumab, and the number of constructed hydrogen bonds was identified as well. The data indicated that Nb34 and Nb30 both generated seven H-bonds ().
Table 5. Total free energy of interaction between nanobodies (ligand) and human VEGF (receptor).
Table 6. Amino acids involved in interaction, location, and number of hydrophobic bonds [detected by LIGPLOT Plus].
Purification results
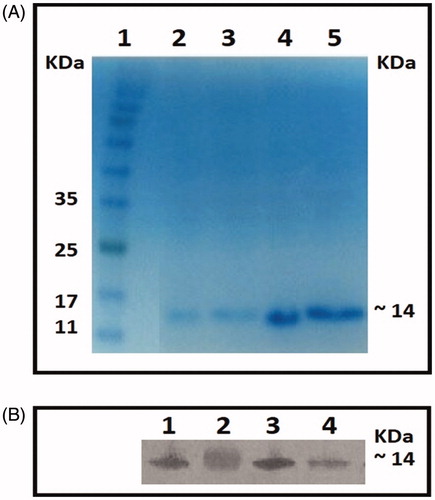
The test nanobodies were purified via nickel affinity chromatography and purification was confirmed by Western blot analyses. Each blot included molecular weight markers. The results indicated that the weight of the purified nanobodies was ≈14 kDa each ().
Figure 2. Purification. (A) 15% polyacrylamide gel stained with Coomassie Blue. Column: 1 = Protein marker, 2 = Nb25, 3 = Nb30, 4 = Nb31, and 5 = Nb34. Molecular weight of four nanobodies was estimated ≈ 14 kDa. (B) Western blots performed using anti-His antibody and developed using 4-CN substrate. Column: 1 = Nb25, 2 = Nb30, 3 = Nb31, and 4 = Nb34.
MTT assay
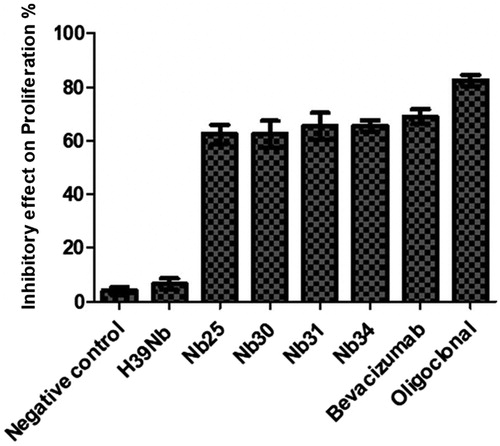
MTT assays were performed to evaluate inhibitory effects of each nanobody, alone and when pooled (oligoclonal). The data indicated that all tested individual nanobodies (Nb25, Nb30, Nb31, and Nb34) were able to inhibit proliferation of HUVEC cells stimulated by VEGF (). The results showed that inhibition (as % vs. control HUVEC cell proliferation) was 69% for bevacizumab, 62.5% for both Nb25 and Nb30, 65.5% for both Nb31, and Nb34, and 82% the oligoclonal mode nanobodies. As individual substances, bevacizumab (positive control) and H39Nb (negative control nanobody) led to the greatest and least inbitory effects, respectively.
Figure 3. Effect of nanobodies (mono- and oligoclonal mode) on HUVEC cell proliferation. Ten µg/ml of all effective factors including, PBS (negative control), H39Nb (negative nanobody control), Bevacizumab (positive control), individual nanobody, and a mixture of all Nanobodies (oligoclonal mode) were combined with a fixed constant concentration (50 ng/ml) of VEGF in wells containing HUVEC cells. Results (% inhibition): Bevacizumab (69%), Nb25 and Nb30 (62.5%), Nb31 and Nb34 (65.5%), oligoclonal nanobodies (82%). Values shown are means ± SD of triplicate evaluations.
