Abstract
The current era of drug discovery has been marked by a significant increase in the development of immune modulating agents to address a range of diseases such as cancer, chronic inflammation, and other conditions of dysregulated immunity. Non-clinical evaluation of these agents in animal models can be challenging, as the presence of an active immune state is often required in order to detect the effects of the test agent. Modulation of interleukin (IL)-10 signaling represents this type of situation in that altering IL-10 action in vivo can be difficult to appreciate in the absence of an ongoing immune response. The study presented here reports on the use of lipopolysaccharide (LPS) challenge in cynomolgus macaques to induce predictable inflammatory cytokine responses. The results showed that IL-10 receptor (IL-10R) blockade with an antagonist monoclonal antibody (mAb) dramatically enhanced the LPS-induced cytokine response, thus demonstrating in vivo pharmacologic activity of this immunomodulatory antibody. We submit that this approach could be applied to other cases where the intent of a candidate therapeutic is to modulate components of inflammatory cytokine responses.
Introduction
In recent years, a large number of immunomodulatory agents have entered clinical development and/or are approved for clinical use in the therapeutic areas of autoimmunity (Tian et al. Citation2014; Polk and Rosenwasser Citation2017), infectious diseases (Vinh Citation2014; Cutino-Moguel et al. Citation2017; Mahon and Hafner Citation2017) and cancer (Chen and Mellman Citation2013; Whiteside et al. Citation2016). These agents include modulators of cytokines or their receptors (e.g. infliximab, anakinra, and tocilizumab) and inhibitors of immune checkpoints such as CTLA-4 (e.g. ipilimumab and tremelimumab) or PD-1/PD-L1 (e.g. nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab), among others. In many cases, the biological pathways that these agents target are only available for modulation in the context of an ongoing immune response. This is most often because the biological target molecule or downstream molecules are not present without immune stimulation. As examples, under “resting” conditions, cytokines associated with inflammatory immune responses [e.g. interleukin (IL)-6, tumor necrosis factor (TNF), IL-1β, and interferon (IFN)-γ] are generally at very low or undetectable levels in the systemic circulation. Similarly, many checkpoint inhibitors (e.g. PD-1 and CTLA-4) and co-stimulatory molecules (e.g. 4-1BB, OX40, and GITR) are at very low or undetectable levels on the surface of unstimulated T-cells. This presents a challenge for evaluating effects of such molecules in non-clinical studies in animals where an overt immune response is not present to modulate.
IL-10 is a cytokine that falls into the category of potential therapeutic targets for which modulation is difficult to assess in the absence of an immune response. IL-10 plays a role in a range of immune functions. Although it can have context-dependent effects on a range of cell types, it is predominantly considered an immunosuppressive cytokine (Moore et al. Citation2001; Pestka et al. Citation2004; Mannino et al. Citation2015; Geginat et al. Citation2016). This is due to its ability to (1) decrease antigen presenting capability of macrophages and dendritic cells by decreasing expression of MHC Class II, co-stimulatory molecules (CD80/CD86), and chemokines, (2) decrease production of and responsiveness to inflammatory cytokines produced by macrophages and T-cells (particularly T-helper [TH]-1 cells), and (3) inhibit the activity of inflammatory cytokines (Moore et al. Citation2001; Pestka et al. Citation2004). In particular, IL-10 can inhibit the production of IL-6, TNF, IL-1β, and IL-12 by macrophages (Moore et al. Citation2001; Saraiva and O'Garra Citation2010), and it can limit the ability of cells to respond to IFN-γ, IL-2, TNF, and IL-4 (Pestka et al. Citation2004).
The receptor for IL-10 consists of two polypeptide chains: IL-10R1 which binds IL-10 with high affinity, and IL-10R2, which together signal via JAK1/TYK2 and STAT3 (Moore et al. Citation2001). A collaboration between Pfizer and Medarex resulted in the development of a fully human IgG4 monoclonal antibody (mAb) (PF-05179147) with antagonist activity against human and cynomolgus monkey IL-10R1, but not IL-10R1 from other species (see Results section and Supplementary Materials). As a result, the only relevant animal model for in vivo non-clinical pharmacologic characterization and safety studies was the macaque. However, as no clear measurable effects of IL-10R blockade were expected in healthy macaques in the absence of immune stimulation, the current study sought to establish an inflammatory response that would be sensitive to IL-10 modulation.
The induction of inflammatory cytokines in response to exposure to bacterial lipopolysaccharide (LPS) has been well-characterized in both humans (Lynn et al. Citation2003; Dillingh et al. Citation2014) and monkeys (Leturcq et al. Citation1996; van der Poll et al. Citation1996; Wagner et al. Citation1997; Ji et al. Citation2004; Picha et al. Citation2004). Because IL-10 limits the production of LPS-induced inflammatory cytokines in rodent models (Berg et al. Citation1995; Moore et al. Citation2001; Pestka et al. Citation2004), intravenous (IV) exposure of LPS was explored in this study to initiate reproducible and measurable cytokine responses in macaques.
