Abstract
In development of peptide therapeutics, rodents are commonly-used preclinical models when screening compounds for efficacy endpoints in the early stages of discovery projects. During the screening process, some peptides administered subcutaneously to rodents caused injection site reactions manifesting as localized swelling. Screening by postmortem evaluations of injection site swelling as a marker for local subcutaneous histamine release, were conducted in rats to select drug candidates without this adverse effect. Histological analysis of skin samples revealed that the injection site reactions were concurrent with mast cell degranulation, resulting in histamine release. Mast cell activation can be mediated by MRGPRX2, a GPCR that induces a pseudo-allergenic immune response. The present study demonstrates that a commercially-available cell-based MRGPRX2 assay reliably identifies compounds that induce histamine release or localized edema in ex vivo human and rodent skin samples. In vitro screening was subsequently implemented using the MRGPRX2 assay as a substitute for postmortem injection site evaluation, thus achieving a significant reduction in animal use. Thus, in cases where injection site reactions are encountered during in vivo screening, to enable faster screening during the early drug discovery process, an MRGPRX2 in vitro assay can be used as an efficient, more ethical tool with human translational value for the development of safer pharmacotherapies for patients.
Introduction
Mast cells (MC) are important modulators of the innate and adaptive immune response and are often located at boundaries between tissues and the external milieu (Shelburne and Abraham Citation2011; Elieh Ali Komi et al. Citation2020). This strategic localization supports the immune system in the early detection of pathogens. MCs circulate in the blood as precursors originating from bone marrow and carry out their differentiation upon arrival at the resident tissue into granulated cells according to tissue specific signals (Elieh Ali Komi et al. Citation2020). Degranulation of MC and release of mediators of inflammation (e.g., histamine, prostaglandins, proteases) occurs upon activation by IgE-dependent or -independent pathways via many different antigens (Gaudenzio et al. Citation2016; Bulfone-Paus et al. Citation2017). The resulting anaphylaxis (IgE-mediated) or anaphylactoid (pseudo-allergenic; non-IgE-mediated) reactions can present with a variety of clinical symptoms (e.g., swelling/edema, hypotension, bronchospasm, skin rashes/hives), depending on the mediators that are released (Theoharides et al. Citation2019).
A Mas-related GPCR, i.e., MRGPRX2, is specifically expressed on mast cells. MRGPRX2 was recently shown to induce pseudo-allergenic reactions to several FDA-approved cationic drugs (McNeil et al. Citation2015; Porebski et al. Citation2018; Grimes et al. Citation2019). MRGPRX2 can also be activated by endogenous peptides such as anti-microbial host defense peptides (human β-defensins, cathelicidin LL-37), neuropeptide Substance P, and opioids (Ali Citation2016, Citation2017). High levels of the MrgprX2 transcript are expressed in human skin mast cells (Porebski et al. Citation2018; Varricchi et al. Citation2019). The Mas-related gene family has ∼50 members in mouse, rat, human, macaque, and rhesus monkey that can be subdivided into several subfamilies (Solinski et al. Citation2014; Ali Citation2017; Porebski et al. Citation2018). In humans, there are four MRGPRX members, MRGPRX1-X4. In rodents, there are several more subtypes of the Mas-related GPCR (Porebski et al. Citation2018). MrgprB has been suggested to be the rodent orthologue of human MRGPRX2 (Solinski et al. Citation2014; Grimbaldeston Citation2015; McNeil et al. Citation2015; Subramanian et al. Citation2016; Wang et al. Citation2020). Many of the drugs and peptides that induce pseudo-allergenic reactions in humans via MRGPRX2, also induce skin irritation and edema in mice (McNeil et al. Citation2015).
Activation of MRGPRX2 induces mast cell degranulation and histamine release (McNeil et al. Citation2015; Ding et al. Citation2019). Histamine is one of the mediators released from mast cell granules upon activation by various toxins and is associated with symptoms of dermal edema/swelling (Garafalo and Kaplan Citation1981; Wei et al. Citation2009; Kimura et al. Citation2015). Histamine release has long been used as a marker of anaphylaxis or pseudo-allergenic reactions (Charitos et al. Citation2020). Quantitative and qualitative measurements of histamine release in the ex vivo setting have been developed to test for allergenic activity induced by a diverse array of test agents with both rodent and human ex vivo skin samples (de Antonio and Rothschild Citation1969; Petersen Citation1997, Citation1998; Petersen et al. Citation1996, Citation1997; Suzuki et al. Citation2020).