Tube formation assay
Tube formation was assessed to examine other forms of inhibitory effects of the test nanobodies (individual and oligoclonal mode) in cells. As the results show, each nanobody significantly inhibited tube formation of HUVEC cells in comparison to what was noted in positive control wells (with only VEGF and no nanobody) (). Results showed that inhibition (vs. control HUVEC cell levels) was evident in treated cells, with only 19% formed branched structures with bevacizumab, 31% with Nb25, 28% with Nb30, 21% with Nb31, 22% with Nb34; and 9% with the oligoclonal mode nanobodies. H39Nb (negative control nanobody) did not cause significant effects on tube formation. No tube-like structures were seen in cells that only received PBS (in place of VEGF).
Figure 4. Effect of nanobodies (mono- and oligoclonal mode) on HUVEC cell tube formation. (A) Nb25. (B) Nb30. (C) Nb31. (D) Nb34. (E) Oligoclonal nanobodies. (F) PBS [negative control]. (G) Bevacizumab [positive control antibody]. (H) H39Nb [negative nanobody control]. (I) VEGF [as stimulator of tube formation]. Cells were observed using a fluorescence inverted microscope. Magnification =20×.
![Figure 4. Effect of nanobodies (mono- and oligoclonal mode) on HUVEC cell tube formation. (A) Nb25. (B) Nb30. (C) Nb31. (D) Nb34. (E) Oligoclonal nanobodies. (F) PBS [negative control]. (G) Bevacizumab [positive control antibody]. (H) H39Nb [negative nanobody control]. (I) VEGF [as stimulator of tube formation]. Cells were observed using a fluorescence inverted microscope. Magnification =20×.](/cms/asset/661bb2aa-4ab0-44b6-9504-a44ac4f83563/iimt_a_1526234_f0004_c.jpg)
Discussion
Treatment of cancer is a major focus of many studies and there is intense competition to achieve this goal (Chabner and Roberts Citation2005). As angiogenesis is a critical step in cancer progression, much research has focused on ways to inhibit this process. Among all molecules involved in angiogenesis, VEGF and its receptors seem to have important roles in the progression of the process (Ferrara et al. Citation2003). This is reflected in the fact that increased levels of serum VEGF have been noted in breast, prostate, and colorectal cancer patients (Duque et al. Citation1999; George et al. Citation2000; Ali et al. Citation2011).
For many years, there have been three primary methods to treat cancer, i.e., surgery followed by radiation therapy and/or chemotherapy. In recent years, immune-based treatments using specific antibody against tumor-based targets have been developed (Trapani and Darcy Citation2017). Bevacizumab is a recombinant mAb developed for treating colorectal cancer. However, there were important limitations (i.e. size resulted in decreased tumor penetrance) that prohibited extensive mAb use in treating this/other cancers. Nanobodies could fill this gap in that they have a small size that makes them capable of penetrating internal layers of solid tumors. Further, their production in bacterial hosts has facilitated their application potential by expediting the formation/testing phase in product development. Lastly, nanobodies have unique characteristics, including high affinity and solubility (Muyldermans et al. Citation2009; Arezumand et al. Citation2017). Accordingly, researchers have used nanobodies for various applications (Kazemi-Lomedasht et al. Citation2015a, Citation2015b, Citation2016, Citation2017). Specifically, Kazemi-Lomedasht et al. developed nanobodies targeting VEGF that potentially inhibited human endothelial cell proliferation and tube formation. The polyclonal antibodies that were generated here recognized several epitopes on the target antigen and elicited stronger responses.
