Abstract
The present study aimed to investigate the protective effect of quercetin on polychlorinated biphenyls (PCB)-induced liver and embryo damage in pregnant Sprague–Dawley rats. Pregnant rats were divided into five groups, and then were orally gavaged daily with peanut oil (vehicle) or a commercial PCB mixture (Aroclor 1254) — with or without co-treatment with 75, 150, or 300 mg/kg quercetin — on gestation days (GD) 4–7. At GD 9, all rats were euthanized, and their blood, liver, and uterus were collected. Expressions of CYP450 mRNA and protein in liver, cytokines (IFNγ, IL-2, IL-4, and IL-6) and IFNγ/IL-4 ratios in liver and sera, liver morphology, and the status of implanted embryos were analyzed. The results showed Aroclor 1254 treatment alone caused hepatic cord damage (i.e. cell disorganization, swelling, decreased cytoplasm, vacuolization), and that quercetin co-treatment appeared to mitigate this damage. Similarly, levels of CYP1A1 and CYP2B1 mRNA in livers of Aroclor 1254-only-treated rats were significantly higher than those in rats co-treated with quercetin. Hepatic and sera levels of IFNγ, IL-2, IL-6, and IFNγ/IL-4 ratios, and the ratio of delayed-development embryos, all increased in Aroclor 1254-treated rats, but were relatively decreased as a result of quercetin co-treatments. IL-4 levels were decreased by Aroclor 1254 and tended to increase back to normal when quercetin was used. The results indicated that quercetin imparted a protective effect against Aroclor 1254-induced toxicity in pregnant rats, in part, by modulating levels of important pro-inflammatory cytokines and reducing induced CYP1A1 and CYP2B1 expression.
Introduction
Polychlorinated biphenyls (PCBs) are a group of persistent lipophilic environmental pollutants that biomagnify in the food chain and bioaccumulate in animals and human tissues (Beyer and Biziuk Citation2009; Wood et al. Citation2016). PCBs have been shown to induce oxidative stress by inducing formation of free radicals (Strużyńska et al. Citation2012) that, in turn, impart toxic effects in the liver through subsequent increases in local lipid peroxidation (Tharappel et al. Citation2008). In addition, PCBs are slowly biotransformed in the liver, leading to a delayed clearance from the body. In general, the first step in PCB metabolism is a cytochrome P450-dependent mono-oxygenation that results in the formation of OH-PCB; these transformations are modulated in particular by CYP1A and CYP2B isoforms (Nomiyama et al. Citation2014; Stamou et al. Citation2015). These hydroxylated metabolites may interact directly with estrogen receptors, as agonists or antagonists (James et al. Citation2008), and impact on reproductive functions in humans and animals (Connor et al. Citation1997; Fielden et al. Citation1997; Hisada et al. Citation2014).
Innate immune cells become activated during pregnancy; this process is characterized by an up-regulation in expression of activation markers on monocytes/granulocytes and changes in cytokine secretion (Groen et al. Citation2015). Of note, the host immune response shifts from a Type 1 (i.e. cellular; T-helper [TH]-1) response toward a Type 2 (i.e. humoral; TH2) one. It is generally accepted that TH1-related cytokines like interleukin (IL)-2 and interferon (IFN)-γ promote cellular immunity while TH2 cytokines like IL-4 exert negative immunoregulatory effects on cellular immunity (while upregulating humoral response). Previous studies showed that both short- and long-term exposure to PCB-153 caused significant immunosuppression and affected TH1/TH2 cell differentiation (Gaspar-Ramírez et al. Citation2012; Kuiper et al. Citation2016). In pregnancy, a shift in TH1/TH2 ratios is associated with recurrent miscarriage and fetal growth retardation (Groen et al. Citation2013).
Flavonoids are polyphenolic compounds found in plants, fruits, and vegetables. Dietary flavonoids have been shown to impart a variety of anti-oxidant, -inflammatory, and -carcinogenic effects in various model systems (Vijayakumar et al. Citation2018). Among the many flavonoids, quercetin has been shown to exhibit potent anti-oxidant effects against several types of liver pathologies (Sekaran et al. Citation2012; Rocha de Oliveira et al. Citation2014). In addition, quercetin was seen to yield no teratogenic effects in rats provided during the entire gestation with 2-2000 mg quercetin/kg (i.e. all had normal fetuses) (Willhite Citation1982).
To ascertain if quercetin could protect against toxicities induced by PCBs, this study examined potential changes in effects of a commercial of PCBs mixture Aroclor 1254 in pregnant rats. To evaluate a broad range of potential protective effects, the livers and embryos of these hosts were evaluated and some potential mechanisms of any protection discerned.
Materials and methods
Material
Aroclor 1254 (Accustandard, New Haven, CT, USA) was suspended in peanut oil and the working stock stored at 4 °C. Quercetin (Sigma, St. Louis, MO, USA) was prepared in sterile saline as 75, 150, and 300 mg/ml stocks. Anti-CYP1A1, -CYP2B1, and -CYC (cytochrome C) antibodies were bought from Chemicon (San Diego, CA, USA). Trizol was purchased from Invitrogen (Carlsbad, CA, USA). NBT (nitroblue tetrazolium), BCIP (5-bromo-4-chloro-3-indolyl-phosphate), and protease inhibitor phenylmethylsulfonylfluoride (PMSF) were bought from Amresco (Solon, OH, USA). M-MLV reverse transcriptase was purchased from Promega (Beijing, China). ELISA kits to assess liver and serum IFNγ, IL-2, IL-4, and IL-6 were purchased from Biovalue (Shanghai, China). RT-PCR primers were synthetized by Sangon Biotech Co. (Shanghai, China).