Injection site reactions during in vivo screening can stall the momentum of the early drug discovery process. Encountering injection site reactions stemming from off-target activation of mast cells during in vivo screening in peptide-based drug discovery programs can also confound interpretation of efficacy parameters and significantly hinder progress to selecting lead candidates. The study reported here describes the establishment of the use of a commercial in vitro assay to predict the occurrence of MRGPRX2-mediated in vivo injection site reactions in rodents and compared it to ex vivo assays (human and rodent). This enables one to abandon the use of animals for screening on this adverse effect, resulting in greatly-reduced animal use in accordance with the 3 R (Replacement, Reduction and Refinement) principles for the more ethical use of animals in drug testing.
Materials and methods
Compounds
Cortistatin-14 (C5808), Substance P (S6883), icatibant (HOE140), sermorelin (G6771) and cetrorelix (C5249) were purchased from Sigma (St. Louis, MO). All other peptides were synthesized and qualified at Novo Nordisk A/S (Maaloev, Denmark).
Animals
Male Sprague Dawley rats (200–250 g, Taconic, city, Germany) were fed ad libitum a standard chow diet (Altromin 1320) along with free access to water. All animals were housed at 23 °C (± 2 °C) with a 55% relative humidity, and maintained on a 12 h light/dark cycle. The rats were mock-handled the days leading up to study initiation, and acclimated to environmental conditions for a minimum of 8 days before study initiation. Group sizes were estimated based on experience from earlier experiments. All experiments were carried out in accordance with the EU Directive 2010/63/EU of the 22 September 2010 on the protection of animals used for scientific purposes, and were approved by The Animal Experimentation Inspectorate, Ministry of Environ-ment and Food, Denmark.
Macroscopic evaluations of rat skin specimens
It has been shown in humans that upon in vivo challenge by intradermal injection of an allergen, histamine is released from cutaneous mast cells into the dermis resulting in localized swelling; the size of the swelling correlates with the local histamine levels (Petersen et al. Citation1997). Similar effects have been observed in rat skin specimens. Therefore, a macroscopic postmortem evaluation of the rat skin specimens in the neck region was performed after subcutaneous injection of drug or vehicle in order to select drug candidates with low degree or without local mast cell degranulation and histamine release.
All rats were shaved in the neck region (an area of 2 × 3 cm) and then randomly allocated into test groups. Each rat received one injection of 100 µl vehicle or drug in the middle of the shaved injection area with a 26-G (0.45 × 12 mm) needle and a 1-ml syringe. Fresh needles were used for each animal and discarded after usage. The insertion of the needle was oriented caudally in the injection site area and marked with permanent ink. At 3 h post-injection, the rats were anesthetized by isoflurane inhalation and immediately euthanized by exsanguination (via abdom-inal aorta). The injection area was then dissected; the subcutaneous tissue was inspected and scored on a 0–5 scale for the following parameters: 1) redness/hemorrhage, 2) swelling/edema, and/or 3) precipitation of test compound. The histologist scoring the injection site was blinded to treatment groups and macroscopic analysis was done in a randomized order. After scoring (∼2–3 min), the demarcated injection site was excised and fixed in 10% neutral buffered formalin in sealed plastic containers for further histologic analysis.
Histological microscopic analysis of rat skin specimens
Each injection site was subsequently trimmed and cut into three sections representing the upper, middle, and lower part of the injection sites. These three sections were further processed and paraffin embedded in one block/injection site. The tissue blocks were then cut in 4–5 µm sections using a microtome in parallel sections and stained with hematoxylin and eosin - or toluidine blue for histamine release evaluation. All prepared sections were evaluated by a trained toxico-pathologist for swelling/edema formation and visible mast cell degranulation using a light microscope.
MRGPRX2 in vitro β-arrestin assay
The commercially-available PathHunter eXpress MRGPRX2 CHO-K1 β-Arrestin GPCR assay (DiscoverX, Fremont, CA) was used to evaluate the potency of test compounds. This assay measured the activation MRGPRX2 by detecting β-arrestin recruitment using a homogeneous, gain-of-signal assay based on Enzyme Fragment Complementation (EFC) technology. The assay employs a β-galactosidase (β-gal) enzyme split into two fragments, the Enzyme Donor (ED) and Enzyme Acceptor (EA). Independently these fragments have no β-gal activity; however, when brought together in in solution, they form an active β-gal enzyme. Since β-arrestin recruitment occurs independent of G-protein coupling, the assay provided a direct measure of receptor activation. As such, MRGPRX2 is tagged with the small fragment of β-gal (a low-affinity version of ED) and co-expressed in cells stably expressing β-arrestin tagged with EA. Activation of MRGPRX2 stimulated binding of β-arrestin to the tagged GPCR, forcing complementation of ED and EA, resulting in the formation of an active β-gal enzyme. The resulting active enzyme hydrolyzed substrate present in the PathHunter detection reagent to generate light.