This report describes the systemic cytokine response that occurs in cynomolgus macaques in response to exposure to LPS and demonstrates that blockade of IL-10R1 with an antagonist mAb can dramatically enhance LPS-induced cytokine responses in vivo. These data not only demonstrate the pharmacologic properties of a candidate anti-IL-10R1 mAb therapeutic, but also show the utility of challenging macaques with LPS to enable evaluation of the effects of candidate therapeutics on inflammatory cytokine responses.
Materials and methods
Animals
Male cynomolgus macaques (Macaca fascicularis) all >2 yr-of-age were imported from Mauritius through a commercial supplier (Shin Nippon Biomedical Laboratories, Everett, WA) and maintained at a Pfizer vivarium facility with primate housing capabilities. All animals weighed ≈ 3–6 kg upon initiation of the study. All procedures performed herein were conducted in accordance with established guidelines and regulations and were reviewed and approved by Pfizer’s Institutional Animal Care and Use Committee. The Pfizer animal care facilities that supported this work are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Antibodies
The anti-human IL-10R1 mAb, PF-05179147, is a fully human IgG4 that was co-developed by Pfizer and Medarex (Redwood City, CA). PF-05179147 was selected based on its high-affinity binding to human and cynomolgus monkey IL-10R1, lack of cross-reactivity to other related cytokine receptors and proteins, and ability to antagonize IL-10-induced signaling in a dose-dependent manner (see Results and Supplemental Materials for mAb characterization). An isotype-matched mAb specific to keyhole limpet hemocyanin (KLH), i.e. PF-05186829, was produced at Pfizer and used as a negative control in experiments.
LPS
Ultrapure-grade LPS prepared from Escherichia coli 0111:B4 (List Biologicals, Campbell, CA) was reconstituted in sterile saline at 10 µg/ml and stored in aliquots at −20 °C until use in experiments. The specific bioactivity for the individual lot (4216A2) used for this work was not available, but the predicted activity provided by the vendor based on a similar lot was ≈6.75 × 106 EU/mg (communication from List Biologicals).
In vitro LPS challenge
Whole blood was collected from animals into sodium heparin tubes (Beckton Dickinson, Caanan, CT) to prevent coagulation. Aliquots (200 µl) of each blood sample were dispensed into 96-well polypropylene plates and placed at 37 °C and 5% CO2 for 30 min. LPS diluted in sterile saline or saline only (control) was added to sample wells (10 µl volume) to achieve final LPS concentrations ranging from 0 to 10 μg/ml. The plates were incubated for an additional 5 h at 37 °C and then centrifuged at 4 °C for 10 min at 600g to pellet the cells. Plasma collected from each well was stored at −80 °C until analyzed. The concentration of TNF in each plasma sample was measured by ELISA using the Monkey TNF ELISA kit (U-CyTech, Utrecht, the Netherlands) according to manufacturer instructions. All samples were measured in duplicate.
In vivo LPS challenge and antibody-based modulation
Animals (n = 3/group) were injected IV with 1.0 µg LPS/kg in a volume of 0.1 ml/kg. The LPS was first administered on three different days – each separated by a 3-week rest period – to establish baseline LPS responses. Prior to the third LPS challenge, blood samples were collected to perform the in vitro LPS challenge (described above) in order to compare in vitro cytokine responses to those induced in vivo. Following another 3-week rest period, animals were injected IV with 0.1, 1, or 10 mg/kg of anti-IL-10R1 mAb or with 10 mg/kg of the isotype control mAb 2 h before a fourth LPS challenge was administered IV. An additional set of control animals was given 10 mg/kg anti-IL-10R1 mAb IV and left unchallenged (no LPS). After another 3-week rest period, a fifth and final LPS challenge was performed on all groups.
Blood samples were collected into serum separator tubes (Greiner Bio-One, Monroe, NC) just prior to and 1, 2, 4, 6, and 24 h after each LPS challenge to measure concentrations of cytokines (all timepoints) and C-reactive protein (CRP) (pre-challenge and 24 h post-challenge only). Blood samples were also collected just prior to and 0.25, 2, 7, 24, 30, 48, 72, 144, 240, 384, and 552 h immediately after antibody administration to measure serum anti-IL10R mAb levels. Sera prepared from the blood samples by centrifugation were stored at −80 °C (for cytokine and CRP assays) or at −20 °C (for antibody assay) until thawed and analyzed.