Monoclonal antibodies compete for the same epitopes on the surface of an antigen. Importantly, mAb binding affinity can be affected by various parameters including interactions with other proteins, post-translational modification (PTM), local pH, temperature, and ion concentrations, etc. Thus, the amount of epitopes is a limiting factor for efficacy. Accordingly, higher doses of monoclonal antibodies would have to be administered to improve efficacy which, in turn, often leads to unwanted side effects (Haurum Citation2006). In the case of bevacizumab, side effects such as bleeding, phlebitis, and emboli formation resulted in a stoppage of patient treatment (Ranpura et al. Citation2011). In comparison, the combined use of antibodies in poly or oligoclonal forms that recognize different epitopes on a target antigen could act more efficiently than a single mAb (Haurum Citation2006) and so enhance efficacy. Several studies have demonstrated that the combined use of noncompetitive antibodies enhanced their function (Logtenberg Citation2007). In one study, it was seen that combining two epitope-distinct mAb (i.e. here, against a receptor) enhanced the overall therapeutic activity (Ben-Kasus et al. Citation2009). Spangler et al. (Citation2010) noted that combinations of anti-EGFR mAb led to inhibition of receptor recycling and decreased levels of EGFR. These findings, and the fact that single domain entity nanobodies make them good options for use in antibody mixtures (Harmsen and De Haard Citation2007), have led to the performance of several studies using mixtures of nanobodies (oligoclonal mode) to target distinct epitopes on tumor cells. In those studies, improved anti-tumor activity with an oligoclonal mixture of nanobodies was achieved compared to that from each nanobody alone (Jamnani et al. Citation2012; Sharifzadeh et al. 2012). Along these lines of reasoning, our group has developed nanobodies targeting various epitopes on VEGF in order to inhibit angiogenesis in tumors. This would be in line with the studies by Nikkhoi et al. (Citation2017, Citation2018) who used oligoclonal nanobodies to inhibit HER2 receptors on MC4L2 HER2+ cells in ovarian and breast cancers. The current studies showed that indeed, use of oligoclonal modes of nanobodies generated against VEGF (or receptor) imparted the greatest effects on normal dividing epithelial cells (i.e. HUVEC) as compared to effects from any of the individual nanobodies.
The present study also reflected the many techniques for the production and amplification of nanobodies from extended libraries, i.e. phage display, ribosome display, mRNA display, and yeast display. Overall, phage display was our first choice because of a possibility of immunizing the animal host (Camelidae) by multiple antigens at the same time (Qi et al. Citation2012; De Meyer et al. Citation2014; Hammers and Stanley Citation2014). By comparing the results of molecular docking and findings of experimental assays with some of the isolated nanobodies, it was found that docking predictions were well matched, and that results could be useful in subsequent studies on different proteins. Still, it was interesting to note that compared to the various nanobodies, Bevacizumab had the higher inhibitory effect on cell proliferation however, the molecular docking predicted a small amount of higher binding affinity for Nb34. This difference might result from using the Fab fragments (1BJ1) instead of complete Bevacizumab molecule in docking analysis. Bevacizumab has two binding sites that could potentially impart more inhibitory effects on the VEGF molecule (Fuh et al. Citation2006; Walker et al. Citation2016).
Conclusions
This study using nanobodies targeting distinct epitopes on VEGF, found for the first time that there was a greater efficacy when using an oligoclonal mode rather than a single nanobody. These outcomes were borne out from analyses of the inhibition of proliferation and ability to undergo tube formation by human endothelial cells in vitro. These results are helpful in that they point toward a potential for using oligoclonal nanobodies as novel agents to target distinct epitopes on VEGF in order to mitigate VEGF-dependent pathologies. However, this needs to be further tested in in vivo model studies.
Disclosure statement
The authors declare no conflicts of interested. The authors alone are responsible for the content of this manuscript.
Additional information
Funding
References
- Ali E, Sheta M, Mohsen M. 2011. Elevated serum and tissue VEGF associated with poor outcome in breast cancer patients. Alexandria J Med. 47:217–224.
- Arezumand R, Alibakhshi A, Ranjbari J, Ramazani A, Muyldermans S. 2017. Nanobodies as novel agents for targeting angiogenesis in solid cancers. Front Immunol. 8:1746.
- Bannas P, Hambach J, Koch-Nolte F. 2017. Nanobodies and nanobody-based human heavy chain antibodies as anti-tumor therapeutics. Front Immunol. 8:1603.
- Beatty J, Beatty B, Vlahos W. 1987. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Meth. 100:173–179.
