Abstract
The coronavirus SARS-CoV-2 of 2019 (COVID-19) causes a pandemic that has been diagnosed in more than 70 million people worldwide. Mild-to-moderate COVID-19 symptoms include coughing, fever, myalgia, shortness of breath, and acute inflammatory lung injury (ALI). In contrast, acute respiratory distress syndrome (ARDS) and respiratory failure occur in patients diagnosed with severe COVID-19. ARDS is mediated, at least in part, by a dysregulated inflammatory response due to excessive levels of circulating cytokines, a condition known as the “cytokine-storm syndrome.” Currently, there are FDA-approved therapies that attenuate the dysregulated inflammation that occurs in COVID-19 patients, such as dexamethasone or other corticosteroids and IL-6 inhibitors, including sarilumab, tocilizumab, and siltuximab. However, the efficacy of these treatments have been shown to be inconsistent. Compounds that activate the vagus nerve-mediated cholinergic anti-inflammatory reflex, such as the α7 nicotinic acetylcholine receptor agonist, GTS-21, attenuate ARDS/inflammatory lung injury by decreasing the extracellular levels of high mobility group box-1 (HMGB1) in the airways and the circulation. It is possible that HMGB1 may be an important mediator of the “cytokine-storm syndrome.” Notably, high plasma levels of HMGB1 have been reported in patients diagnosed with severe COVID-19, and there is a significant negative correlation between HMGB1 plasma levels and clinical outcomes. Nicotine can activate the cholinergic anti-inflammatory reflex, which attenuates the up-regulation and the excessive release of pro-inflammatory cytokines/chemokines. Therefore, we hypothesize that low molecular weight compounds that activate the cholinergic anti-inflammatory reflex, such as nicotine or GTS-21, may represent a potential therapeutic approach to attenuate the dysregulated inflammatory responses in patients with severe COVID-19.
Introduction
The coronavirus disease of 2019 (COVID-19) caused by SARS-CoV-2 is a complex disease that can produce acute and chronic dysfunction in pulmonary, cardiovascular, neuropsychological, renal, musculoskeletal, and gastrointestinal tissues (Farsalinos, Angelopoulou, et al. Citation2020; Rothan and Byrareddy Citation2020). Current CDC estimates suggest that 81% of patients with COVID-19 experience mild-to-moderate symptoms, including cough, shortness of breath, and mild forms of pneumonia (To et al. Citation2020). In addition, 14 and 5% of patients diagnosed with COVID-19 experience severe-to-critically severe symptoms, respectively, which include dyspnea, hypoxia, respiratory failure, and multiple organ failure (To et al. Citation2020). In the United States, ≈19% of all COVID-19 patients require hospitalization, of which 6% are admitted to intensive care units (ICU) (Li, et al. Citation2020). Although there is a wide range of symptoms for COVID-19, ≈3 to 17% of COVID-19 patients are diagnosed with one of the hallmarks of severe COVID-19 (Guan et al. Citation2020; Huang et al. Citation2020; Wu et al. Citation2020), acute respiratory distress syndrome (ARDS), that requires patients to be treated with high flow oxygen as a life-saving intervention (Coperchini et al. Citation2020). Ultimately, of the critically severe COVID-19 patients admitted into ICU, the mortality rate is 39–72% (Huang et al. Citation2020; Wu et al. Citation2020; Yang et al. Citation2020).
During the early stages of the COVID-19 pandemic, clinical data suggested that ≈14% of all patients were shown to have bilateral ground-glass opacities in the lung and 75% of all patients were diagnosed with pneumonia (Chen, Zhou, et al. Citation2020). Moreover, this hallmark symptom of lung inflammation in severe COVID-19 has recently been hypothesized to result from a “cytokine-storm”, characterized by excessive levels of pro-inflammatory cytokines and chemokines that exacerbate lung injury and promote multiple organ failure (Coperchini et al. Citation2020).
The cytokine storm is mediated, in part, by a dysregulated response of the innate immune system that results in high plasma levels of tumor necrosis factor (TNF)-α and interleukins, including but not limited to, IL-1β, IL-8, and IL-6, and certain chemokines (Lee et al. Citation2014; Fajgenbaum and June Citation2020). In the context of COVID-19, five separate cohort studies have reported that the plasma levels of IL-6 range from 7–64 ng/ml in patients with severe disease phenotypes, which has previously been thought to be a major mediator of acute lung injury and ARDS (Sinha et al. Citation2020). However, as reported in three large cohort lung study groups (i.e. ALVEOLI, FACT, SAILSS), non-COVID-19 patients diagnosed with a hyper-inflammatory phenotype of ARDS had IL-6 plasma levels that were 10- to 200-fold higher than in patients with severe COVID-19 (Sinha et al. Citation2020). Currently, the IL-6 monoclonal antibodies tocilizumab, sarilumab, and siltuximab, which decrease IL-6 levels, are authorized by the United States Food and Drug Administration for use in COVID-19 patients (COVID-19 Treatment Guidelines Panel Citation2020a, 2020b) (clinical trials NCT04306705 and NCT04322773). Internationally, there are concerns about the use of anti-IL-6 therapies as they increase the risk/rate of opportunistic infections (Calabrese et al. Citation2020). Moreover, clinically-relevant models that simulate the use of oxygen therapy for prolonged periods of time, such as in those with severe COVID-19 infection, suggest that the over-expression of IL-6 is protective for oxygen-induced lung injury in mice (Ward et al. Citation2000). Thus, it is still unclear what definitive role IL-6 has in the pathophysiology of COVID-19, prompting investigators to continue searching for other possible therapeutic targets.