PathHunter MRGPRX2 expressing CHO-K1 cell suspension were transferred to 96-well plates, incubated for 48 h in media supplied by the manufacturer and thereafter, treated for 90 min with varying concentrations of test compounds at 37 °C. Upon addition of the provided detection reagent cocktail, plates were incubated at room temperature, protected from light, for 1 h to generate the assay signal. Plates were then evaluated with a standard luminescence plate reader (Mithras, Berthold Technologies, Bad Wildbad, Germany). Data were analyzed in Prism 7 software (GraphPad, San Diego, CA) and EC50 values were calculated by nonlinear regression analysis of sigmoidal dose response curves. Mean pEC50 with the 95% confidence interval are stated in the tables, as well as the mean EC50 values that were calculated by 10-pEC50, respectively, and presented in nM.
Ex vivo assay of human skin samples
Donor skin
Abdominal skin specimens were obtained from five individual patients undergoing cosmetic surgery for removal of excess skin. The skin was transported at ambient temperature to RefLab (Copenhagen, Denmark) in a container supplied with sterile saline immediately after surgical removal. As the skin donors were fully anonymized, the tissue was obtained and used in this study in accordance with the Danish Act on Research Ethics Review of Health Research Projects, Section 14 (3) - “Notification obligation” concerning the use of anonymized human tissue for research purposes. Note that at the time of performing the study, the use of human skin was exempt from ethical approval because donors were fully anonymized. This means that while an ethical approval was obtained, but no informed consent from each skin donor was required; thus, no approval number is available.
Preparation of skin
Smaller skin specimens measuring ≈ 4 × 15 cm were excised from the parent tissue. Subcutaneous fat was removed before the skin specimens were pinned onto Styrofoam covered with moist tissue paper to keep the skin hydrated from the dermal side. Twelve microdialysis probes with a 2 kDa molecular weight cutoff (EP Medical, Copenhagen, Denmark) were inserted into each skin specimens using 23 G guide cannulas. The length of the microdialysis membranes was adjusted to 40 mm, with 2 cm of the membrane being implanted intradermally. To avoid any bias from tissue excision and potential spillover, the probes were spaced a minimum of 1 cm apart and at least 1 cm from the edge of the skin specimen. The inlet tubing of the microdialysis probes was connected to syringes mounted in a micro-perfusion pump (AL1200, World Precision Instruments, Hertfordshire, UK) enabling perfusion of the probes.
Microdialysis
The intradermal microdialysis probes were perfused with Pipes buffer at a flow rate of 3.0 µl/min for 30 min to wash out trauma-induced mediators caused by probe implantation. Next, dialysates were collected for 30 min to assess basal histamine levels in the tissue before the skin was subjected to 2 × 25 µl intradermal injections with the test compounds at alternating sides of each microdialysis membrane. Microdialysis sampling was carried out in 10-min intervals for 60 min to assess the amount of histamine released from each injection. The microdialysis sampling procedure was conducted at room temperature.
Histamine detection system
Histamine detection was performed at RefLab ApS as described in Jensen et al. (Citation2020). In brief, the assay was based on the selective binding of histamine to a glass fiber matrix that can be detected fluorometrically and quantified using a Histareader 501® (RefLab, Copenhagen, Denmark) after coupling to o-phthalaldehyde (OPA). Significant histamine release was deter-mined as occurring in a sample that yielded a value 3X the SD of the background fluorescence. Assay sensitivity for histamine was 5 ng/ml (CV% < 7%). Histamine AUC were calculated by the trapezoidal rule.
Results
Cationic compounds can activate MRGPRX2
Sermorelin, Substance P, cetrorelix and icatibant were tested in the DiscoverX β-arrestin assay in order to confirm that compounds reported (Chen et al. Citation2008; Olivennes et al. Citation2003; Amatya et al. Citation2010; Baş et al. Citation2015; McNeil et al. Citation2015) to induce skin irritation in humans also activated MRGPRX2. Cortistatin (a somatostatin receptor agonist) - reported to also be a potent agonist at MRGPRX2 - was used as a comparator (Allia et al. Citation2005; Sieler et al. Citation2008; Solinski et al. Citation2014). Cetrorelix was found to be a full agonist (pEC50 = 6.6 nM) at MRGPRX2 with comparable potency to cortistatin (pEC50 = 6.6, ). In contrast, sermorelin and Substance P displayed lower estimated potencies (pEC50 = 5.0 and 4.8, respectively), and icatibant was inactive ().