- Ben-Kasus T, Schechter B, Lavi S, Yarden Y, Sela M. 2009. Persistent elimination of ErbB-2/HER2-over-expressing tumors using combinations of monoclonal antibodies: Relevance of receptor endocytosis. Proc Natl Acad Sci USA. 106:3294–3299.
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Cassarino T, Bertoni M, Bordoli L, et al. 2014. SWISS-MODEL: Modelling protein tertiary and quarter-nary structure using evolutionary information. Nucleic Acids Res. 42:W252–W258.
- BIOVIA. 2017. BIOVIA Discovery Studio 2017 R2: A comprehensive predictive science application for Life Sciences. San Diego (CA). Dassault SystÉmes, 2017. http://accelrys.com/products/collaborative-science/biovia-discovery-studio.
- Carter P, Lazar G. 2018. Next generation antibody drugs: Pursuit of the ‘high-hanging fruit’. Nat Rev Drug Discov. 17:197.
- Ceradini D, Kulkarni A, Callaghan M, Tepper O, Bastidas N, Kleinman M, Capla J, Galiano R, Levine J, Gurtner G. 2004. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 10:858.
- Chabner B, Roberts T. 2005. Chemotherapy and the war on cancer. Nat Rev Cancer. 5:65.
- Cortez-Retamozo V, Backmann N, Senter P, Wernery U, De Baetselier P, Muyldermans S, Revets H. 2004. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res. 64:2853–2857.
- Corti D, Kearns J. 2016. Promises and pitfalls for recombinant oligoclonal antibodies-based therapeutics in cancer and infectious disease. Curr Opin Immunol. 40:51–61.
- de Meyer T, Muyldermans S, Depicker A. 2014. Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 32:263–270.
- Detalle L, van Heeke G, Allosery K, de Brabandere V, de Smedt T, de Fougerolles A. 2017. Nanobodies as inhaled biotherapeutics for lung diseases. Pharmacol Ther. 169:47–56.
- Duque J, Loughlin K, Adam R, Kantoff P, Zurakowski D, Freeman M. 1999. Plasma levels of vascular endothelial growth factor are increased in patients with metastatic prostate cancer. Urology. 54:523–527.
- Ebrahimizadeh W, Gargari S, Javidan Z, Rajabibazl M. 2015. Production of novel VHH nanobody inhibiting angiogenesis by targeting binding site of VEGF. Appl Biochem Biotechnol. 176:1985–1995.
- Ecker D, and Jones S. (Eds.) 2015. The therapeutic monoclonal antibody market. New York: Taylor & Francis.
- Ferrara N, Gerber H, LeCouter J. 2003. The biology of VEGF and its receptors. Nat Med. 9:669.
- Ferrara N, Hillan K, Novotny W. 2005. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 333:328–335.
- Flajnik M, Deschacht N, Muyldermans S. 2011. A case of convergence: Why did a simple alternative to canonical antibodies arise in sharks and camels?. PLoS Biol. 9:e1001120.
- Fuh G, Wu P, Liang W, Ultsch M, Lee C, Moffat B, Wiesmann C. 2006. Structure-function studies of two synthetic anti-vascular endothelial growth factor Fab and comparison with the Avastin™ Fab. J Biol Chem. 281:6625–6631.
- George M, Eccles S, Tutton M, Abulafi A, Swift R. 2000. Correlation of plasma and serum VEGF levels with platelet count in colorectal cancer: Clinical evidence of platelet scavenging? Clin Cancer Res. 6:3147–3152.
- Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hammers C, Songa E, Bendahman N, Hammers R. 1993. Naturally-occurring antibodies devoid of light chains. Nature. 363:446.
- Hammers C, Stanley J. 2014. Antibody phage display: Technique and applications. J Invest Dermatol. 134:1.
- Hanahan D, Weinberg R. 2011. Hallmarks of cancer: The next generation. Cell. 144:646–674.
- Harmsen M, De Haard H. 2007. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol. 77:13–22.