A recent epidemiological study reported that Chinese smokers were statistically less likely to be hospitalized due to COVID-19 (Gonzalez-Rubio et al. Citation2020). Non-peer-reviewed clinical observations in France indicated that current smokers were less likely (<5% of all COVID-19 patients) to be infected with SARS-CoV-2, although they did worse once infected (Changeux et al. Citation2020). These studies have been widely debated in light of the harm caused by smoking but have led to the hypothesis that inhaled nicotine from tobacco may attenuate the rates of infection or the severity of COVID-19 (Gonzalez-Rubio et al. Citation2020; Hartmann-Boyce and Lindson Citation2020; Karanasos et al. Citation2020; Leung et al. Citation2020; Tindle et al. Citation2020). Conversely, several meta-analyses of published research suggest that smoking is positively correlated with the risk of severe disease in hospitalized COVID-19 patients and progression of COVID-19 (Karanasos et al. Citation2020; Patanavanich and Glantz Citation2020). Our group independently analyzed 41 international studies that included complete patient data for active smokers never smokers, and markers for severe COVID-19 symptoms, including ICU admission, invasive ventilation, and death (). Using a univariate odds ratio analysis, the analyses confirmed that smokers had a 232.85% higher risk of experiencing severe COVID-19 symptoms (OR: 2.699; 95% CI: 1.627–2.954; p < 0.0001) compared to nonsmokers (OR: 0.3705; 95% CI: 0.338–0.615) (). However, while smoking increased the risk of severe COVID-19, nicotine has anti-inflammatory efficacy which activates the cholinergic anti-inflammatory pathway by stimulating α7 nicotinic cholinergic receptors (α7nAChR) (Borovikova et al. Citation2000; Tracey Citation2007; Ni et al. Citation2011; Hartmann-Boyce and Lindson Citation2020; Tindle et al. Citation2020).
Figure 1. Meta-Analysis of the prevalence of severe COVID-19 in smoking and nonsmoking patients from 41 international studies. Severe and non-severe COVID-19 patient data were collected from 41 different worldwide studies from current smokers and nonsmokers (n = 49,156). Patients were considered to have severe disease if they required ICU admission, invasive mechanical ventilation, or died from COVID-19. The odds ratio of smoking vs. severity and nonsmoking vs. severity was determined using a contingency analysis. Odds ratio of severity for COVID-19 smoker patients is 2.699 (95% CI: 1.627–2.954), which is 232.85% higher than COVID-19 nonsmoker patients with the odds ratio of 0.3705 (95% CI: 0.338–0.615; p < 0.0001).
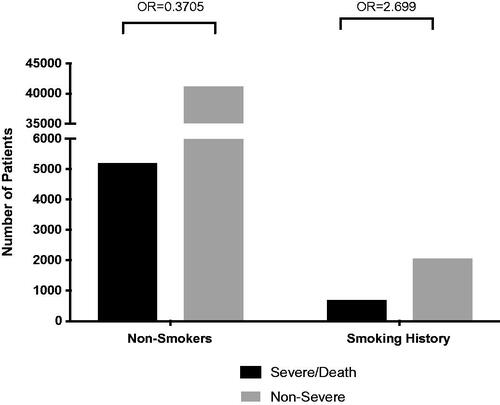
Table 1. Number of COVID-19 patients with severe symptoms and smoking history from 41 international studies.
The hyperinflammation in COVID-19 patients has been postulated to result from, in part, the dysfunction of the cholinergic anti-inflammatory pathway (Andersson et al. Citation2020; Farsalinos, Angelopoulou, et al. Citation2020; Gonzalez-Rubio et al. Citation2020; Mazloom Citation2020). The fundamental foundation of this hypothesis is the COVID-19-induced imbalance in cytokine secretion is normally regulated by vagus nerve-mediated cholinergic signaling (Andersson et al. Citation2020; Gonzalez-Rubio et al. Citation2020; Sinha et al. Citation2020). Cholinergic anti-inflammatory activity is initiated by the afferent vagus nerve following the detection of pathogens, damage-associated molecular patterns or certain cytokines/chemokines (Pavlov et al. Citation2003). The afferent vagus nerve relays signals to the central nervous system, which communicates regulatory activity via the efferent vagus nerve that signals effector organs. In the lungs, neuroendocrine cells receive efferent vagus nerve innervation that forms the parasympathetic nervous system relay (Wu et al. Citation2014). The activation of these pulmonary neuroendocrine cells in the distal airways induces the release of acetylcholine and modulates the local inflammatory environment by binding and activating α7nAChR on alveolar macrophages and other cells and tissues (Borovikova et al. Citation2000; Pavlov et al. Citation2003; Wang et al. Citation2003; Wu et al. Citation2014).