Animals
Sprague–Dawley rats (females and males [just for mating], 8-week old) were purchased from the Experimental Animal Center of Hebei Medical University, China. All rats were housed in polypropylene cages maintained in specific pathogen-free rooms maintained at 20–25 °C with a 55–65% relative humidity, and with a 12 h light-dark cycle. All rats had ad libitum access to standard rodent chow and filtered water. All experiments followed a protocol that was approved by the Animal Ethics Committee of the Agricultural University of Hebei, China.
For the studies, rats were acclimated for 10 days prior to the experiment. Timed pregnant rats were obtained by housing one estrous female with one male overnight. The rats were required to be pregnant (i.e. spermatozoids found in vaginal smears; Gestation Day [GD] 0) at the same time for the study to occur. Once confirmed, all GD 0 rats were randomly allocated into five experimental groups: Group A = control group, peanut oil [morning], and quercitin vehicle (sterile saline) [afternoon]; Group B = 10 mg/kg Aroclor 1254 [morning] + sterile saline [afternoon]; Group C = 10 mg/kg Aroclor 1254 [morning] + 75 mg/kg quercetin [afternoon]; Group D = 10 mg/kg Aroclor 1254 [morning] + 150 mg/kg quercetin [afternoon]; and, Group E = 10 mg/kg Aroclor 1254 [morning] + 300 mg/kg quercetin [afternoon]. All groups received their respective treatments by oral gavage (no anesthesia used) from GD 4–7 of pregnancy (implantation period). Gavage volumes never exceeded 1 ml/kg at any dosing. These doses of quercetin were selected based upon previous studies (Choi and Kim Citation2010).
At GD 9 (two days after final exposure), rats were anesthetized using xylazine (Sheng Da Pharmaceuticals, Ji Lin, China) and after decapitation, blood was collected and immediately processed to prepare serum that was isolated and then stored at –80 °C until analysis. At necropsy, each liver was collected and processed as described below. The gravid uterus was also excised and total implanted embryos and delay-development embryo numbers counted. Rate of delay-development was calculated as: 100% × (number of delay-development embryos/number of implanted embryos).
Liver histolopathology
Each isolated liver was blot dried and weighed. Liver sections were then prepared as follows: a section of liver tissue was put into Bouin’s solution, dehydrated in graded ethanol, embedded in paraffin, serial-sectioned at 5-μm thick, and stained by hematoxylin and eosin (HE). The samples were then evaluated in a blinded manner for any changes in morphology by a certified histologist using a light microscope.
CYP450 expression (RT-PCR)
Liver tissue (50 mg) used to analyze CYP450 mRNA was snap frozen in liquid nitrogen, pulverized, and its mRNA then isolated using Trizol (manufacturer protocols). Isolated mRNA was evaluated for content/purity using a Nano Drop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Reverse transcription was then done using a M-MLV reverse transcription system and resulting cDNA was subjected to PCR amplification (total volume, 50 μl). The primers used for amplification were: CYP1A1 = 5′-CTGGTTCTGGATACCCAGCTG-3′ (forward), and 5′-CCTAGGGTTGGTTACCAGG-3′ (reverse); CYP2B1 = 5′-TATCTTGCTC- CTCCTTGCTCT-3′ (forward), and 5′-GCCTCCTTT ATGGTGTCTGTC-3′ (reverse). CYC = 5′-CTTCGACATCACGGCTGATGG-3′ (forward), and 5′- CAGGACCTGTATGCTTCAGG-3′ (reverse). RT–PCR reaction volumes were 50 μl (25 μl 2 × Taq PCR Master Mix, 2 μl 10 μM forward primer, 2 μl 10 μM reverse primer, 5 μl template, 16 μl de-ionized water). PCR was performed as follows: 30 cycles of template denaturation at 94 °C for 30 s, primer annealing at Tm 57 °C for 30 s, and primer extension at 72 °C for 1 min, followed by a final extension at 72 °C for 5 min. All PCR-amplified products were then evaluated under UV light on a 2% agarose gel containing ethidium bromide by scanning with a Gel Doc image scanner (Bio-Rad, Hercules, CA, USA).
CYP450 expression (Western blot)
Liver tissue (50 mg) from each rat was homogenized in 10 vol ice-cold phosphate- buffered saline (pH = 7.2) using a Teflon homogenizer. The homogenate was then centrifuged at 4000 rpm (30 min, 4 °C) to separate supernatant from cellular debris. The resulting supernatant was isolated and measured for total protein using a commercial BCA kit (Pierce, Rockford, IL, USA). Total protein (10 µg/sample) were then loaded into a 10% acrylamide gel, resolved using SDS-PAGE, and ultimately electrotransferred to a PVDF membrane. The membrane was blocked in 5% nonfat milk in TBST (Tris-Buffered Saline containing 0.05% Tween-20) for 4 h at room temperature to block nonspecific binding events, and then incubated with anti-CYP1A1, -CYP2B1, or -CYC antibodies (each 1:1000 in TBST) at 4 °C overnight. The membrane was then washed three times with TBST before being incubated in a solution of TBST containing horse-radish peroxidase (HRP)-conjugated anti-rabbit IgG (1:500) secondary antibody for 1 h at room temperature. After a final series of rinses using TBST, signal was generated with an NBT/BCIP kit (Solarbio, Beijing, China). After normalization for loading, density of each protein band was quantified in a scanning densitometer using Image J software (NIH, Bethesda, MD, USA); all values were then calculated as relative to control sample value.
Tissue levels of IFNγ, IL-2, IL-4, and IL-6
Levels of IFNγ, IL-2, IL-4, and IL-6 in each liver lysate (same homogenate supernatant as above) and in sera were assessed using commercial kits, according to manufacturer instructions. The level of sensitivity of the kits was 4 pg IFNγ/ml, 1 pg IL-2/ml, 4 pg IL-4/ml, and 4 pg IL-6/ml. All samples were run in triplicate; mean values were reported as pg/ml.