Cytokine and CRP measurements
Levels of IL-1β, IL-6, IFN-γ, and TNF were simultaneously quantified in thawed serum samples using a Milliplex MAP Non-Human Primate Cytokine Bead Panel reagent kit (Millipore, Burlington, MA) according to manufacturer instructions. In brief, cytokine standards or test samples were incubated with distinct sets of beads, each coated with a cytokine-specific capture antibody. Beads were washed, incubated with matched-pair biotinylated antibodies to each cytokine, and then incubated with a streptavidin–phycoerythrin (PE) conjugate. After a final wash, PE fluorescence levels were recorded for each distinct bead type in a Luminex 100 system using associated software (Bio-Rad, Hercules, CA). IL-10 was quantified using a Monkey IL-10 Cytokine ELISA kit (U-CyTech Biosciences) according to manufacturer protocols. The lower limit of quantification for the individual cytokine readouts was 5.15 pg IL-10/ml, 16.0 TNF pg/ml, and 3.2 pg/ml for IL-1β, IL-6, and IFN-γ. All samples were measured in duplicate.
Serum concentrations of CRP were measured using a using a Siemens CardioPhase™ hsCRP assay kit and a Siemens Advia 1800 clinical chemistry platform and instrumentation (Siemens Healthcare Diagnostics, Tarrytown, NY), according to manufacturer instructions. In brief, serum samples were incubated with a suspension of uniform polystyrene latex particles coated with anti-CRP antibody. Agglutination resulting from CRP binding to the antibody was detected by increases in turbidity measured at 571 nm. The serum CRP concentration (in mg/L) was extrapolated from a standard curve generated using calibrators on the platform. The limit of detection was 0.1 mg CRP/L.
Measurement of IL-10R1 mAb concentration in serum
A colorimetric ELISA was employed to measure serum concentrations of the anti-IL-10R1 mAb PF-05179147. Wells of 96-well Nunc plates (Maxisorp, VWR, Radnor, PA) were each coated with 50 μl of capture antigen (recombinant human IL-10R1 extracellular domain) at 2.5 μg/ml in phosphate-buffered saline (PBS, pH 7.4) and incubated at ≈4 °C overnight. The wells were then washed with wash buffer (10 mM phosphate buffer [pH 7.4], 150 mM NaCl, 0.05% [v/v] Tween 20) and blocked with 200 μl assay buffer (StartingBlock T20 [PBS]; Thermo Scientific, Waltham, MA) for 1 h. After washing, samples, standards, and controls were diluted 10-fold in assay buffer, aliquots of 50 µl were added to dedicated wells, and the plates incubated for 1 h at room temperature. After washing the wells, a horseradish (HRP)-conjugated mouse anti-human IgG4 secondary antibody (Invitrogen, Carlsbad, CA) was added to each well and the plates were incubated a further 1 h at room temperature. The presence of secondary antibody in each well was detected using TMB substrate (Sigma, St. Louis, MO) and then measurement of absorbance in each well at 450 nm in a Spectra Max M5 plate reader (Molecular Devices, San Jose, CA). The PF-05179147 concentration in each sample was determined using a 4-parameter fit model of the assay calibration curve in the range of 15.6–1000 ng/ml.
Results
The candidate antagonistic anti-IL-10R1 mAb (PF-05179147) was generated by immunizing humanized immunoglobulin mice against human IL-10R1 and then screening hybridomas for production of mAb reactive to both human and cynomolgus monkey IL-10R1 (see Supplemental Materials for details). The resulting mAb binds human and cynomolgus monkey IL-10R1 with similar high affinity in both soluble recombinant (Supplemental Figure S1A) and cell surface-expressed (Supplemental Figure S1B) forms. The mAb is also able, in a dose-dependent fashion, to block the ability of IL-10 to induce STAT-3-dependent intracellular signaling by inhibiting IL-10 binding to IL-10R1 on peripheral blood mononuclear cells from human and cynomolgus monkey donors (Supplemental Figure S2).
Despite its in vitro activity, the mAb was not expected to induce measurable effects in vivo in the absence of an active immune response. Thus, this work attempted to establish predictable LPS-induced cytokine responses in cynomolgus macaques in order to evaluate the pharmacologic properties of the mAb. A first step was to determine an effective dose of LPS for IV delivery that would elicit measurable cytokine responses without adversely affecting the health of the animals. Based on a previous publication (Picha et al. Citation2004) that explored macaque responses to different IV doses of LPS, administration of up to 100 µg LPS/kg was predicted to be tolerated. However, different LPS preparations can have different potencies (Erridge et al. Citation2002). Taking this into account, an in vitro dose–response experiment on cynomolgus macaque blood samples was also performed to characterize the commercially supplied batch of LPS intended for in vivo use. It was predicted that the dose–response determined in vitro would help guide dose level selection as well as identify animals likely to respond in vivo.