Severely ill COVID-19 patients receive high flow oxygen, noninvasive, or invasive mechanical ventilation. It is unclear whether cumulative lung injury in these patients is due only to viral pathogenesis or from a combination of viral infection (Fan et al. Citation2020) and oxygen therapy. In clinically relevant laboratory models that simulate the use of oxygen therapy, prolonged exposure to high concentrations of oxygen (i.e. hyperoxia) can directly injure the lungs (Entezari et al. Citation2014; Patel et al. Citation2020; Sitapara, Gauthier, Patel, et al. Citation2020; Sitapara, Gauthier, Valdés-Ferrer, et al. Citation2020; Ward et al. Citation2000). Furthermore, as in certain COVID-19 patients, the cytokine storm occurs in animal models of hyperoxia-induced inflammatory lung injury (Entezari et al. Citation2014; Patel et al. Citation2013; Moon et al. Citation2015). In mice with hyperoxia-induced inflammatory lung injury, the systemic administration of the α7 nicotinic acetylcholine receptor agonist, GTS-21, activates α7nAChR-mediated cholinergic anti-inflammatory signaling and attenuates lung injury in mice (Andersson et al. Citation2020; Sitapara, Gauthier, Patel, et al. Citation2020; Sitapara, Gauthier, Valdés-Ferrer, et al. Citation2020). GTS-21 can inhibit the inflammatory response by activating anti-inflammatory and antioxidant pathways (Uteshev Citation2014; Wang, Cai, et al. Citation2020). GTS-21 has been previously evaluated in animal models of inflammatory diseases, including experimental sepsis (Pavlov et al. Citation2007; Kang et al. Citation2014; Tarnawski et al. Citation2018; Mavropoulos et al. Citation2017). In addition, GTS-21 has been evaluated in human clinical trials and has a favorable safety profile at doses up to 450 mg/day (Kitagawa et al. Citation2003; Kox et al. Citation2011). Furthermore, ivermectin, an FDA-approved anti-parasitic drug, has also been reported to have positive allosteric modulator activity on α7nAChR (Krause et al. Citation1998; de Melo et al. Citation2020). Interestingly, ivermectin also inhibits the nuclear import of SARS-CoV-2 proteins, which inhibits viral replication (Pandey et al. Citation2020). Therefore, a better understanding the effects of α7nAChR activation on the regulation of the hyper-inflammatory phenotype in COVID-19, using compounds such as GTS-21, may provide more insightful information about the treatment of COVID-19.
GTS-21 attenuates inflammation by significantly decreasing plasma and airway levels of HMGB1 (Sitapara, Gauthier, Patel, et al. Citation2020; Sitapara, Gauthier, Valdés-Ferrer, et al. Citation2020). Importantly, increased plasma HMGB1 has recently been shown to be significantly correlated with more adverse clinical COVID-19 outcomes and increased ICU hospitalizations and death rates (Chen, Long, et al. Citation2020; Chen, Yu, et al. Citation2020). Thus, compounds, such as nicotine or GTS-21, that activate α7nAChR-mediated cholinergic anti-inflammatory signaling or other modulators that decrease HMGB1 secretion, could potentially decrease the complications associated with COVID-19 disease ().
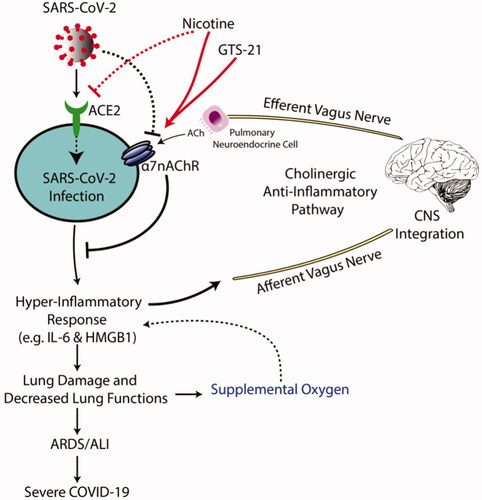
Figure 2. Proposed mechanisms of GTS-21 in α7nAChR-mediated cholinergic anti-inflammatory pathway to attenuate inflammatory lung injury produced by SARS-CoV-2 infection. SARS-CoV-2 infects target cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor. Subsequently, SARS-CoV-2 is taken into the cell by endocytosis and ultimately produces a hyper-inflammatory response, known as the “cytokine storm response syndrome.” The SARS-CoV-2-induced inflammation can damage lung tissue, producing impaired lung function, which is treated with supplemental oxygen therapy (OT). However, a subset of these patients developed acute respiratory distress syndrome (ARDS)/acute lung injury (ALI), which can occur in severe cases of SARS-CoV-2 infection. Patients with ARDS/ALI require prolonged supplemental OT using non-invasive and invasive mechanical ventilation. Although supplemental OT can be a life-saving intervention, prolonged exposure to high concentrations of oxygen (i.e. hyperoxia) can also exacerbate the hyper-inflammatory response, establishing a pathological inflammatory cycle. Under homeostatic conditions, inflammatory signals can be regulated by the cholinergic anti-inflammatory pathway. In response to pathogens, damage-associated molecular patterns and cytokines, the afferent vagus nerve senses these signals and relays information to the central nervous system. the efferent vagus nerve communicates with the effector cells in the lung, the pulmonary neuroendocrine cell, and releases acetylcholine (ACh). Acetylcholine then binds to lung cell α7nAChR on parenchyma and innate immune cells. The activation of α7nAChR by acetylcholine down-regulates the excessive production/secretion of pro-inflammatory cytokines and chemokines (including IL-6 and HMGB1). Therefore, α7nAChR agonists, like nicotine and GTS-21, may have a protective role against the severe symptoms of SARS-CoV-2 infection.