- Hassanzadeh-Ghassabeh G, Devoogdt N, De Pauw P, Vincke C, Muyldermans S. 2013. Nanobodies and their potential applications. Nanomedicine. 8:1013–1026.
- Haurum J. 2006. Recombinant polyclonal antibodies: Next generation of antibody therapeutics? Drug Discov Today. 11:655–660.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. 2004. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl J Med. 350:2335–2342.
- Jamnani F, Rahbarizadeh F, Shokrgozar M, Ahmadvand D, Mahboudi F, Sharifzadeh Z. 2012. Targeting high affinity and epitope-distinct oligoclonal nanobodies to HER2 over-expressing tumor cells. Exp Cell Res. 318:1112–1124.
- Jamnani F, Rahbarizadeh F, Shokrgozar M, Mahboudi F, Ahmadvand D, Sharifzadeh Z, Parhamifar L, Moghimi S. 2014. T-Cells expressing VHH-directed oligoclonal chimeric HER2 antigen receptors: Towards tumor-directed oligoclonal T-cell therapy. Biochim Biophys Acta. 1840:378–386.
- Kazemi-Lomedasht F, Behdani M, Bagheri K, Habibi-Anbouhi M, Abolhassani M, Arezumand R, Shahbazzadeh D, Mirzahoseini H. 2015a. Inhibition of angiogenesis in human endothelial cell using VEGF specific nanobody. Mol Immunol. 65:58–67.
- Kazemi-Lomedasht F, Behdani M, Rahimpour A, Habibi-Anbouhi M, Poshang-Bagheri K, Shahbazzadeh D. 2015b. Selection and characterization of specific nanobody against human IgG. Monoclon Antib Immunodiagn Immunother. 34:201–205.
- Kazemi-Lomedasht F, Behdani M, Habibi-Anbouhi M, Shahbazzadeh D. 2016. Production and characterization of novel camel single domain antibody targeting mouse VEGF. Monoclon Antib Immunodiagn Immunother. 35:167–171.
- Kazemi-Lomedasht F, Behdani M, Bagheri K, Anbouhi M, Abolhassani M, Khanahmad H, Shahbazzadeh D, Mirzahoseini H. 2014. Expression and purification of functional human vascular endothelial growth factor-a121; The most important angiogenesis factor. Adv Pharm Bull. 4:323.
- Kazemi-Lomedasht F, Pooshang-Bagheri K, Habibi-Anbouhi M, Hajizadeh-Safar E, Shahbazzadeh D, Mirzahosseini H, Behdani M. 2017. In vivo immunotherapy of lung cancer using cross-species reactive vascular endothelial growth factor nanobodies. Iran J Basic Med Sci. 20:489.
- Kolkman J, Law D. 2010. Nanobodies - From llamas to therapeutic proteins. Drug Discov Today Technol. 7:e139–e146.
- Könning D, Zielonka S, Grzeschik J, Empting M, Valldorf B, Krah S, Schröter C, Sellmann C, Hock B, Kolmar H, et al. 2017. Camelid and shark single domain antibodies: Structural features and therapeutic potential. Curr Opin Struct Biol. 45:10–16.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. 2015. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New Engl J Med. 373:23–34.
- Laskowski R, and Swindells M., editors. 2011. LigPlot+: Multiple Ligand-Protein Interaction Diagrams for Drug Discovery Roman A. Laskowski and Mark B. Swindells Journal of Chemical Information and Modeling. 2011 51 (10), 2778–2786 DOI: 10.1021/ci200227u.
- Lee C, Iorno N, Sierro F, Christ D. 2007. Selection of human antibody fragments by phage display. Nat Protocols. 2:3001.
- Logtenberg T. 2007. Antibody cocktails: Next-generation biopharmaceuticals with improved potency. Trends Biotechnol. 25:390–394.