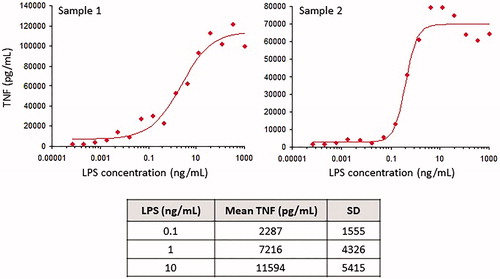
In all blood samples tested, LPS induced production of TNF in a concentration-dependent manner; the linear portion of the dose–response curve fell between ≈ 0.1 and 10 ng/ml (). As successful antagonism of immunosuppressive IL-10 signaling in vivo was expected to exacerbate LPS-induced cytokine responses, a dose of 1 µg LPS/kg was selected. This dose was crudely estimated using the assumptions of 3 kg for the smallest animals and 300-ml blood volume, such that 1 μg/kg (or 3 μg/animal) would theoretically result in a maximal blood concentration of 10 ng/ml, which was within the linear portion of the in vitro dose-response curve. The anticipated response level, if reproduced in vivo, would potentially allow detection of enhanced cytokine responses following IL-10R1 antagonism.
Figure 1. In vitro cytokine responses to LPS. Cynomolgus macaque blood samples were stimulated with various concentrations of LPS for 5 h and then plasma was collected from each well. TNF concentrations in the plasma samples were determined by ELISA. Top panels show dose–response curves from two different animals; the table shows the mean concentrations of TNF (±SD) from samples from 17 individual animals incubated with 0.1, 1, or 10 ng/ml LPS.
To characterize the in vivo cytokine responses of cynomolgus macaques to LPS, 15 animals were challenged with 1 μg LPS/kg by IV injection every 3 weeks (a total of three separate challenges; see for study design). Repeat LPS exposures were included in the study design to assess cytokine response reproducibility and determine whether multiple LPS exposures would result in blunted cytokine responses, a phenomenon reported previously in other species when animals are given multiple endotoxin challenges within a short timeframe (Fraker et al. Citation1988; Randow et al. Citation1995; Döcke et al. Citation1997; Grutz, Citation2005; Lopez-Collazo and del Fresno Citation2013). The LPS injections were spaced apart by 3 weeks with the intent to minimize this known desensitization phenomenon. Following the first LPS challenge, all 15 animals showed measurable increases in serum levels of multiple cytokines (). The hierarchy of the magnitude of peak cytokine concentrations was: IL-6 > TNF > IL-10 > IFN-γ > IL-1β. The timing of the peak cytokine responses depended on the cytokine, with TNF peaking at 1 h, IL-6 at 2 h, IL-1β and IL-10 at 1–2 h, and IFN-γ at 2–4 h after LPS challenge. Additionally, all 15 animals showed increases at 24 h in serum CRP (), an acute phase protein which is induced by inflammatory cytokines such as IL-6 (Li et al. Citation1990).
Figure 2. LPS challenge study design. Cynomolgus macaques (n = 3/group) were challenged IV with LPS (1.0 µg/kg) on three occasions – each separated by 3 weeks – to establish a baseline LPS response. After an additional 3 weeks, a fourth LPS challenge was administered 2 h after IV injection of 0.1, 1, or 10 mg/kg of the anti-IL-10R1 mAb PF-05179147, 10 mg/kg of isotype control mAb PF-05186829, or vehicle control. As an additional control, the anti-IL-10R1 mAb was injected IV into animals that were not challenged with LPS. After another 3 weeks, a fifth and final LPS challenge was given to all groups. Serum cytokine concentrations were measured in samples collected just prior to each LPS challenge and 1, 2, 4, 6, and 24 h after each LPS challenge; serum CRP concentrations were assessed just prior to and 24 h after each LPS challenge. The serum concentration of anti-IL-10R1 mAb was measured before and at various timepoints after its injection.
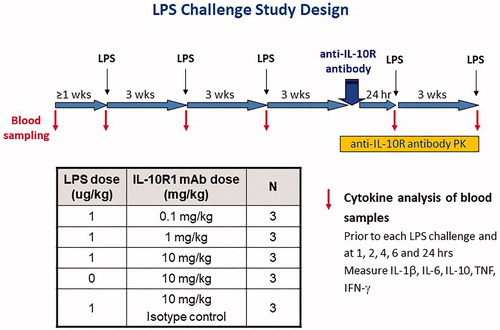
Figure 3. Baseline cytokine and CRP responses to LPS in vivo. Cynomolgus macaques were administered three LPS challenges (indicated by blue arrows), each separated by 3 weeks, and serum cytokines were measured at timepoints before (time “0”) and after LPS challenge as indicated on the x-axis of each plot. Each plot shows the serum concentration (in pg/ml) of a given cytokine or CRP (in mg/L); each line represents data from an individual animal (n = 15).
Though the LPS injections were spaced 3 weeks apart, there were some cytokines (IL-6 and IFN-γ) for which serum concentrations following the second or third challenges were lower than the concentrations observed after the first LPS challenge (). However, there did not appear to be a further diminution of the cytokine response from the second to third challenge. The clear exception was IL-10 whose levels continued to increase with each LPS challenge. IL-10 also peaked earlier after the second and third challenges (1 h) as compared with the first challenge (2 h in most animals).