Figure 1. Cationic compounds can activate MRGPRX2. Compounds were tested in the DiscoverX β-arrestin assay and compared to cortistatin (somatostatin receptor agonist) that is a potent agonist at MRGPRX2. Representative graph on potency of compounds tested (left) and table (right) of mean pEC50 that are calculated as the mean of three independent experiments, and the 95% confidence interval (CI) is given as [lower; upper]. The mean EC50 is calculated from the mean pEC50.
![Figure 1. Cationic compounds can activate MRGPRX2. Compounds were tested in the DiscoverX β-arrestin assay and compared to cortistatin (somatostatin receptor agonist) that is a potent agonist at MRGPRX2. Representative graph on potency of compounds tested (left) and table (right) of mean pEC50 that are calculated as the mean of three independent experiments, and the 95% confidence interval (CI) is given as [lower; upper]. The mean EC50 is calculated from the mean pEC50.](/cms/asset/7729dabc-bd6b-42f3-82e1-e759b536dc17/iimt_a_1877375_f0001_c.jpg)
Histamine release in ex vivo human skin samples
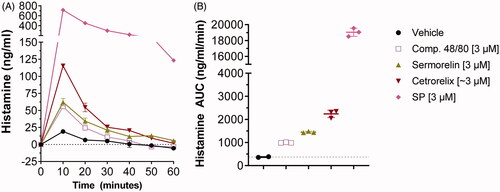
To determine if the potencies of the compounds tested in the cell-based MRGPRX2 assay correlated in a translationally-relevant setting, histamine release was assessed in freshly-acquired abdominal human skin specimens from patients undergoing cosmetic surgery. Exposure of skin specimens to sermorelin, cetrorelix and Substance P (tested on MRGPRX2 potency) and Compound 48/80 (widely-used in animal and tissue models as a mast cell activator [Rothschild Citation1970; Kumar et al. Citation2020]) induced histamine release with peak values occurring at 10 min post-administration of compound (). Based on technical replicates for each compound, all compounds induced a greater excursion in histamine release at 10 min compared to the vehicle (19.0 [± 2.8] ng/mL), with Substance P (714.3 [± 25.7] ng/mL) and cetrorelix (115.3 [± 2.1] ng/mL) causing the largest peak values, followed by sermorelin (62.0 [± 5.3] ng/mL) and Compound 48/80 (55.7 [± 0.6] ng/mL). AUC values over the 60-min measurement period reflected the rank order of histamine release observed at peak histamine release (). Note: the extent of histamine release induced by Substance P () was not mirrored in the rank ordering on in vitro response to MRGPRX2 (), possibly due to additional activation of the neurokinin-1 receptor (NK1R) or other receptors on mast cells by this compound (Erin et al. Citation2004; Subramanian et al. Citation2016; Green et al. Citation2019; Zhan et al. Citation2019).
Figure 2. Compounds active on MRGPRX2 induce histamine release in human skin specimens. All compounds were injected at 3 µM and histamine release was measured for 60 min (in 10-min intervals) using microdialysis sampling. Basal levels (0 min measurement) have been subtracted from the remaining timepoints. (A) Histamine recovery depicted in ng/ml. Points are shown as mean ± SD of technical replicates. (B) Histamine AUC for the 60-min period shown in A. Substance P is off scale, with a mean of 714 ng/ml. The graph depicts mean ± SD of technical replicates.
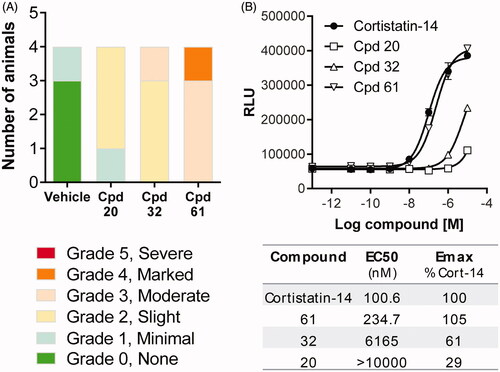
Postmortem evaluation of injection site reactions in rat skin specimens
Ex vivo screening of compounds by postmortem evaluations of injection site swelling was conducted in rats to select drug candidates with low effect or no adverse effect. Macroscopic inspection was first performed to score skin specimens (3 h post-injection) on the severity of swelling/edema induced by a specific compound (, right panel) compared to vehicle controls (, left panel). To determine whether the observed edema was associated with degranulation and thereby histamine release, skin specimens were processed for microscopic evaluation of degranulation. Specimens from vehicle-dosed animals (, left panel) were compared with that of rats dosed with compound (, right panel). Compounds were ranked according to severity score from macroscopic evaluations of injection site reactions (). Compounds that ranked high on the macroscopic evaluation of edema and induced degranulation and histamine release did not progress into in vivo efficacy screening.