Statistical analysis
Results were expressed as mean ± SD of three independent experiments. Significant differences across treatment versus control groups were determined by using one-way analysis of variance (ANOVA). Dunnett’s multiple comparison tests were conducted when analytic results were significant. All evaluations were performed using SPSS software (v.18.0, SPSS, Chicago, IL, USA). A p values < 0.05 was considered statistically significant.
Results
Liver histology
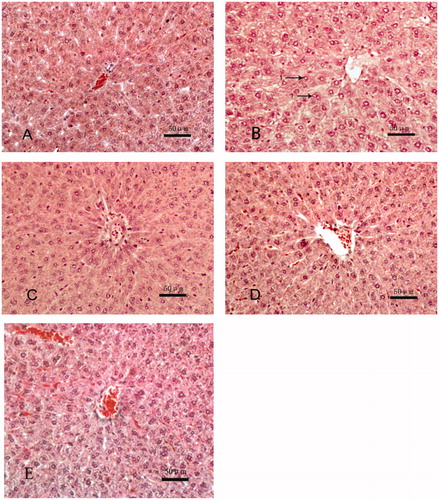
Control dams () presented liver tissues with normal hepatocytes (most with well-preserved cytoplasm) and normal hepatic cords. In Aroclor 1254-only rats (), there was evidence of cord disorganization and swollen hepatocytes with reduced levels of cytoplasm; there was also an occasional presence of lipid droplets (arrows). Among Group C rats that received quercetin, expected Aroclor 1254-induced changes in liver cells were not noticeable (), and liver tissue structures tended to “normalize” with quercetin treatments of 150 () and 300 mg/kg (). From this, it was deemed 150 mg quercetin/kg was an optimal dose to protect against liver damage induced with this dose of Aroclor 1254 — in this particular regimen.
Figure 1. Histology of liver tissues. Samples were isolated from dams in each experimental group and processed and analyzed as noted in the methods. Representative slides from each group of animals is shown. (A) Control (peanut oil), (B) Aroclor 1254 (10 mg/kg) only, (C) Aroclor + 75 mg/kg quercetin, (D) Aroclor + 150 mg/kg quercetin, and (C) Aroclor + 300 mg/kg quercetin. Arrows indicate lipid droplet(s) presence. Hematoxylin-eosin (HE); magnification = 200×.
Effects on embryo implants
The average number of implanted embryos in control dams was 13.75 (± 0.6) and the rate of delay-development embryos was 1.6% (± 0.3)% (). Treatment with Aroclor 1254 resulted in significantly-fewer implanted embryos (8.8 [± 1.0]) and a rate of delay-development embryo that was significantly increased (28.2 [± 5.7])%. Treatment with the lowest quercetin dose resulted in no mitigation of Aroclor 1254-induced effects. In comparison, there was no significant different in values for each parameter between control dams and those rats in Groups D and E.
Table 1. Rat embryo implantation and delayed-development ratios.
CYP1A1 and CYP2B1 mRNA
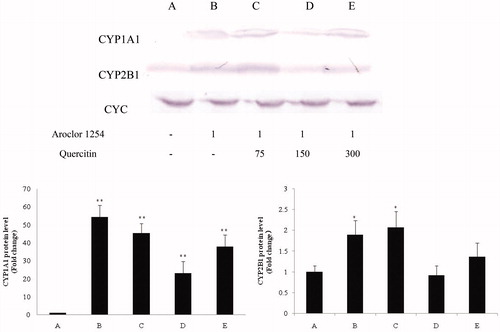
CYP1A1 mRNA expression in control rat liver tissues was nominal (expression relative to that of CYC housekeeping gene = 0.02 [± 0.00]; ). CYP1B1 mRNA expression in these tissues were 0.41 [± 0.04]. Aroclor 1254 treatment alone induced significant 55.9- and 1.7-fold increases in, respectively, CYP1A1 (0.91 [± 0.08]) and CYP2B1 (0.71 [± 0.05]) expression. The lowest dose of quercetin again had no obvious impact on CYP1A1 (0.80 [± 0.07]) and CYP2B1 (0.72 [± 0.04]) relative (to CYC housekeeping gene) expression levels. However, a higher dose of quercetin (150 mg/kg) significantly blocked these inductions, with expression of each in the rat livers being decreased to now just 0.35- (CYP1A1; 0.60 [± 0.06]) and 0.27-fold (CYP2B1; 0.52 [± 0.03]) (each relative to CYC housekeeping gene). Values in the highest quercetin dose group (300 mg/kg) also indicated reduction in dam liver CYP1A1 (0.66 [± 0.04]) and CYP2B1 (0.62 [± 0.02]) expression levels. However, as these remained on par with the values in the Group D rats, this indicated no consistent quercitin dose-related effect. Interestingly, even with the mitigation, values of CYP1A1 expression in the Group D and E dams were still significantly higher than in the control rat liver samples.
Figure 2. CYP1A1 and CYP2B1 mRNA expression in rat liver samples (RT-PCR). All PCR-amplified products were evaluated under UV in a 2% agarose gel [containing ethidium bromide] by scanning with a Gel Doc image scanner. Evaluations of CYC housekeeping gene were done to permit normalization as needed for loading. Values shown in bar charts are in terms of relative intensity (i.e. vs. CYC level). Values shown are means ± SD (n = 8/group). *Value significantly different from control (*p < 0.05, **p < 0.01). Each experiment was repeated three times; figure shown is representative outcome. Doses in upper figure in terms of mg/kg. Treatments A-E described in legend to .