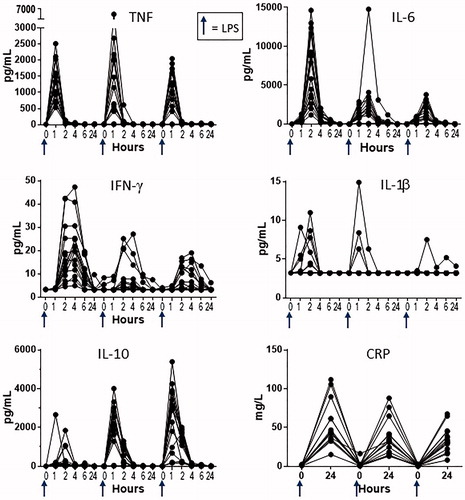
To determine whether cytokine responses induced by LPS in vitro correlated with those induced in vivo, blood samples were collected from each animal just prior to the third LPS challenge and stimulated with LPS. The concentrations of IL-6 and TNF obtained in plasma samples following in vitro LPS challenge showed no correlation (p > 0.4) with the peak cytokine concentrations measured in serum from the same animals following in vivo LPS challenge (). The in vitro vs. in vivo correlation for IFN-γ and IL-1β was not determined because only in a small minority of cases were there detectable levels of cytokine in both in vitro and in vivo samples.
Figure 4. In vitro vs. in vivo cytokine responses. In vitro cytokine responses: whole blood from the same cynomolgus macaques as described in was collected (just prior to the third in vivo LPS challenge) and incubated 5 h with different concentrations of LPS in vitro. Plasma was then collected and cytokine concentrations in the plasma were measured. In vivo cytokine responses: the same animals were challenged IV with LPS (immediately after sample collection for in vitro responses) and cytokines were measured from serum prepared from blood collected 1, 2, 4, 6, and 24 h after the LPS challenge. Each plot shows a different cytokine (as indicated). Each symbol represents the cytokine concentration obtained in vitro (x-axis) vs. the highest concentration observed in vivo (y-axis) from an individual animal (n = 15). IL-6 and TNF were not significantly correlated (p > 0.4, Pearson correlation). ND = not determined (cytokine concentration in in vitro and/or in vivo sample was below limit of detection of the assay for most animals).
Having established the levels of cytokines to expect with LPS challenge (), it was evaluated whether blocking signaling through IL-10R with the anti-IL-10R1 mAb would block the suppressive effect of IL-10 and therefore enhance inflammatory cytokine responses to LPS (). Following administration of anti-IL-10R1 mAb at 0.1, 1, and 10 mg/kg, a dramatic dose-dependent increase was observed in the LPS-induced concentrations of IL-6, TNF, IFN-γ, and IL-1β, when compared to LPS-induced cytokine concentrations measured in the absence of anti-IL-10R1 mAb treatment (). Furthermore, elevated cytokine responses in animals administered LPS in the presence of 10 mg/kg anti-IL-10R1 mAb correlated with clinical signs, including emesis, decreased activity, hunched posture, and decreased skin turgor. These clinical signs were not observed in any other group.
Figure 5. Impact of IL-10R1 blockade on LPS-induced cytokine responses. As outlined in , the 15 cynomolgus macaques described in and were split into five separate groups of three animals. Animals were challenged with LPS at timepoints indicated by blue arrows. Just prior to the fourth LPS administration, the anti-IL-10R1 mAb PF-05179147 (larger green arrows) or an isotype control mAb (open larger arrows) was administered at the doses indicated in column labels. Additional control animals shown in the second column from the right were administered PF-05179147 (larger green arrow) without an LPS challenge. Serum cytokines indicated by row labels were measured following dosing. Each line shows cytokine concentrations in serum samples collected from an individual animal (n = 3/group) over time. Note: data for the first three LPS challenges (see ) are included as a comparison and are often not visible because of the scale adjustment to capture the cytokine concentrations obtained in the presence of the anti-IL-10R1 mAb.
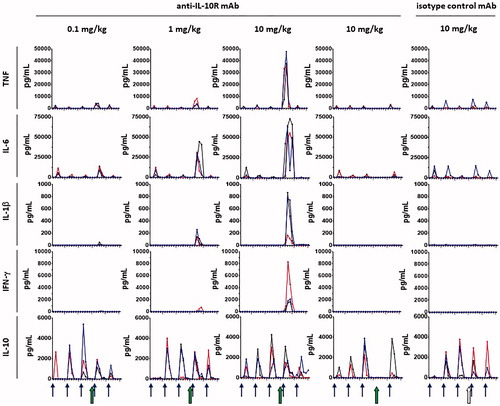
In contrast, no increase in cytokines was observed over the previous LPS challenges when an isotype control mAb was administered (at 10 mg/kg) prior to LPS, confirming that the effect of anti-IL-10R1 mAb was related to its specificity for IL-10R1. Also, when anti-IL-10R1 mAb was administered without the LPS challenge, there was little to no cytokine induced, indicating that anti-IL-10R1 mAb was potentiating the cytokine response to LPS, rather than directly inducing cytokine release. This latter observation also confirmed that the effect of the anti-IL-10R1 mAb was difficult to appreciate in the absence of an LPS-induced cytokine response.