Figure 3. Acute induction of swelling/edema in postmortem evaluation of rat skin specimens. Representative examples of rat skin specimens excised postmortem 3 h after single subcutaneous dose of vehicle (left panel) or a compound (right panel) that was found to induce injection site reactions in sub-chronic studies.
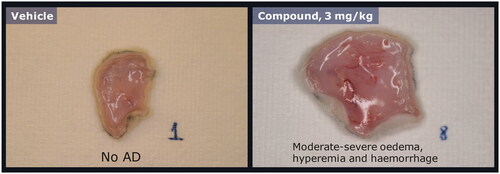
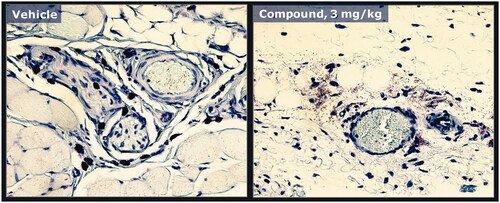
Figure 4. Mast cell degranulation caused by a peptide analogue that induces swelling/edema. Rat skin specimens shown in were processed and stained with tolui-dine blue and nucleus counterstain to confirm degranulation of mast cells in rats dosed subcuta-neously with either vehicle (left panel) or the peptide analogue (right panel). Example of a mast cell with histamine containing granules (red/dark stain) contained within the cytoplasm (left panel). Injection of the peptide analogue induced degranulation and extracellular dispersion of histamine containing granule contents (right panel).
MRGPRX2 activation predicts injection site reactions in rat skin specimens
To determine whether the postmortem ex vivo rankings of compounds could be linked to MRGPRX2, these compounds were screened on activation of MRGPRX2 in the DiscoverX assay (). The rank order of scoring severity from postmortem macroscopic evaluations () was found to correlate with the rank order of potency on MRGPRX2 ().
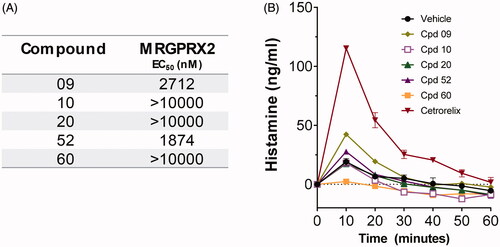
Compounds selected with MRGPRX2 assay do not induce histamine release in human skin specimens
To confirm the translational value of the MRGPRX2 assay, front-runner compounds inactive on MRGPRX2 and qualifying for the relevant criteria for lead candidate selection were tested in the ex vivo assay for histamine release from human skin specimens. Compounds were injected in equimolar concentrations and were tested on the same skin donor as in . Front-runner compounds that showed weak activity on MRGPRX2 () also induced slightly greater histamine release in human skin specimens () and were de-selected.
Discussion
Peptide drug discovery projects may encounter injection site reactions with sub-chronic subcutaneous dosing in rodents. The present study provides an example of identifying the cause of injections site reactions in a discovery project, and the implementation of a translationally-relevant in vitro counter-screen that predicted the occurrence of injection site reactions. This effect on local tolerance was demonstrated to be off-target and the extent/severity of the reaction was found to be species-specific, with rats being more sensitive than mice. Although internal compounds that induced injection site reactions tended to be cationic in nature, it was uncertain what aspect of charge, structure or symmetry of a compound induced the onset of injection site reactions in rodent efficacy models.
Meanwhile, in order to progress projects and evaluate efficacy endpoints preclinically, histological evaluations in rats dosed acutely were conducted prior to proceeding with sub-chronic efficacy studies. The rat had been used as a preclinical safety model in previous injection site reactions studies, where histamine release had been determined to have been the cause of observed changes. Local histamine release causes irritation at the injection site, which then presents as swelling or edema upon macroscopic histological evaluation. McNeil et al. (Citation2015) first reported that activation of the mast cell receptor MRGPRX2 by cationic compounds induces a pseudo-allergenic response in mice. Following up on the report, internal in vitro studies with the DiscoverX MRGPRX2 β-arrestin assay established a parallel association between the occurrence and severity of injection site reactions in rats and activation of and potency on MRGPRX2 for these compounds. A comparison of the evaluations of rodent skin specimens and the MRGPRX2 assay to induction of histamine release in freshly acquired human skin samples confirmed the translational relevance of these findings, thus leading to the selection of safer therapeutics that progress to the clinic.