![Figure 2. CYP1A1 and CYP2B1 mRNA expression in rat liver samples (RT-PCR). All PCR-amplified products were evaluated under UV in a 2% agarose gel [containing ethidium bromide] by scanning with a Gel Doc image scanner. Evaluations of CYC housekeeping gene were done to permit normalization as needed for loading. Values shown in bar charts are in terms of relative intensity (i.e. vs. CYC level). Values shown are means ± SD (n = 8/group). *Value significantly different from control (*p < 0.05, **p < 0.01). Each experiment was repeated three times; figure shown is representative outcome. Doses in upper figure in terms of mg/kg. Treatments A-E described in legend to Figure 1.](/cms/asset/3a7746fc-ef0b-443f-80b6-d9c47cdc3b96/iimt_a_1604585_f0002_b.jpg)
CYP1A1 and CYP2B1 protein expression
The levels of CYP1A1 and CYP2B1 protein expression in the liver samples as measured by Western blot analyses were similar (trends) to the mRNA expression patterns reported above (). Aroclor 1254 alone induced 54.3- and 1.9-fold increases (vs. corresponding control liver values) in, respectively, CYP1A1 and CYP2B1 protein levels. Quercetin-related outcomes as above with mRNA were again noted. With 75 mg/kg quercetin, CYP1A1 and CYP2B1 protein levels in the livers did not reflect any obvious impact compared with liver levels in the Aroclor 1254-only rats. In comparison, in Group D rats (150 mg/kg quercetin), liver CYP1A1 and CYP2B1 protein levels were now just 23.3- and 0.9-fold higher than in control rat livers, with each significantly decreased correspondingly from those in the Aroclor 1254-only rats. With Group E rats (300 mg/kg quercetin), these values were 38.6- and 1.3-fold higher versus control, respectively. These values for the Groups D and E rats once again demonstrated there was no dose-related effect for quercitin at the higher end of the range of quercetin doses tested.
Figure 3. CYP1A1 and CYP2B1 protein in rat liver samples (homogenates; Western blot). Evaluation of CYC was performed to permit normalization as needed for loading. Values shown in bar charts are in terms of relative intensity (i.e. after normalizing, vs. control rat value). Values shown are means ± SD (n = 8/group). *Value significantly different from control (*p < 0.05, **p < 0.01). Each experiment repeated three times; figure shown is representative outcome. Doses in upper figure in terms of mg/kg. Treatments A–E described in legend to .
IFNγ, IL-2, IL-4, and IL-6 in rat liver lysate and sera
In it illustrates that the levels of IFNγ in liver and sera of Aroclor 1254-only treated dams increased significantly relative to corresponding values in control rats. Serum IFNγ values in these rats increased to 1433.8 [± 52.3] pg/ml from 1246.2 [± 45.3] pg/ml (control) and liver values to 394.2 [± 37.3] pg/ml from 106.5 [± 21.9] pg/ml (control). In dams that were co-treated with quercetin, serum IFNγ levels all returned to control values even with the lowest dose of quercetin, but this low dose did not fully mitigate the induction in the context of liver IFNγ. Almost identical outcome patterns were noted with regard to IL-2. Sera values in Aroclor 1254-only treated rats increased to 238.5 [± 20.3] pg/ml from 137.9 [± 17.6] pg/ml (control) and liver values to 42.6 [± 6.3] pg/ml from 23.8 [± 2.0] pg/ml (control). However, in this case, the lowest quercetin dose used in co-treatments was unable to cause any reduction in serum IL-2 levels to one no longer significantly different from control dam values.
Figure 4. IFNγ, IL-2, IL-4, and IL-6 levels in rat liver tissue homogenates and sera. IFNγ/IL-4 ratio for each sample group is also presented (bottom). Values shown are means ± SD (n = 8/group). *Value significantly different from control (*p < 0.05, **p < 0.01). Treatments A–E described in legend to .
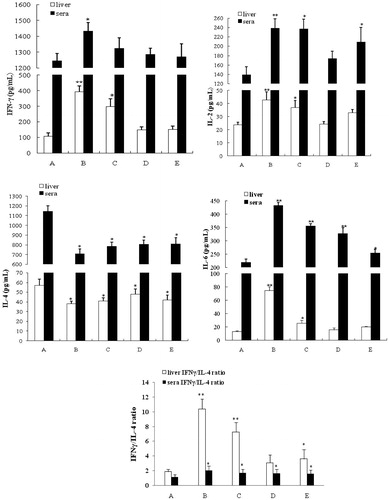
With regard to IL-6, serum levels in all Aroclor 1254-treated rats remained significantly higher than that in controls (432.7 [± 12.3] pg/ml vs. 219.9 [± 12.9] pg/ml) regardless of the level of quercetin used (Group D = 327.6 [± 21.8] pg/ml, Group E = 254.6 [± 13.5] pg/ml). Still, there seemed to be a dose-trend toward lowering of these values. On the other hand, hepatic IL-6 levels were all induced by Aroclor 1254 (74.8 [± 5.7] pg/ml from 12.9 [± 1.6] pg/ml), but as with IL-2, the rats in Groups D and E had levels no longer significantly different than control dam tissues. Lastly, with IL-4, there was a consistent significant lowering of serum IL-4 levels in Aroclor 1254-only-treated rats (706.5 [± 52.3] pg/ml from 1143.4 [± 60.3] pg/ml). Co-treatment with quercetin appeared to have no impact on this change. Liver IL-4 levels followed the same trend patterns, with quercetin imparting no mitigating effect at any tested level.
To assess if the outcomes might have reflected any shifts in the TH1/TH2 balance in these dams, IFNγ/IL-4 ratios in each liver homogenate and serum sample were calculated. The impact of Aroclor 1254 in the livers was clear, with significant increases in these ratios relative to in control rat samples (i.e. 1.90 [± 0.32]); this was driven by the increases in IFNγ combined with decreases in IL-4. In nearly every case, even with quercitin co-treatments, the hepatic ratios declined relative to the Aroclor 1254-only values, but remained significantly elevated relative to controls (only Group D had lost significance). Specifically, Aroclor 1254 alone resulted in a mean hepatic IFNγ/IL-4 ratio of 10.40 [± 1.37]; values for Groups D and E rats were, respectively, 3.07 [± 1.04] and 3.59 [± 1.28].