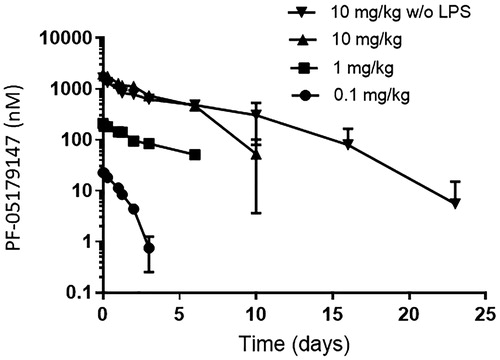
A final fifth LPS challenge was performed to test the ability of animals to mount an inflammatory cytokine response 3 weeks after the anti-IL-10R1 mAb was administered. Based on the pharmacokinetic profiles of the anti-IL-10R1 mAb administered at 0.1, 1.0, or 10 mg/kg (), it was not anticipated that effects would persist for more than 3 weeks. Indeed, the serum cytokine concentrations detected after the fifth LPS challenge were generally similar to those induced by the first three LPS challenges in the absence of the anti-IL-10R1 mAb (), indicating that the effects of the anti-IL-10R1 mAb persisted less than 3 weeks.
Figure 6. Serum concentration of anti-IL-10R1 mAb. At the indicated timepoints following IV administration of PF-05179147 to cynomolgus macaques at 0.1, 1.0, or 10 mg/kg – with or without (w/o) LPS, the concentration of PF-05179147 was measured in serum samples. Plots show the mean (±SD) concentration vs. time profiles for each dose group (n = 3/group) as indicated.
Discussion
As described in this report, LPS challenge in cynomolgus macaques has the potential to be used for testing effects of molecules intended to modulate immune responses characterized by inflammatory cytokines. In this setting, the anti-IL-10R1 mAb, PF-05179147, dramatically potentiated the production of several inflammatory cytokines (IL-1β, IL-6, TNF, and IFN-γ), revealing clear pharmacologic activity that was not observed in normal healthy animals maintained under standard care conditions.
The LPS induced an increase of at least one cytokine in samples from all animals tested, both in vitro and in vivo. However, the concentrations of cytokines produced in vitro following exposure of blood samples to LPS did not correlate with peak serum cytokine concentrations produced in vivo. This may be in part because cell types not represented in blood (e.g. tissue macrophages and Kupffer cells) are able to produce cytokines in response to LPS, or certain cells may produce cytokines secondarily upon exposure to other LPS-induced cytokines in vivo. Regardless, the data show that in vitro cytokine responses cannot quantitatively predict in vivo cytokine responses. Therefore, in vitro assays could not be used as a substitute for in vivo challenge, at least in this setting.
Peak serum cytokine concentrations in cynomolgus macaques were generally higher following an initial challenge with LPS when compared with the concentrations following subsequent LPS challenges. This phenomenon of desensitization of cytokine responses is well-established in models of endotoxin challenge and sepsis in rodents and other species (Grutz Citation2005). Interestingly, this phenomenon is now being exploited clinically as a way to mitigate cytokine release with immune stimulatory therapeutics such as T-cell-recruiting bi-specific molecules (Benjamin and Stein Citation2016). The inverse relationship observed here between inflammatory cytokines (lower upon subsequent doses) and IL-10 (higher upon subsequent doses), as well as the earlier and more robust IL-10 production after multiple LPS challenges, suggests that IL-10 contributes to the blunting of inflammatory cytokines upon multiple LPS challenges. Indeed, the data showing that IL-10R1 blockade dramatically increases LPS-induced levels of IL-1β, IL-6, TNF, and IFN-γ clearly demonstrate that IL-10 limits the production of inflammatory cytokines. Therefore, as has been observed in mice (Berg et al. Citation1995) and rhesus macaques (Asiedu et al. Citation2007), IL-10 is a key negative regulator of inflammatory cytokines in cynomolgus macaques.
Consistent with this, IL-10 is important for limiting the detrimental effects of inflammatory cytokines and subsequent pathology during infection (Jakobshagen et al. Citation2015; Claser et al. Citation2017), and polymorphisms in IL-10 leading to lower IL-10 expression are associated with increased susceptibility to sepsis (Gallagher et al. Citation2003; Schaaf et al. Citation2003). These findings, along with the data presented here, suggest that the anti-IL-10R1 mAb could enhance inflammatory responses in disease settings where IL-10 actively suppresses the responses. Such an effect has been shown to be beneficial in animal models of persistent infection (Brooks et al. Citation2006) and cancer (Vicari et al. Citation2002; Guiducci et al. Citation2005; Newton et al. Citation2014).