Recently, HMGB1 has gained attention as a target for treating COVID-19 (Andersson et al. Citation2020). In other disease models, extracellular HMGB1 has been shown to form complexes with viral and bacterial pathogens and damage-associated molecules (Hreggvidsdottir et al. Citation2009; Wang et al. Citation2019). These HMGB1 complexes are endocytosed by the protein, receptor for advanced glycation end-products, on innate immune cells, such as macrophages (Yang et al. Citation2019). Internalized HMGB1-complexes can activate cytosolic receptors which induce the formation of inflammasomes and cell death pathways (Lu et al. Citation2014; Andersson et al. Citation2020). It has been hypothesized HMGB1 can form complexes with SARS-CoV-2 RNA fragments that can be taken up into innate immune cells to further exacerbate the host’s innate immune system-mediated hyper-inflammatory response to SARS-CoV-2 infection. In addition to decreasing HMGB1 secretion, the activation of macrophage α7nAChR by small molecule agonists (e.g. acetylcholine and GTS-21) inhibits inflammasome activation by attenuating the endocytosis of HMGB1-LPS complexes into certain cells (Lu et al. Citation2014; Yang et al. Citation2019).
Recent epidemiological studies have reported that men and women have similar prevalence rates for COVID-19; however, men are more susceptible to developing severe COVID-19 symptoms and have higher mortality rates (Jin et al. Citation2020). In pulmonary arterial hypertension patients, men have higher mortality rates due to an increased level of pulmonary vascular necrosis and an increased amount of HMGB1 in the extracellular milieu (Rafikov et al. Citation2019; Zemskova et al. Citation2020). Thus, increased HMGB1 levels could explain why men with COVID-19 have a higher mortality rate compared to women. Therefore, the activation of the cholinergic anti-inflammatory pathway with α7nAChR agonists offers at least two hypothetical mechanisms for protection against SARS-CoV-2 infection: (1) attenuation of SARS-CoV-2-induced hyper-cytokinemia, particularly by decreasing the secretion of HMGB1; and (2) inhibition of endocytosis of HMGB1-SARS-CoV-2 RNA complexes, thus decreasing hyper-inflammatory responses. Furthermore, in silico-based investigations have reported a potential interaction between the α7nAChR and the SARS-CoV-2 glycoprotein spike protein (Farsalinos et al. Citation2020a). The interaction between α7nAChR and the spike protein was predicted to be similar to that of α-bungarotoxin, a molecule in the venom of the snake, Bungarus multicinctus multicinctus, that selectively antagonizes α7nAChR (Farsalinos et al. Citation2020b). This interaction may play a critical role in disrupting the cholinergic anti-inflammatory system in COVID-19 patients, resulting in lung inflammation and severe ARDS. α7nAChR agonists, such as nicotine or GTS-21, could also compete with the binding of the spike protein for α7nAChR, representing a third protective mechanism.
Depending on the organ and cell type, nicotine has pleiotropic effects on angiotensin 2 (ACE2) receptor expression (Leung et al. Citation2020; Tindle et al. Citation2020). Currently, there are conflicting data as to whether nicotine modulation of ACE2 expression is protective or harmful in patients with SARS-CoV-2 infection (Hartmann-Boyce et al. Citation2020). The ACE2 receptor is the primary cellular entry point for SARS-CoV-2 (Hoffmann et al. Citation2020). In silico molecular docking studies indicate that nicotine may bind to ACE2 and competitively inhibit the formation of the ACE2-SARS-CoV-2 complex (Chellasamy et al. Citation2020). If this observation is supported by direct binding experiments, the direct competition of nicotine or GTS-21 the with the spike protein for binding to ACE2, could be a fourth potential mechanism to protect patients from being infected with COVID-19.
Nicotine-containing products are widely available and already in use by large numbers of people worldwide. Nicotine could potentially decrease the cellular entry of SARS-CoV-2, and nicotine has anti-inflammatory efficacy that may be useful in the treatment of COVID-19-associated ARDS. Unfortunately, nicotine is habit-forming and long-term use of cigarettes and similar products have been shown to increase the risk of cancer, lung, and heart disease. This reality presents several moral dilemmas. If used under the strict supervision and guidance of a physician, nicotine products could possibly provide a potential therapeutic strategy in the current pandemic but withdrawing some patients from nicotine after SARS-CoV-2 is controlled, will prove difficult. Furthermore, a large number of people worldwide are still active smokers.
Given the potential short-term benefits of nicotine in COVID-19 infection, would it still be ethical for physicians to actively promote smoking cessation in the midst of the current viral pandemic? Furthermore, instituting active smoking cessation therapy in current smokers at present is a problematic issue. Public health groups will undoubtedly feel compelled to weigh in decidedly on this question but the ultimate solution will probably need to be decided by a physician and how she/he informs each patient. This problem, that of long-term addiction, could be circumvented by the rapid development of α7nAChR agonists, such as GTS-21, which has already been clinically evaluated for the treatment of certain neurodegenerative diseases (Kitagawa et al. Citation2003).