Similar patterns of shift in ratio were evident in the sera, but the net changes were not as great relative to the control values, that is, control = 1.10 [± 0.35], Aroclor 1254 only = 2.00 [± 0.56], Group E = 1.57 [± 0.48]). In Groups D and E, these shifts appeared to be driven primarily by the reductions in IL-4 induced by the Aroclor 1254.
Discussion
It is known PCBs can cause damage in several organs, particularly the liver; in the latter, there are toxicities induced in hepatocytes and often the induction of hepatocellular steatosis (Fernandes et al. Citation2011; Maisano et al. Citation2016). As always, the liver acts as a depot for PCB sequestration; this becomes problematic in the case of a pregnant host exposed to this toxicant (Bonfanti et al. Citation2009). In the present study, pregnant rats treated with Aroclor 1254 displayed various toxicities in their livers.
In healthy hosts, agents in the PCB family are metabolized primarily in the liver through the activities of various types of cytochrome P450 (CYP450) (Zhu and Silverman Citation2008; Mise et al. Citation2016). Of all the isozymes, CYP1 and CYP2 are the major forms involved in PCB metabolism (Hirakawa et al. Citation2018). Because of this, CYP1A1 is often used a biomarker of host exposure to certain persistent organic pollutants (POP) (Foltz et al. Citation2014; Wimmerová et al. Citation2016). This use as a biomarker is valid in that other studies have previously shown that in the hepatic microsomes of untreated (control) rats, CYP1A1 protein was undetectable (Sidorova et al. Citation2016). Pollutant-induced responses have also been noted with CYP2B (Wakui et al. Citation2006).
The first step in PCB metabolism via CYP1A and CYP2B is a mono-oxygenation that results in formation of a hydroxylated congener (i.e. OH-PCB). Earlier studies showed hepatic expression levels of CYP1 genes positively correlated with the concentration of each OH-PCB congener in the tissues (Nomiyama et al. Citation2014; Stamou et al. Citation2015). There metabolites then can impart their own distinct toxicities, including inducing causing peroxidative damage (Dreiem et al. Citation2009), neurotoxicity (Lesmana et al. Citation2014), and increases in fetal malformations (Juchau et al. Citation1985). Because treatment here with Aroclor 1254 significantly increased CYP1A1 and CYP2B1 mRNA expressions and protein levels relative to those in control rat livers, it is most likely certain there were corresponding local increases in OH-PCB formation as well (Martin and Klaassen Citation2010). Although not directly evaluated here, these expected increases in a presence of various toxic metabolites of the PCBs present in the Aroclor 1254 likely, in turn, impacted on the ability of each Aroclor 1254-treated dam here to nurture development of their embryos.
Lastly, it is also known that PCBs induce NF-κB activation that can lead to significant increase in inflammatory agent mRNA and protein expression (Eske et al. Citation2014). In vitro and in vivo studies showed that POPs (which could include PCBs) trigger secretion of pro-inflammatory cytokines and alter lymphocyte function — in part, via disruption of T-cell receptor (TCR) signaling and cytokine production (Imbeault et al. Citation2012; Wahlang et al. Citation2016; Rousselet et al. Citation2017). In many cases, induced increases in local levels of inflammatory agents (including cytokines such as tumor necrosis factor [TNF]-α, interferon [IFN]-γ, interleukin [IL]-1β and IL-2, and reactive intermediates generated by local immune cells, etc.) can subsequently give rise to secondary toxicities/pathologies in the liver (Godoy et al. Citation2013; Wahlang et al. Citation2014). Since many of these same agents are also released into the general circulation, distal toxicities may also manifest and could harm an embryo/fetus (Mori et al. Citation2016). IL-6 is also functionally associated with embryonic development; elevated IL-6 concentrations were associated with a decline in blastocyst formation rates (Yu et al. Citation2018). Here, hepatic/serum levels of IFNγ, IL-2, and IL-6 in Aroclor 1254-treated rats were significantly increased and there were significant increases in host IFNγ/IL-4 (TH1/TH2) ratios, suggesting that the immune balance of TH1/TH2 moves toward TH1. Since excess levels of circulating TH1 cytokines may induce early pregnancy loss/abortion peri-implantation (Chaouat et al. Citation2007), it is possible that dysregulation of normal embryo development induced by PCBs in the current study could be related to the changes induced in the formation of these inflammatory agents/products.
Quercetin, a major flavonoid in several plants/herbs/herbal medicines, is known to impart multiple biological effects (including anti-inflammatory and anti-tumor ones) and strengthen the functions of certain types of immune cells (Massi et al. Citation2017). Reports have shown that quercetin imparted protective effects in hepatocytes, including in those injured by lipopolysaccharide or ethanol (Casella et al. Citation2014; Li et al. Citation2014). It was previously noted in Choi et al. (Citation2010) that quercetin significantly blocked induction of CYP1A1 that could be triggered by PCB77 or PCB126. In the current study, quercetin (especially at higher doses tested) consistently resulted in a liver configuration that reflected a normalization in hepatic structure and CYP1A1 and CYP2B1 expressions away from that modified by the Aroclor 1254. As noted earlier, alterations in CYP450 levels in the liver due to PCB exposures would likely also affect levels of OH-PCB formation. Because quercetin was seen here to inhibit CYP450 induction (by Aroclor 1254), this implied then that levels of OH-PCB formation in the co-treated hosts were also likely decreased, to the benefit of the host. It could also be that an increased removal of any OH-PCBs that had formed in the rats provided an additional protective effect imparted by the quercitin. This would be in keeping with findings of James et al. (Citation2008) who noted that continual dosing of quercitin could lead to altered expression of UDP-glucuronosyltransferases (UGTs, specifically, UGT1A1 isoform). These enzymes could then, in turn, via Phase II glucuronidation reactions help detoxify any OH-PCBs that still managed to be formed. Accordingly, although changes both in levels of OH-PCB-forming CYP isoforms and increases in OH-PCB-detoxifying UGT isoforms, any overall toxic effect from the PCB given to a host would be expected to be weakened. In the present study, such an outcome was borne out by the findings that quercetin co-treatment of the rat dams led to a protective effect against liver damage and embryo toxicity from the Aroclor 1254.