The use of LPS challenge enabled a clear demonstration of pharmacologic activity of the anti-IL-10R1 mAb, which was not appreciated in “resting” animals, without an ongoing cytokine response to modulate. In this case, the increase of cytokines was sufficiently robust so that in retrospect, it would not have been necessary to establish a baseline response with multiple LPS challenges prior to evaluating the anti-IL-10R1 mAb. However, this and other aspects of experimental design may have to be considered when using LPS challenge to test effects of other immunomodulatory molecules. Of note, LPS challenge of cynomolgus macaques has previously been shown to have either no effect, or a modest and/or transient effect on most parameters commonly measured in non-clinical studies, including most hematology and clinical chemistry parameters (Picha et al. Citation2004). As a result, LPS challenge may be feasible in the context of non-clinical toxicity evaluation in cynomolgus macaques, since the response to LPS is anticipated to minimally impact measures of toxicity.
The LPS challenge procedure in cynomolgus macaques presented here highlights that in cases where it is required, the use of a relevant immune response can be a valuable tool for revealing pharmacologic activity of immunomodulatory therapeutics. Given the increased recent focus on modulating the immune system for therapeutic benefit, the need for such non-clinical models to evaluate new candidate therapies is expected to increase in parallel.
Supplemental Material
Download PDF (375 KB)Acknowledgements
The authors wish to acknowledge: John M. Marcek for his role as study director for the monkey study; Haichun Huang and the hybridoma generation group at Medarex for producing the anti-IL-10R1 mAb; Jing Min, Danielle Meyer, and Barrett Thiele at Pfizer for antibody re-engineering efforts to produce PF-05179147; Simon Bergqvist, Dayna Kappenman, Fausto Maldonado, Qi Guan, and George Smith at Pfizer for the in vitro characterization of the binding and neutralization properties of PF-05179147; James Alvey and Elsa Barbacci-Tobin at Pfizer for assisting with the cytokine analyses; and Wenlin Li at Pfizer for pharmacokinetic analysis of PF-05179147 in cynomolgus macaque serum.
Disclosure statement
All the authors are current employees of Pfizer Inc. and stockholders.
Additional information
Funding
References
- Asiedu C, Guarcello V, Deckard L, Jargal U, Gansuvd B, Acosta E, Thomas J. 2007. Cloning and characterization of recombinant rhesus macaque IL-10/Fc(ala-ala) fusion protein: A potential adjunct for tolerance induction strategies. Cytokine. 40(3):183–192.
- Benjamin J, Stein A. 2016. The role of blinatumomab in patients with relapsed/refractory acute lymphoblastic leukemia. Ther Adv Hematol. 7(3):142–156.
- Berg DJ, Kühn R, Rajewsky K, Müller W, Menon S, Davidson N, Grünig G, Rennick D. 1995. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 96(5):2339–2347.
- Brooks D, Trifilo M, Edelmann K, Teyton L, McGavern D, Oldstone M. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 12(11):1301–1309.
- Chen D, Mellman I. 2013. Oncology meets immunology: The cancer-immunity cycle. Immunity. 39(1):1–10.
- Claser C, de Souza J, Thorburn S, Grau G, Riley E, Renia L, Hafalla J. 2017. Host resistance to plasmodium-induced acute immune pathology is regulated by IL-10 receptor signaling. Infect Immun. 85(6):1–9.
- Cutino-Moguel M, Eades C, Rezvani K, Armstrong-James D. 2017. Immunotherapy for infectious diseases in hematological immunocompromise. Br J Haematol. 177(3):348–356.
- Dillingh M, van Poelgeest E, Malone K, Kamper E, Stroes E, Moerland M, Burggraaf J. 2014. Characterization of inflammation and immune cell modulation induced by low-dose LPS administration to healthy volunteers. J. Inflamm. 11:1–9.
- Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. 1997. Monocyte deactivation in septic patients: Restoration by IFN-γ treatment. Nat. Med. 3(6):678–681.
- Erridge C, Bennett-Guerrero E, Poxton I. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4(8):837–851.
- Fraker D, Stovroff M, Merino M, Norton J. 1988. Tolerance to tumor necrosis factor in rats and the relationship to endotoxin tolerance and toxicity. J Exp Med. 168(1):95–105.
- Gallagher P, Lowe G, Fitzgerald T, Bella A, Greene C, McElvaney N, O'Neill S. 2003. Association of IL-10 polymorphism with severity of illness in community acquired pneumonia. Thorax. 58(2):154–156.
- Geginat J, Larghi P, Paroni M, Nizzoli G, Penatti A, Pagani M, Gagliani N, Meroni P, Abrignani S, Flavell RA. 2016. The light and the dark sides of IL-10 in immune-mediated diseases and cancer. Cytokine Growth Factor Rev. 30:87–93.
- Grutz G. 2005. New insights into the molecular mechanism of IL-10-mediated immunosuppression. J. Leukocyte Biol. 77:3–15.