Acknowledgments
The authors would like to thank Maleka T. Stewart, David Mathieu, and Miranda M. Bogdanowicz for their critical reading of the manuscript.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
Additional information
Funding
References
- Andersson U, Ottestad W, Tracey K. 2020. Extracellular HMGB1: A therapeutic target in severe pulmonary inflammation including COVID-19? Mol Med. 26(1):42.
- Argenziano M, Bruce S, Slater C, Tiao J, Baldwin M, Barr R, Chang B, Chau K, Choi J, Gavin N, et al. 2020. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ. 369:1–9.
- Azar K, Shen Z, Romanelli R, Lockhart S, Smits K, Robinson S, Brow S, Pressman A. 2020. Disparities in outcomes among COVID-19 patients in a large health care system in California: Study estimates the COVID-19 infection fatality rate at U.S. county level. Health Aff. 39(7):1253–1262.
- Bhargava A, Fukushima E, Levine M, Zhao W, Tanveer F, Szpunar S, Saravolatz L. 2020. Predictors for severe COVID-19 infection. Clin Infect Dis May. 30:1–7.
- Bi X, Su Z, Yan H, Du J, Wang J, Chen L, Peng M, Chen S, Shen B, Li J. 2020. Prediction of severe illness due to COVID-19 based on an analysis of initial fibrinogen to albumin ratio and platelet count. Platelets. 31(5):674–679.
- Borovikova L, Ivanova S, Zhang M, Yang H, Botchkina G, Watkins L, Wang H, Abumrad N, Eaton J, Tracey K. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 405(6785):458–462.
- Buckner F, McCulloch D, Atluri V, Blain M, McGuffin S, Nalla A, Huang M, Greninger A, Jerome K, Cohen S, et al. 2020. Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin Infect Dis May. 22:1–7.
- Calabrese C, Rajendram P, Sacha G, Calabrese L. 2020. Practical aspects of targeting IL-6 in COVID-19 disease. Cleveland Clin J Med. 2020:1–5.
- Cao M, Zhang D, Wang Y, Lu Y, Zhu X, Li Y, Xue H, Lin Y, Zhang M, Sun Y, et al. 2020. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. Resp Med. DOI:https://doi.org/10.1101/2020.03.04.20030395.
- CDC COVID-19 Response Team. 2020. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019-United States, February 12–March 28, 2020. MMWR. 69:382–386.
- Changeux J, Amoura Z, Rey F, Miyara M. 2020. A nicotinic hypothesis for COVID-19 with preventive and therapeutic implications. C R Biol. 343(1):33–39.
- Chellasamy S, Kumar S, Wei H. 2020. A computational insight of the improved nicotine binding with ACE2-SARS-CoV-2 complex with its clinical impact. Biomolecules. arXiv:2004.14943.
- Chen L, Long X, Xu Q, Tan J, Wang G, Cao Y, Wei J, Luo H, Zhu H, Huang L, et al. 2020. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell Mol Immunol. 17(9):992–994.
- Chen L, Yu J, He W, Chen L, Yuan G, Dong F, Chen W, Cao Y, Yang J, Cai L, et al. 2020. Risk factors for death in 1859 subjects with COVID-19. Leukemia. 34(8):2173–2183.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 395(10223):507–513.
- Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, et al. 2020. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 368:1–10.
- Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. 2020. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 53:25–32.
- COVID-19 Treatment Guidelines Panel. 2020a. Immune-based therapy under evaluation for treatment of COVID-19. Bethesda (MD): National Institutes of Health; [accessed 2020 Sep 17]. https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/interleukin-6-inhibitors/
- COVID-19 Treatment Guidelines Panel. 2020b. Coronavirus disease 2019 (COVID-19) treatment guidelines. Bethesda (MD): National Institutes of Health; [accessed 2020 Dec 9]. https://www.covid19treatmentguidelines.nih.gov/
- de Melo G, Lazarini F, Larrous F, Feige L, Kergoat L, Marchio A, Pineau P, Lecuit M, Lledo P, Changeux J, et al. 2020. Anti-COVID-19 efficacy of ivermectin in the golden hamster. bioRxiv. DOI:https://doi.org/10.1101/2020.11.21.392639.
- Entezari M, Javdan M, Antoine DJ, Morrow DMP, Sitapara R, Patel V, Wang M, Sharma L, Gorasiya S, Zur M, et al. 2014. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol. 2:314–322.
- Fajgenbaum D, June C. 2020. Cytokine storm. N Engl J Med. 383(23):2255–2273.
- Fan E, Beitler J, Brochard L, Calfee C, Ferguson N, Slutsky A, Brodie D. 2020. COVID-19-associated acute respiratory distress syndrome: Is a different approach to management warranted? Lancet Resp Med. 8(8):816–821.
- Farsalinos K, Angelopoulou A, Alexandris N, Poulas K. 2020. COVID-19 and the nicotinic cholinergic system. Eur Respir J. 56(1):2001589.
- Farsalinos K, Eliopoulos E, Leonidas D, Papadopoulos G, Tzartos S, Poulas K. 2020a. Molecular modelling and docking experiments examining the interaction between SARS-CoV-2 spike glycoprotein and neuronal nicotinic acetylcholine receptors. Preprints. DOI:https://doi.org/10.20944/preprints202005.0365.v1.
- Farsalinos K, Eliopoulos E, Leonidas D, Papadopoulos G, Tzartos S, Poulas K. 2020b. Nicotinic cholinergic system and COVID-19: In silico identification of an interaction between SARS-CoV-2 and nicotinic receptors with potential therapeutic targeting implications. Int J Mol Sci. 21(16):5807.
- Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, Xiong W, Yang D, Chen R, Lu F, et al. 2020. COVID-19 with different severities: A multicenter Study of Clinical Features. Am J Respir Crit Care Med. 201(11):1380–1388.
- Gavin W, Campbell E, Zaidi S, Gavin N, Dbeibo L, Beeler C, Kuebler K, Abdel-Rahman A, Luetkemeyer M, Kara A. 2020. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID-19. Am J Infect Control. 49(2):158–165.
- Gonzalez-Rubio J, Navarro-Lopez C, Lopez-Najera E, Lopez-Najera A, Jimenez-Diaz L, Navarro-Lopez J, Najera A. 2020. Cytokine release syndrome (CRS) and nicotine in COVID-19 patients: Trying to calm the storm. Front Immunol. 11:1359.
- Goyal P, Choi J, Pinheiro L, Schenck E, Chen R, Jabri A, Satlin M, Campion T, Nahid M, Ringel J, et al. 2020. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 382(24):2372–2374.
- Gu T, Mack J, Salvatore M, Sankar S, Valley T, Singh K, Nallamothu B, Kheterpal S, Lisabeth L, Fritsche L, et al. 2020. COVID-19 outcomes, risk factors and associations by race: A comprehensive analysis using electronic health records data in Michigan Medicine. MedRxiv. DOI:https://doi.org/10.1101/2020.06.16.20133140.
- Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, Liu L, Shan H, Lei C, Hui D, et al. 2020. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 382(18):1708–1720.
- Hartmann-Boyce J, Lindson N. 2020. The role of nicotine in COVID-19 infection. Oxford (UK): CEBM (Centre for Evidence-Based Medicine); [accessed 2020 Aug 8]. https://www.cebm.net/covid-19/nicotine-replacement-therapy/
- Hartmann-Boyce J, Morris E, Goyder C, Kinton J, Perring J, Nunan D, Mahtani K, Buse J, Prato S, Ji L, et al. 2020. Diabetes and COVID-19: Risks, management, and learnings from other national disasters. Diabetes Care. 43(8):1695–1703.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens T, Herrler G, Wu N, Nitsche A, et al. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181(2):271–280.
- Hreggvidsdottir H, Ostberg T, Wähämaa H, Schierbeck H, Aveberger A, Klevenvall L, Palmblad K, Ottosson L, Andersson U, Harris H. 2009. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 86(3):655–662.
- Hu L, Chen S, Fu Y, Gao Z, Long H, Ren H, Zuo Y, Wang J, Li H, Xu Q, et al. 2020. Risk factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID-19) hospitalized patients in Wuhan. China Clin Infect Dis. 3:1–10.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395(10223):497–506.
- Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, Chen G, Cheng G, Wang Y, Bi J, et al. 2020. Prediction for progression risk in patients with COVID-19 pneumonia: The CALL score. Clin Infect Dis. 71(6):1393–1399.
- Jin J, Bai P, He W, Wu F, Liu X, Han D, Liu S, Yang J. 2020. Gender differences in patients with COVID-19: Focus on severity and mortality. Front Publ Health. 9:152.
- Kalligeros M, Shehadeh F, Mylona E, Benitez G, Beckwith C, Chan P, Mylonakis E. 2020. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 28(7):1200–1204.
- Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan X, Yan Z, et al. 2014. HMGB1 in health and disease. Mol Aspects Med. 40:1–116.
- Karanasos A, Aznaouridis K, Latsios G, Synetos A, Plitaria S, Tousoulis D, Toutouzas K. 2020. Impact of smoking status on disease severity and mortality of hospitalized patients with COVID-19 infection: A systematic review and meta-analysis. Nicotine Tob Res. 22(9):1657–1659.
- Kim E, Chin B, Kang C, Kim N, Kang Y, Choi J, Oh D, Kim J, Koh B, Kim S, et al. 2020. Clinical course and outcomes of patients with Severe Acute Respiratory Syndrome corona-virus 2 infection: A preliminary report of the first 28 patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 35:e142.
- Kitagawa H, Takenouchi T, Azuma R, Wesnes K, Kramer W, Clody D, Burnett A. 2003. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 28(3):542–551.
- Klang E, Kassim G, Soffer S, Freeman R, Levin M, Reich D. 2020. Severe obesity as an independent risk factor for COVID‐19 mortality in hospitalized patients younger than 50. Obesity. 28(9):1595–1599.
- Kox M, Pompe J, Peters E, Vaneker M, van der Laak J, van der Hoeven J, Scheffer G, Hoedemaekers C, Pickkers P. 2011. α7 Nicotinic acetylcholine receptor agonist GTS-21 attenuates ventilator-induced TNFα production and lung injury. Br J Anaesth. 107(4):559–566.
- Krause R, Buisson B, Bertrand S, Corringer P, Galzi K, Changeux J, Bertrand D. 1998. Ivermectin: A positive allosteric effector of the α7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 53(2):283–294.
- Kuderer N, Choueiri T, Shah D, Shyr Y, Rubinstein S, Rivera R, Shete S, Hsu C, Desai A, de Lima Lopes G, et al. 2020. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet. 395(10241):1907–1918.