Normal systemic adaptations to pregnancy include a shift toward a Type 2 immune profile among T-lymphocytes and concurrent increases in levels of natural killer (NK) and T-regulatory (Treg) cells (Gui et al. Citation2012; Groen et al. Citation2015). It is now known that IL-4 plays a crucial role in the success of pregnancy; there is strong evidence that IL-4 deficiency contributes to infertility and spontaneous abortions (reviewed in Chatterjee et al. Citation2014). In keeping with those findings, the data in the current study showed that Aroclor 1254 alone led to significant reductions in hepatic and circulating IL-4 levels in the dams and concomitant decreases in levels of fetal implantations/increases in delay-development among their embryos. The observed effects on these fetal parameters were reversed for the most part by quercitin co-treatments. However, most of these occurred in the absence of any substantive rebound in IL-4 in the serum of the co-treated hosts. Thus, this means IL-4 was not likely the main factor for the observed protective effect of quercitin in the fetuses of these co-treated dams.
As noted above, excess levels of circulating TH1 cytokines may induce early pregnancy loss/abortion peri-implantation. It has been shown repeatedly that an immunologic challenge of pregnant rats induces production of inflammatory cytokines, including IFNγ (Si et al. Citation2013), IL-6 (Pan et al. Citation2018), and IL-2 (Sharashenidze et al. Citation2014). From the data in the present study, it is possible to infer that the dysregulation of normal embryo development being driven by the Aroclor 1254 might be more dependent upon circulating levels of IFNγ, IL-2, and IL-6 than those of IL-4. Here, the only cytokines whose serum levels seemed to parallel the trends in changes in numbers of implanted embryos and in delayed-development embryos were IFNγ, IL-2 and IL-6. This finding indicated to us that quercetin affected the secretion and thus the toxicity of IFNγ, IL-2, and IL-6. In so doing, this led to adjustment in the TH1/TH2 balance in the pregnant Aroclor 1254-treated rats.
Conclusions
During Aroclor 1254-induced hepatotoxicity and embryo damage, co-administration with quercetin imparted synergistic protective effects on the liver and embryos of pregnant rats. These outcomes were mediated, in part, by reductions in levels of PCB metabolites and potentially by restoration of the maternal TH1/TH2 balance. Our own earlier experiments confirmed quercetin had protective effect on the endometrial cells injured by Aroclor 1254 (Xu et al. Citation2014); this experiment also showed that quercetin helped protect against Aroclor 1254-induced embryo-toxicity in utero. Taken together, we conclude quercetin might be beneficial during pregnancy in a host at risk of exposure to Aroclor 1254/potentially other PCBs.
Acknowledgment
The authors wish to sincerely thank Professor Man Hu (Agricultural University of Hebei College of Veterinary Medicine) for help in the analysis of liver pathomorphology.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
Additional information
Funding
References
- Beyer A, Biziuk M. 2009. Environmental fate and global distribution of polychlorinated biphenyls. Rev Environ Contam Toxicol. 201:137–158.
- Bonfanti P, Colombo A, Villa S, Comelli F, Costa B, Santagostino A. 2009. The effects of accumulation of an environmentally relevant polychlorinated biphenyl mixture on cytochrome P450 and P-glycoprotein expressions in fetuses and pregnant rats. Chemosphere. 75:572–579.
- Casella M, Parody J, Ceballos M, Quiroga A, Ronco M, Francés D, Monti J, Pisani G, Carnovale C, Carrillo M, et al. 2014. Quercetin prevents liver carcinogenesis by inducing cell cycle arrest, decreasing cell proliferation, and enhancing apoptosis. Mol Nutr Food Res. 58:289–300.
- Chaouat G, Dubanchet S, Ledée N. 2007. Cytokines: Important for implantation? J Assist Reprod Genet. 24:491–505.
- Chatterjee P, Chiasson V, Bounds K, Mitchell B. 2014. Regulation of the anti-inflammatory cytokines IL-4 and IL-10 during pregnancy. Front Immunol. 5:253.
- Choi E, Kim G. 2010. Quercetin accumulation by chronic administration causes the caspase-3 activation in liver and brain of mice. Biofactors. 36:216–221.
- Choi Y, Arzuaga X, Kluemper C, Caraballo A, Toborek M, Hennig B. 2010. Quercetin blocks caveolae-dependent pro-inflammatory responses induced by co-planar PCBs. Environ Intl. 36:931–934.
- Connor K, Ramamoorthy K, Moore M, Mustain M, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P. 1997. Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: Structure-activity relationships. Toxicol Appl Pharmacol. 145:111–123.
- Dreiem A, Rykken S, Lehmler H, Robertson L, Fonnum F. 2009. Hydroxylated polychlorinated biphenyls increase reactive oxygen species formation and induce cell death in cultured cerebellar granule cells. Toxicol Appl Pharmacol. 240:306–313.
- Eske K, Newsome B, Han S, Murphy M, Bhattacharyya D, Hennig B. 2014. PCB-77 dechlorination products modulate pro-inflammatory events in vascular endothelial cells. Environ Sci Pollut Res Int. 21:6354–6364.
- Fernandes A, Foxall C, Lovett A, Rose M, Dowding A. 2011. The assimilation of dioxins and PCBs in conventionally reared farm animals: Occurrence and biotransfer factors. Chemosphere. 83:815–822.