It must also be acknowledged that not all injection site reactions encountered in preclinical studies are governed by MRGPRX2. Nonetheless, the in vitro screen provides a quick way to eliminate this possibility, while the ex vivo human skin model allows for a more complex and representative evaluation of ‘the real world’ where a compound may have agonistic effects on several receptors. The plethora of recent literature reports further substantiating the role of MRGPRX2 in drug-induced allergenic responses, i.e., drugs having off-target activity on MRGPRX2, suggests that the probability of encountering this preclinical safety signal is not uncommon (Porebski et al. Citation2018; Grimes et al. Citation2019; Jiang et al. Citation2019; Zheng et al. Citation2019; Hu et al. Citation2020; Mencarelli et al. Citation2020; Ogasawara et al. Citation2020; Sahid et al. Citation2020). As an example, LaFleur et al. (Citation2020) similarly grappled with injection site reactions in preclinical animal studies and used human CD34+ stem cell-derived mature human mast cells expressing MRGPRX2 to validate the translational relevance of using in vitro screens against MRGPRX2 and provide a comparison between two (Ca2+ based and β-arrestin) commercially-available assays for this purpose.
Lansu et al. (Citation2017) used in silico modeling tools to assess the structure-activity nature of drugs interacting with MRGPRX2. Based on extensive in vitro screening on LAD2 cells (a human mast cell line) the authors asserted that (A) MRGPRX2 is a Gαq coupled GPCR with a preference for peptides with positive charges, and (B) while structurally distinct from canonical opioid receptors, MRGPRX2 does bind (but not exclusively) to many opioid receptor agonists (Lansu et al. Citation2017). However, a consensus pattern on structure or sequence could not be determined on peptides unrelated to opioid receptor agonists. As such, based on current know-ledge, relying on confirmation of activity on MRGPRX2 with the in vitro screen is the most efficient and reliable method to avoid advancing compounds with off-target activity on this receptor. In addition, the in vitro test replaces the ex vivo rodent test, thereby eliminating use of animals for prescreening.
Thus, when faced with pseudo-allergenic reactions caused by MRGPRX2 in preclinical animal studies, this study provides evidence demonstrating that early drug discovery projects can use the in vitro MRGPRX2 activation as a counter-screen to deselect suboptimal compounds from progressing to the clinic. Results from the in vitro assay may be further corroborated using a more complex human test system such as the ex vivo skin model, which may also reveal other off-target effects of lead candidates not related to MRGPRX2.
Acknowledgements
The authors would like to thank Jette Platou, Birgitte Björkenberg and Louise Nielsen for the contribution of their technical expertise on the in vitro and ex-vivo assays
Disclosure statement
LMJ, CMD, KCF, CBJ and BSW are full-time employees of Novo Nordisk and hold minor share portions as part of their employment.
References
- Ali H. 2016. Mas-related G protein coupled receptor-X2: A potential new target for modulating mast cell-mediated allergic and inflammatory diseases. J Immunobiol. 2016(1):115.
- Ali H. 2017. Emerging roles for MAS-related g protein-coupled receptor-X2 in host defense peptide, opioid, and neuropeptide-mediated inflammatory reactions. Adv Immunol. 136:123–162.
- Allia E, Tarabra E, Volante M, Cerrato M, Ghigo E, Muccioli G, Papotti M. 2005. Expression of cortistatin and MrgX2, a specific cortistatin receptor, in human neuroendocrine tissues and related tumours. J Pathol. 207(3):336–345.
- Amatya B, Nordlind K, Wahlgren C. 2010. Responses to intradermal injections of Substance P in psoriasis patients with pruritus. Skin Pharmacol Physiol. 23(3):133–138.
- Baş M, Greve J, Stelter K, Havel M, Strassen U, Rotter N, Veit J, Schossow B, Hapfelmeier A, Kehl V, et al. 2015. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 372(5):418–425.
- Bulfone-Paus S, Nilsson G, Draber P, Blank U, Levi-Schaffer F. 2017. Positive and negative signals in mast cell activation. Trends Immunol. 38(9):657–667.