- Lee D, Gardner R, Porter D, Louis C, Ahmed N, Jensen M, Grupp S, Mackall C. 2014. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 124(2):188–195.
- Leung J, Yang C, Sin D. 2020. COVID-19 and nicotine as a mediator of ACE-2. Eur Respir J. 55(6):2001261.
- Li R, Pei S, Chen B, Song Y, Zhang T, Yang W, Shaman J. 2020. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 368(6490):489–493.
- Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, et al. 2020. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 146(1):110–118.
- Liu D, Wang Y, Wang J, Liu J, Yue Y, Liu W, Zhang F, Wang Z. 2020. Characteristics and outcomes of a sample of patients with COVID-19 identified through social media in Wuhan, China: Observational study. J Med Internet Res. 22(8):e20108.
- Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, et al. 2020. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 55:102763.
- Lu B, Kwan K, Levine Y, Olofsson P, Yang H, Li J, Joshi S, Wang H, Andersson U, Chavan S, et al. 2014. α7 Nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol Med. 20:350–358.
- Ma K, Liu Z, Cao C, Liu M, Liao J, Zou J, Kong L, Wan K, Zhang J, Wang Q, et al. 2020. COVID-19 myocarditis and severity factors: An adult cohort study. Medrxiv. 2020:1–60.
- Mavropoulos S, Khan N, Levy A, Faliks B, Sison C, Pavlov V, Zhang Y, Ojamaa K. 2017. Nicotinic acetylcholine receptor-mediated protection of the rat heart exposed to ischemia reperfusion. Mol Med. 23(1):120–133.
- Mazloom R. 2020. Feasibility of therapeutic effects of the cholinergic anti-inflammatory pathway on COVID-19 symptoms. J Neuroimmune Pharmacol. 15(2):165–166.
- Moon H, Cao Y, Yang J, Lee J, Choi H, Jin Y. 2015. Lung epithelial cell-derived extracellular vesicles activate macrophage-mediated inflammatory responses via ROCK1 pathway. Cell Death Dis. 6:e2016.
- Ni Y, Tian F, Lu Z, Yang G, Fu H, Wang J, Yan X, Zhao Y, Wang Y, Jiang T. 2011. Protective effect of nicotine on lipopolysaccharide-induced acute lung injury in mice. Respiration. 81(1):39–46.
- Pandey S, Pathak S, Pandey A, Salunke A, Chawla J, Sharma A, Sharma S, Thivari P, Ratna H. 2020. Ivermectin in COVID-19: What do we know? Diabetes Metab Syndr. 14(6):1921–1922.
- Patanavanich R, Glantz S. 2020. Smoking is associated with COVID-19 progression: A meta-analysis. medRxiv. 2020:1–16.
- Patel V, Dial K, Wu J, Gauthier A, Wu W, Lin M, Espey M, Thoma D, Ashby C, Mantell L. 2020. Dietary anti-oxidants significantly attenuate hyperoxia-induced acute inflammatory lung injury by enhancing macrophage function via reducing the accumulation of airway HMGB1. Int J Mol Sci. 21(3):977.
- Patel V, Sitapara R, Gore A, Phan B, Sharma L, Sampat V, Li J, Yang H, Chavan S, Wang H, et al. 2013. High Mobility Group Box-1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. Am J Respir Cell Mol Biol. 48(3):280–287.
- Pavlov V, Ochani M, Yang L, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish W, Rosas-Ballina M, Czura C, et al. 2007. Selective α7 nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 35:1139–1144.
- Pavlov V, Wang H, Czura C, Friedman S, Tracey K. 2003. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol Med. 9(5–8):125–134.
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, et al. 2020. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 71(15):762–768.
- Rafikov R, Nair V, Sinari S, Babu H, Sullivan J, Yuan J, Desai A, Rafikova O. 2019. Gender difference in damage-mediated signaling contributes to pulmonary arterial hypertension. Antioxid Redox Signal. 31(13):917–932.
- Rentsch C, Kidwai-Khan F, Tate JP, Park L, King J, Skanderson M, Hauser R, Schultze A, Jarvis C, Holodniy M, et al. 2020. Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54-75 years. MedRxiv. 2020:1–32.
- Rothan H, Byrareddy S. 2020. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 109:102433.
- Şenkal N, Meral R, Medetalibeyoğlu A, Konyaoğlu H, Kose M, Tukek T. 2020. Association between chronic ACE inhibitor exposure and decreased odds of severe disease in patients with COVID-19. Anatol J Cardiol. 24(1):21–29.
- Sinha P, Matthay M, Calfee C. 2020. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. 180(9):1152–1154.
- Sitapara R, Gauthier A, Patel V, Lin M, Zur M, Ashby C, Mantell L. 2020. The α7 nicotinic acetylcholine receptor agonist GTS-21 improves bacterial clearance in mice by restoring hyperoxia-compromised macrophage function. Mol Med. 26(1):98.
- Sitapara R, Gauthier A, Valdés-Ferrer S, Lin M, Patel V, Wang M, Martino A, Perron J, Ashby C, Tracey K. 2020. The α7 nicotinic acetylcholine receptor agonist, GTS-21, attenu-ates hyperoxia-induced acute inflammatory lung injury by alleviating the accumulation of HMGB1 in the airways and the circulation. Mol Med. 26:63.