- Fielden M, Chen I, Chittim B, Safe S, Zacharewski T. 1997. Examination of the estrogenicity of 2,4,6,2′,6′-pentachlorobiphenyl (PCB 104), its hydroxylated metabolite 2,4,6,2′,6′-pentachloro-4-biphenylol (HO-PCB 104), and a further chlorinated derivative, 2,4,6,2′,4′,6′-hexa- chlorobiphenyl (PCB 155). Environ Health Perspect. 105:1238–1248.
- Foltz K, Baird R, Ylitalo G, Jensen B. 2014. Cytochrome P4501A1 expression in blubber biopsies of endangered false killer whales (Pseudorca crassidens) and nine other odontocete species from Hawaii. Ecotoxicology. 23:1607–1618.
- Gaspar-Ramírez O, Pérez-Vázquez FJ, Pruneda-Álvarez LG, Orta-García ST, González-Amaro R, Pérez-Maldonado IN. 2012. Effect of polychlorinated biphenyls 118 and 153 on Th1/Th2 cells differentiation. Immunopharmacol Immunotoxicol. 34:627–632.
- Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger J, et al. 2013. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 87:1315–1530.
- Groen B, Links T, Lefrandt J, van den Berg P, de Vos P, Faas M. 2013. Aberrant pregnancy adaptations in the peripheral immune response in Type 1 diabetes: A rat model. PLoS One. 8:e65490.
- Groen B, van der Wijk A, van den Berg P, Lefrandt J, van den Berg G, Sollie K, de Vos P, Links T, Faas M. 2015. Immunological adaptations to pregnancy in women with Type 1 diabetes. Sci Rep. 5:13618.
- Gui J, Xiong F, Li J, Huang G. 2012. Effects of acupuncture on TH1 and TH2 cytokines in rats with implantation failure. Evid Based Complement Alternat Med. 2012:893023.
- Hirakawa S, Miyawaki T, Hori T, Kajiwara J, Katsuki S, Hirano M, Yoshinouchi Y, Iwata H, Mitoma C, Furue M. 2018. Accumulation properties of polychlorinated biphenyl congeners in Yusho patients and prediction of their cytochrome P450-dependent metabolism by in silico analysis. Environ Sci Pollut Res Int. 25:16455–16463.
- Hisada A, Shimodaira K, Okai T, Watanabe K, Takemori H, Takasuga T, Koyama M, Watanabe N, Suzuki E, Shirakawa M, et al. 2014. Associations between levels of hydroxylated PCBs and PCBs in serum of pregnant women and blood thyroid hormone levels and body size of neonates. Int J Hyg Environ Health. 217:546–553.
- Imbeault P, Findlay C, Robidoux M, Haman F, Blais J, Tremblay A, Springthorpe S, Pal S, Seabert T, Krümmel E, et al. 2012. Dysregulation of cytokine response in Canadian First Nations communities: Is there an association with persistent organic pollutant levels? PLoS One. 7:e39931.
- James M, Sacco J, Faux L. 2008. Effects of food natural products on the biotransformation of PCBs. Environ Toxicol Pharmacol. 25:211–217.
- Juchau M, Bark D, Shewey L, Greenaway J. 1985. Generation of reactive dysmorphogenic intermediates by rat embryos in culture: Effects of cytochrome P450 inducers. Toxicol Appl Pharmacol. 81:533–544.
- Kuiper J, Moran M, Cetkovic-Cvrlje M. 2016. Exposure to polychlorinated biphenyl-153 decreases incidence of autoimmune Type 1 diabetes in non-obese diabetic mice. J Immunotoxicol. 13:850–860.
- Lesmana R, Shimokawa N, Takatsuru Y, Iwasaki T, Koibuchi N. 2014. Lactational exposure to hydroxylated polychlorinated biphenyl (OH-PCB 106) causes hyperactivity in male rat pups by aberrant increase in dopamine and its receptor. Environ Toxicol. 29:876–883.
- Li Y, Deng Y, Tang Y, Yu H, Gao C, Liu L, Liu L, Yao P. 2014. Quercetin protects rat hepatocytes from oxidative damage induced by ethanol and iron by maintaining intercellular liable iron pool. Hum Exp Toxicol. 33:534–541.
- Maisano M, Cappello T, Oliva S, Natalotto A, Giannetto A, Parrino V, Battaglia P, Romeo T, Salvo A, Spanò N, et al. 2016. PCB and OCP accumulation and evidence of hepatic alteration in the Atlantic bluefin tuna, T. thynnus, from the Mediterranean Sea. Mar Environ Res. 121:40–48.
- Martin L, Klaassen C. 2010. Differential Effects of polychlorinated biphenyl congeners on serum thyroid hormone levels in rats. Toxicol Sci. 117:36–44.
- Massi A, Bortolini O, Ragno D, Bernardi T, Sacchetti G, Tacchini M, De Risi C. 2017. Research progress in the modification of quercetin leading to anti-cancer agents. Molecules. 22:1270.
- Mise S, Haga Y, Itoh T, Kato A, Fukuda I, Goto E, Yamamoto K, Yabu M, Matsumura C, Nakano T, et al. 2016. From the cover: Structural determinants of the position of 2,3′,4,4′,5-pentachlorobiphenyl (CB118) hydroxylation by mammalian cytochrome P450 monooxygenases. Toxicol Sci. 152:340–348.
- Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J. 2016. The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin Immunopathol. 38:635–649.
- Nomiyama K, Hirakawa S, Eguchi A, Kanbara C, Imaeda D, Yoo J, Kunisue T, Kim E, Iwata H, Tanabe S. 2014. Toxicological assessment of polychlorinated biphenyls and their metabolites in the liver of Baikal seal (Pusa sibirica)). Environ Sci Technol. 48:13530–13539.