- Lovell S, Davis I, Arendall W, De Bakker P, Word J, Prisant M, Richardson J, Richardson D. 2003. Structure validation by Cα geometry: ϕ, ψ and Cβ deviation. Protein Structure Function Bioinform. 50:437–450.
- Muyldermans S, Baral T, Retamozzo V, De Baetselier P, De Genst E, Kinne J, Leonhardt H, Magez S, Nguyen VK, Revets H, et al. 2009. Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol. 128:178–183.
- Nikkhoi S, Rahbarizadeh F, Ahmadvand D. 2017. Oligoclonal nanobodies as an innovative targeting agent for cancer therapy: New biology and novel targeting systems. Protein Express Purific. 129:115–121.
- Nikkhoi S, Rahbarizadeh F, Ahmadvand D, Moghimi S. 2018. Multivalent targeting and killing of HER2 over-expressing breast carcinoma cells with methotrexate-encapsulated tetra-specific non-overlapping variable domain heavy chain anti-HER2 antibody-PEG-liposomes: In vitro proof-of-concept. Eur J Pharm Sci.
- Nowakowski A, Wang C, Powers D, Amersdorfer P, Smith T, Montgomery V, Sheridan R, Blake R, Smith L, Marks J. 2002. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc Natl Acad Sci USA. 99:11346–11350.
- Oliveira S, Schiffelers R, van der Veeken J, van der Meel R, Vongpromek R, en Henegouwen P, Storm G, Roovers R. 2010. Down-regulation of EGFR by a novel multivalent nanobody-liposome platform. J Controlled Release. 145:165–175.
- Otrock Z, Mahfouz R, Makarem J, Shamseddine A. 2007. Understanding the biology of angiogenesis: Review of the most important molecular mechanisms. Blood Cells Mol Dis. 39:212–220.
- Qi H, Lu H, Qiu H, Petrenko V, Liu A. 2012. Phagemid vectors for phage display: Properties, characteristics and construction. J Mol Biol. 417:129–143.
- Ranpura V, Hapani S, Wu S. 2011. Treatment-related mortality with bevacizumab in cancer patients: A meta-analysis. JAMA. 305:487–494.
- Rissiek B, Koch-Nolte F, Magnus T. 2014. Nanobodies as modulators of inflammation: Potential applications for acute brain injury. Front Cell Neurosci. 8:344.
- Scott A, Wolchok J, Old L. 2012. Antibody therapy of cancer. Nat Rev Cancer. 12:278.
- Sharifzadeh Z, Rahbarizadeh F, Shokrgozar M, Ahmadvand D, Mahboudi F, Jamnani F, Aghaee Bakhtiari S. 2013. Development of oligoclonal nanobodies for targeting the tumor-associated glycoprotein 72 antigen. Mol Biotechnol. 54:590–601.
- Shibuya M. 2001. Structure and function of VEGF/VEGF-receptor system involved in angio-genesis. Cell Structure Funct. 26:25–35.
- Spadiut O, Capone S, Krainer F, Glieder A, Herwig C. 2014. Microbials for the production of monoclonal antibodies and antibody fragments. Trends Biotechnol. 32:54–60.
- Spangler J, Neil J, Abramovitch S, Yarden Y, White F, Lauffenburger D, Wittrup K. 2010. Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc Natl Acad Sci USA. 107:13252–13257.
- Swain S, Baselga J, Kim S, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero J, Schneeweiss A, Heeson S, et al. 2015. Pertuzumab, trastuzumab, and docetaxel in HER2+ metastatic breast cancer. New Engl J Med. 372:724–734.
- Trapani J, Darcy P. 2017. Immunotherapy of cancer. Austral Family Physician. 46:194.
- Walker A, Chung C, Neu M, Burman M, Batuwangala T, Jones G, Tang CM, Steward M, Mullin M, Tournier N, et al. 2016. Novel interaction mechanism of a domain antibody based inhibitor of human vascular endothelial growth factor with greater potency than ranibizumab and bevacizumab and improved capacity over aflibercept. J Biol Chem. 291:5500–5511.