- Soares R, Mattos L, Raposo L. 2020. Risk factors for hospitalization and mortality due to COVID-19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 103(3):1184–1190.
- Tabata S, Imai K, Kawano S, Ikeda M, Kodama T, Miyoshi K, Obinata H, Mimura S, Kodera T, Kitagaki M, et al. 2020. The clinical characteristics of COVID-19: A retrospective analysis of 104 patients from the outbreak on board the Diamond Princess cruise ship in Japan. MedRxiv. 2020:1–21.
- Tarnawski L, Reardon C, Caravaca A, Rosas-Ballina M, Tusche M, Drake A, Hudson L, Hanes W, Li J, Parrish W, et al. 2018. Adenylyl cyclase-6 mediates inhibition of TNF in the inflammatory reflex. Front Immunol. 9:2648.
- Tindle H, Newhouse P, Freiberg M. 2020. Beyond smoking cessation: Investigating medicinal nicotine to prevent and treat COVID-19. Nicotine Tob Res. 22(9):1669–1671.
- To K, Tsang O, Leung W, Tam A, Wu T, Lung D, Yip C, Cai J, Chan J, Chik T, et al. 2020. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect Dis. 20(5):565–574.
- Tracey K. 2007. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 117(2):289–296.
- Uteshev V. 2014. The therapeutic promise of positive allosteric modulation of nicotinic receptors. J Med Virol. 92:797–806.
- Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, Lang C, Huang D, Sun Q, Xiong Y, et al. 2020. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. Eur J Phar-Macol. 727:181–185.
- Wang B, Wang Z, Zhao J, Zeng X, Wu M, Wang S, Wang T. 2020. Epidemiological and clinical course of 483 patients with COVID-19 in Wuhan, China: A single-center, retrospective study from the mobile cabin hospital. Eur J Clin Microbiol Infect Dis. 39(12):2309–2315.
- Wang H, Cai D, Chen Z, Wang Y. 2020. GTS-21 promotes α7nAChR to alleviate intestinal ischemia-reperfusion-induced apoptosis and inflammation of enterocytes. Med Sci Monit. 26:e921618-1–e921618-7.
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li J, Wang H, Yang H, Ulloa L, et al. 2003. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 421(6921):384–388.
- Wang K, Zhang Z, Yu M, Tao Y, Xie M. 2020. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: An ambispective observational cohort study. Intensive Care Med. 46(7):1472–1474.
- Wang M, Gauthier A, Daley L, Dial K, Wu J, Woo J, Lin M, Ashby C, Mantell L. 2019. The role of HMGB1, a nuclear damage-associated molecular pattern molecule, in the pathogenesis of lung diseases. Antioxid Redox Signal. 31(13):954–993.
- Wang R, Pan M, Zhang X, Han M, Fan X, Zhao F, Miao M, Xu J, Guan M, Deng X, et al. 2020. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. 95:421–428.
- Ward N, Waxman A, Homer R, Mantell L, Einarsson O, Du Y, Elias J. 2000. Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol. 22(5):535–542.
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, et al. 2020. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 180(7):934–943.
- Wu H, Li L, Su X. 2014. Vagus nerve through α7nAChR modulates lung infection and inflammation: Models, cells, and signals. BioMed Res Intl. 2014:283525.
- Xu Y, Li Y, Zeng Q, Lu Z, Li Y, Wu W, Dong S, Huang G, Wang X. 2020. Clinical characteristics of SARS-CoV-2 pneumonia compared to controls in Chinese Han population. Medrxiv. 2020:1–20.
- Yang H, Liu H, Zeng Q, Imperato G, Addorisio M, Li J, He M, Cheng K, Al-Abed Y, Harris H, et al. 2019. Inhibition of HMGB1/RAGE-mediated endocytosis by HMGB1 antagonist box A, anti-HMGB1 antibodies, and cholinergic agonists suppresses inflammation. Mol Med. 25:13.
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. 2020. Clinical course and outcomes of critically-ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Resp Med. 8(5):475–481.
- Yanover C, Mizrahi B, Kalkstein N, Marcus K, Akiva P, Barer Y, Shalev V, Chodick G. 2020. What factors increase the risk of complications in SARS-CoV-2-infected patients? A cohort study in a Nationwide Israeli Health Organization. JMIR Public Health Surveill. 6(3):e20872.
- Yao Q, Wang P, Wang X, Qie G, Meng M, Tong X, Bai X, Ding M, Liu W, Liu K, et al. 2020. Retrospective study of risk factors for severe SARS-Cov-2 infections in hospitalized adult patients. Polish Arch Intern Med. 130:390–399.
- Zemskova M, Kurdyukov S, James J, McClain N, Rafikov R, Rafikova O. 2020. Sex-specific stress response and HMGB1 release in pulmonary endothelial cells. PLoS One. 15(4):e0231267.
- Zhang J, Dong X, Cao Y, Yuan Y, Yang Y, Yan Y, Akdis C, Gao Y. 2020. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan. China Allergy. 75(7):1730–1741.
- Zheng Y, Xiong C, Liu Y, Qian X, Tang Y, Liu L, Leung E, Wang M. 2020. Epidemiological and clinical characteristics analysis of COVID-19 in the surrounding areas of Wuhan, Hubei Province in 2020. Pharmacol Res. 157:104821.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 395(10229):1054–1062.