- Charitos I, Castellaneta F, Santacroce L, Bottalico L. 2020. Historical anecdotes and breakthroughs of histamine: From discovery to date. Endocr Metab Immune Disord Drug Targets. 2020. DOI:https://doi.org/10.2174/1871530320666200729150124.
- Chen R-G, Shen Y-N, Yei J, Wang C-F, Xie D-H, Wang X-H, Zhou J-D, Chen C-Y, Wu Y-L, Gunnarsson R, et al. 2008. A comparative study of growth hormone (GH) and GH-releasing hormone(1-29)-NH2 for stimulation of growth in children with GH deficiency. Acta Paediatr Suppl. 82:32–35.
- de Antonio M, Rothschild A. 1969. Histamine release and mast cell degranulation induced by 2,4-dinitrophenol in rat tissues. Experientia. 25(3):244–245.
- Ding Y, Che D, Li C, Cao J, Wang J, Ma P, Zhao T, An H, Zhang T. 2019. Quercetin inhibits MRGPRX2-induced pseudo-allergic reaction via PLCγ-IP3R related Ca2+ fluctuations. Int Immunopharmacol. 66:185–197.
- Elieh Ali Komi D, Wohrl S, Bielory L. 2020. Mast cell biology at molecular level: A comprehensive review. Clin Rev Allergy Immunol. 58(3):342–365.
- Erin N, Ersoy Y, Ercan F, Akici A, Oktay S. 2004. NK-1 antagonist CP99994 inhibits stress-induced mast cell degranulation in rats. Clin Exp Dermatol. 29(6):644–648.
- Garafalo J, Kaplan AP. 1981. Histamine release and therapy of severe dermatographism. J Allergy Clin Immunol. 68(2):103–105.
- Gaudenzio N, Sibilano R, Marichal T, Starkl P, Reber LL, Cenac N, McNeil BD, Dong X, Hernandez JD, Sagi-Eisenberg R, et al. 2016. Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest. 126(10):3981–3998.
- Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. 2019. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron. 101(3):412–420.
- Grimbaldeston M. 2015. Mast cell-MrgprB2: sensing secretagogues or a means to over-react? Immunol Cell Biol. 93(3):221–223.
- Grimes J, Desai S, Charter N, Lodge J, Santos R, Isidro-Llobet A, Mason A, Wu Z, Wolfe L, Anantharaman L, et al. 2019. MrgX2 is a promiscuous receptor for basic peptides causing mast cell pseudo-allergic and anaphylactoid reactions. Pharmacol Res Perspect. 7(6):e00547.
- Hu T, Hou Y, Lu J, Wang X, Wei D, Wang C. 2020. Dextromethorphan, a widely-used cough suppressant, induces local anaphylaxis via MRGPRX2 on mast cells. Chem Biol Interact. 2020:109248.
- Jensen B, Bartko E, Baumann K, Skov P. 2020. Measuring histamine and cytokine release from basophils and mast cells. Meth Mol Biol. 2163:247–262.
- Jiang W, Hu S, Che D, An H, Liu R. 2019. A mast-cell-specific receptor mediates Iopamidol-induced immediate IgE-independent anaphylactoid reactions. Int Immunopharmacol. 75:105800.
- Kimura L, Prezotto-Neto J, Tavora B, Faquim-Mauro E, Pereira N, Antoniazzi M, Jared S, Teixeira C, Santoro M, Barbaro K. 2015. Mast cells and histamine play an important role in edema and leukocyte recruitment induced by Potamotrygon motoro stingray venom in mice. Toxicon. 103:65–73.
- Kumar M, Singh K, Duraisamy K, Ajarem J, Kwok C, Chow B. 2020. Protective effect of genistein against Compound 48/80-induced anaphylactoid shock via inhibiting MAS related G protein-coupled receptor X2 (MRGPRX2). Molecules. 25(5):1028.
- Lafleur M, Werner J, Fort M, Lobenhofer E, Balazs M, Goyos A. 2020. MRGPRX2 activation as a rapid, high-throughput mechanistic-based approach for detecting peptide-mediated human mast cell degranulation liabilities. J Immunotoxicol. 17(1):110–121.
- Lansu K, Karpiak J, Liu J, Huang X-P, McCorvy JD, Kroeze WK, Che T, Nagase H, Carroll FI, Jin J, et al. 2017. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol. 13(5):529–536.
- McNeil B, Pundir P, Meeker S, Han L, Undem B, Kulka M, Dong X. 2015. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 519(7542):237–241.
- Mencarelli A, Gunawan M, Yong K, Bist P, Tan W, Tan S, Liu M, Huang E, Fan Y, Chan J, et al. 2020. A humanized mouse model to study mast cells mediated cutaneous adverse drug reactions. J Leukoc Biol. 107(5):797–807.