- Pan J, Zhan C, Yuan T, Wang W, Shen Y, Sun Y, Wu T, Gu W, Chen L, Yu H. 2018. Effects and molecular mechanisms of intrauterine infection/inflammation on lung development. Respir Res. 19:93.
- Rocha de Oliveira C, Ceolin J, Rocha de Oliveira R, Gonçalves Schemitt E, Raskopf Colares J, de Freitas Bauermann L, Hilda Costabeber I, Morgan-Martins M, Mauriz J, Da Silva J, et al. 2014. Effects of quercetin on polychlorinated biphenyls-induced liver injury in rats. Nutr Hosp. 29:1141–1148.
- Rousselet E, Levin M, Gebhard E, Higgins B, DeGuise S, Godard-Codding C. 2017. Polychlorinated biphenyls (PCBs) modulate both phagocytosis and NK cell activity in vitro in juvenile loggerhead sea turtles (Caretta caretta). J Toxicol Environ Health. 80:556–561.
- Sekaran S, Kandaswamy S, Gunasekaran K, Perumal E, Afsar F, Madhan B, Jagadeesan A. 2012. Protective role of quercetin on polychlorinated biphenyls (Aroclor-1254) induced oxidative stress and apoptosis in liver of adult male rats. J Biochem Mol Toxicol. 26:522–532.
- Sharashenidze AD, Kikalishvili LA, Kintaraia PI, Sanikidze TB, Turmanidze TR. 2014. Alterations of immunebalance in the rats blood during experimental preeclampsia. Georgian Med News. 236:97–101.
- Si LF, Zhang SY, Gao CS, Chen SL, Zhao J, Cheng XC. 2013. Effects of IFN-γ on IL-18 expression in pregnant rats and pregnancy outcomes. Asian-Australas J Anim Sci. 26:1399–1405.
- Sidorova Y, Perepechaeva M, Pivovarova E, Markel A, Lyakhovich V, Grishanova A. 2016. Menadione suppresses benzo(α)pyrene-induced activation of cytochrome P4501A: Insights into a possible molecular mechanism. PLoS One. 11:e0155135.
- Stamou M, Uwimana E, Flannery B, Kania-Korwel I, Lehmler H, Lein P. 2015. Subacute nicotine co-exposure has no effect on 2,2′,3,5′,6-pentachlorobiphenyl disposition but alters hepatic cytochrome P450 expression in the male rat. Toxicology. 2:59–68.
- Strużyńska L, Sulkowski G, Dąbrowska-Bouta B. 2012. Aroclor 1254 selectively inhibits expression of glial GLT-1 glutamate transporter in the forebrain of chronically exposed adult rat. Toxicology. 300:12–18.
- Tharappel J, Lehmler H, Srinivasan C, Robertson L, Brett T, Spear B, Glauert H. 2008. Effect of anti-oxidant phytochemicals on hepatic tumor promoting activity of 3,3′,4,4′-tetrachlorobi- phenyl (PCB)-77. Food Chem Toxicol. 46:3467–3474.
- Vijayakumar K, Rengarajan R, Radhakrishnan R, Anand A. 2018. Hypolipidemic effect of Psidium guajava leaf extract against hepatotoxicity in rats. Pharmacogn Mag. 14:4–8.
- Wahlang B, Petriello M, Perkins J, Shen S, Hennig B. 2016. Polychlorinated biphenyl exposure alters the expression profile of microRNAs associated with vascular diseases. Toxicol In Vitro. 35:180–187.
- Wahlang B, Song M, Beier J, Cameron Falkner K, Al-Eryani L, Clair H, Prough R, Osborne T, Malarkey D, Christopher J, et al. 2014. Evaluation of Aroclor 1260 exposure in a mouse model of diet-induced obesity and non-alcoholic fatty liver disease. Toxicol Appl Pharmacol. 279:380–390.
- Wakui S, Yokoo K, Takahashi H, Muto T, Suzuki Y, Kanai Y, Hano H, Furusato M, Endou H. 2006. Prenatal 3,3′,4,4′,5-pentachlorobiphenyl exposure modulates induction of rat hepatic CYP1A1, CYP1B1, and AhR by 7,12-dimethylbenz[a]anthracene. Toxicol Appl Pharmacol. 210:200–211.
- Willhite C. 1982. Teratogenic potential of quercetin in the rat. Food Chem Toxicol. 20:75–79.
- Wimmerová S, van den Berg M, Chovancová J, Patayová H, Jusko T, van Duursen M, Palkovičová Murínová L, Canton R, van Ede K, Trnovec T. 2016. Relative effect potency estimates of dioxin-like activity for dioxins, furans, and dioxin-like PCBs in adults based on cytochrome P4501A1 and -1B1 gene expression in blood. Environ Int. 96:24–33.
- Wood S, Armitage J, Binnington M, Wania F. 2016. Deterministic modeling of the exposure of individual participants in the National Health and Nutrition Examination Survey (NHANES) to polychlorinated biphenyls. Environ Sci Process Impacts. 18:1157–1168.
- Xu L, Sun L, Lu L, Zhong X, Ma Y, Qin J. 2014. Effects of quercetin on CYP450 and cytokines in Aroclor 1254 injured endometrial cells of the pregnant rats. Biomed Res Int. 2014:497508.
- Yu C, Zhang X, Wang L, Liu Y, Li N, Li M, Chen L, Liu Y, Yao Y. 2018. Interleukin-6 regulates expression of Fos and Jun genes to affect development of mouse pre-implantation embryos. J Obstet Gynaecol Res. 44:253–262.
- Zhu Y, Silverman R. 2008. Revisiting heme mechanisms. A perspective on the mechanisms of nitric oxide synthase (NOS), heme oxygenase (HO), and cytochrome P450s (CYP450s)). Biochemistry. 47:2231–2243.