- Guiducci C, Vicari A, Sangaletti S, Trinchieri G, Colombo M. 2005. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 65(8):3437–3446.
- Jakobshagen K, Ward B, Baschuk N, Huss S, Brunn A, Malecki M, Fiolka M, Rappl G, Corogeanu D, Karow U, et al. 2015. Endogenous IL-10 alleviates systemic anti-viral cellular immune response and T-cell-mediated immunopathology in select organs of acutely LCMV-infected mice. Am J Pathol. 185(11):3025–3038.,
- Ji X, Sun K, Feng Y, Yin G. 2004. Changes of inflammation-associated cytokine expressions during early phase of experimental endotoxic shock in macaques. World J Gastroenterol. 10(20):3026–3033.
- Leturcq D, Moriarty A, Talbott G, Winn R, Martin T, Ulevitch R. 1996. Antibodies against CD14 protect primates from endotoxin-induced shock. J Clin Invest. 98(7):1533–1538.
- Li S, Liu T, Goldman N. 1990. Cis-acting elements responsible for IL-6-inducible C-reactive protein gene expression. J Biol Chem. 265(7):4136–4142.
- Lopez-Collazo E, del Fresno C. 2013. Pathophysiology of endotoxin tolerance: Mechanisms and clinical consequences. Crit Care. 17(6):242
- Lynn M, Rossignol DP, Wheeler JL, Kao RJ, Perdomo CA, Noveck R, Vargas R, D'Angelo T, Gotzkowsky S, McMahon FG. 2003. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J Infect Dis. 187(4):631–639.
- Mahon R, Hafner R. 2017. Applying precision medicine and immunotherapy advances from oncology to host-directed therapies for infectious diseases. Front Immunol. 8:688.
- Mannino M, Zhu Z, Xiao H, Bai Q, Wakefield M, Fang Y. 2015. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 367(2):103–107.
- Moore K, de Waal Malefyt R, Coffman R, O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 19:683–765.
- Newton MR, Askeland EJ, Andresen ED, Chehval VA, Wang X, Askeland RW, O'Donnell MA, Luo Y. 2014. Anti-IL-10R1 monoclonal antibody in combination with bacillus Calmette-Guerin is protective against bladder cancer metastasis in a murine orthotopic tumor model and demonstrates systemic specific anti-tumor immunity. Clin Exp Immunol. 177(1):261–268.
- Pestka S, Krause C, Sarkar D, Walter M, Shi Y, Fisher P. 2004. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 22:929–979.
- Picha K, Tam S, Khandekar V, Soderman A, Martin E, Jordan R, Bugelski P. 2004. Effects of LPS on toxicologically-important endpoints in cynomolgus macaques. Preclinica. 2:427–434.
- Polk B, Rosenwasser L. 2017. Biological therapies of immunologic diseases: Strategies for immunologic interventions. Immunol Allergy Clin North Am. 37(2):247–259.
- Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk H. 1995. Mechanism of endotoxin desensitization: Involvement of IL-10 and TGFβ. J Exp Med. 181(5):1887–1892.
- Saraiva M, O'Garra A. 2010. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 10(3):170–181.
- Schaaf BM, Boehmke F, Esnaashari H, Seitzer U, Kothe H, Maass M, Zabel P, Dalhoff K. 2003. Pneumococcal septic shock is associated with the IL-10-1082 gene promoter polymorphism. Am J Respir Crit Care Med. 168(4):476–480.
- Tian G, Li J, Wang D, Zhou D. 2014. Targeting IL-10 in autoimmune diseases. Cell Biochem Biophys. 70(1):37–49.
- van der Poll T, Jansen J, Levi M, ten Cate H, Hack CE, Aarden LA, ten Cate JW, van Deventer SJ. 1996. Interleukin-10 release during endotoxemia in chimpanzees: Role of platelet-activating factor and IL-6. Scand J Immunol. 43(1):122–125.
- Vicari AP, Chiodoni C, Vaure C, Aït-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP, et al. 2002. Reversal of tumor-induced dendritic cell paralysis by CpG immuno-stimulatory oligonucleotide and anti-IL-10 receptor antibody. J Exp Med. 196(4):541–549.
- Vinh D. 2014. Cytokine immunomodulation for the treatment of infectious diseases: Lessons from primary immunodeficiencies. Exp Rev Clin Immunol. 10(8):1069–1100.
- Wagner T, Horton V, Carlson G, Myhre P, Gibson S, Imbertson L, Tomai M. 1997. Induction of cytokines in cynomolgus monkeys by the immune response modifiers, imiquimod, S-27609, and S-28463. Cytokine. 9(11):837–845.
- Whiteside T, Demaria S, Rodriguez-Ruiz M, Zarour H, Melero I. 2016. Emerging opportunities and challenges in cancer immunotherapy. Clin Cancer Res. 22(8):1845–1855.