- Ogasawara H, Furuno M, Edamura K, Noguchi M. 2020. Peptides of major basic protein and eosinophil cationic protein activate human mast cells. Biochem Biophys Rep. 21:100719.
- Olivennes F, Diedrich K, Frydman R, Felberbaum R, Howles C. 2003. Safety and efficacy of a 3 mg dose of the GnRH antagonist cetrorelix in preventing premature LH surges: Report of two large multi-centre, multi-national, Phase IIIb clinical experiences. Reprod Biomed Online. 6(4):432–438.
- Petersen L, Brasso K, Pryds M, Skov P. 1996. Histamine release in intact human skin by mono-cyte chemoattractant factor-1, RANTES, macrophage inflammatory protein-1α, stem cell factor, anti-IgE, and codeine as determined by an ex vivo skin microdialysis technique. J Allergy Clin Immunol. 98(4):790–796.
- Petersen L, Church M, Skov P. 1997. Histamine is released in the wheal but not the flare following challenge of human skin in vivo: A microdialysis study. Clin Exp Allergy. 27(3):284–295.
- Petersen L. 1997. Quantitative measurement of extracellular histamine concentrations in intact human skin in vivo by the microdialysis technique: methodological aspects. Allergy. 52(5):547–555.
- Petersen L. 1998. Measurement of histamine release in intact human skin by microdialysis technique. Clinical and experimental findings. Dan Med Bull. 45(4):383–401.
- Porebski G, Kwiecien K, Pawica M, Kwitniewski M. 2018. Mas-related G protein-Coupled Receptor-X2 (MRGPRX2) in drug hypersensitivity reactions. Front Immunol. 9:3027.
- Rothschild A. 1970. Mechanisms of histamine release by Compound 48/80. Br J Pharmacol. 38(1):253–262.
- Sahid M, Liu S, Mogi M, Maeyama K. 2020. Tachykinin-1 receptor antagonism suppresses Substance-P- and Compound 48/80-induced mast cell activation from rat mast cells expressing functional mas-related GPCR B3. Inflamm Res. 69(3):289–298.
- Shelburne C, Abraham S. 2011. The mast cell in innate and adaptive immunity. Adv Exp Med Biol. 716:162–185.
- Siehler S, Nunn C, Hannon J, Feuerbach D, Hoyer D. 2008. Pharmacological profile of somatostatin and cortistatin receptors. Mol Cell Endocrinol. 286(1–2):26–34.
- Solinski H, Gudermann T, Breit A. 2014. Pharmacology and signaling of MAS-related G protein-coupled receptors. Pharmacol Rev. 66(3):570–597.
- Subramanian H, Gupta K, Ali H. 2016. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. 138(3):700–710.
- Suzuki Y, Liu S, Ogasawara T, Sawasaki T, Takasaki Y, Yorozuya T, Mogi M. 2020. A novel MRGPRX2-targeting antagonistic DNA aptamer inhibits histamine release and prevents mast cell-mediated anaphylaxis. Eur J Pharmacol. 878:173104.
- Theoharides T, Tsilioni I, Ren H. 2019. Recent advances in our understanding of mast cell activation - or should it be mast cell mediator disorders? Expert Rev Clin Immunol. 15(6):639–656.
- Varricchi G, Pecoraro A, Loffredo S, Poto R, Rivellese F, Genovese A, Marone G, Spadaro G. 2019. Heterogeneity of human mast cells with respect to MRGPRX2 receptor expression and function. Front Cell Neurosci. 13:299.
- Wang J, Zhang Y, Che D, Zeng Y, Wu Y, Qin Q, Wang N. 2020. Baicalin induces Mrgprb2-dependent pseudo-allergy in mice. Immunol Lett. 226:55–61.
- Wei J, Wei X, Mo Y, He S. 2009. Induction of mast cell accumulation, histamine release and skin edema by N49 phospholipase A2. BMC Immunol. 10:21.
- Zhan Y, Ma N, Liu R, Wang N, Zhang T, He L. 2019. Polymyxin B and polymyxin E induce anaphylactoid response through mediation of Mas-related G protein-coupled receptor X2. Chem Biol Interact. 308:304–311.
- Zheng Y, Che D, Peng B, Hao Y, Zhang X, He L, Geng S. 2019. All-trans-retinoic acid activated mast cells via Mas-related G-protein-coupled receptor-X2 in retinoid dermatitis. Contact Dermatitis. 81(3):184–193.