ABSTRACT
Background
Maintaining proper immune function and hormone status is important for athletes to avoid upper respiratory tract infection (URTI) and insufficient recovery, which is detrimental to sport performance and health. The aim of this study was to evaluate whether three-week supplementation of L-glutamine could benefit the mucosal immunity and hormonal status of combat-sport athletes as well as their rates of upper respiratory tract infection (URTI) and subjective feelings of well-being after intensive training.
Methods
Twenty-one combat-sport athletes from the National Taiwan University of Sport were recruited in this study. After intensive training, two groups of the participants were asked to consume powder form of 0.3 g/kg body weight of L-glutamine (GLU group) or maltodextrin (PLA group) with drinking water in a randomized design at the same time every day during 3 weeks. Saliva samples were collected to measure immunoglobulin A (IgA), nitric oxide (NO), testosterone (T) and cortisol (C) before and after three-week supplementation; moreover, Hooper’s index questionnaires were completed for wellness assessment. The incidence and duration of URTI were recorded by using a health checklist throughout the entire study period.
Results
Supplementation of L-glutamine significantly enhanced the concentrations of IgA and NO in saliva; additionally, the incidence of URTI was significantly reduced. Regarding hormones, T concentration was significantly decreased in the PLA group, whereas C concentration was significantly increased, resulting in a significant decrease of T/C ratio. In contrast, the GLU group showed a significant increase of T/C ratio, while the mood scores of the Hooper’s index questionnaire were higher in the PLA group.
Conclusions
Three-week supplementation of L-glutamine after intensive training enhanced the mucosal immunity, improved hormonal status and reduced the rate of URTI of combat-sport athletes while feelings of well-being were also enhanced. Therefore, L-glutamine would be beneficial for the sports performance and recovery of athletes.
1. Background
Repeated strenuous bouts of prolonged exercise for achieving peak performance have been shown to affect athletes’ immunity and increase the risk of upper respiratory tract infections (URTI) [Citation1]. Athletes in combat sports were typically required to meet specific weight categories for competition, which sometimes necessitated rapid weight-loss strategies. Previous studies have reported that the cumulative effects of prolonged intensive training and rapid weight reduction suppressed athletes’ mucosal immunity [Citation2,Citation3], while close contact in the environment of training and competition increased the exposure to pathogens [Citation4]. These features make combat-sport athletes particularly susceptible to infections, which can negatively impact their well-being and sport performance.
Salivary immunoglobulin A (SIgA) is the primary antibody isotype in the mucosal immune system, acting against specific virulence factors and serving as the body’s first line of defense [Citation5,Citation6]. Intensive training has been reported to decrease the secretion of SIgA, making athletes more vulnerable to URTI, which may further interfere with their training and increase the likelihood of missing training days [Citation6]. A study reported that soccer players experienced a significant increase in SIgA secretion rate and a decrease in URTI following a 2-week detraining period after the competitive season [Citation7]. Moreover, another study also indicated that the typical decline of an individual’s relative SIgA over the 3 weeks before an URTI appeared to precede and contribute to the URTI risk [Citation8].
Nitric oxide (NO) plays a major role in the mucosal defense of airways and the oral cavity [Citation9]. The antibacterial effect of NO is expressed in two ways: preventing bacterial growth, and increasing cytotoxicity by macrophages [Citation10]. It has been reported that the increased generation of NO during rhinovirus infections is associated with fewer symptoms and more rapid viral clearance [Citation11], with higher baseline NO being associated with fewer cold symptoms after stress [Citation12]. Based on these studies, boosting NO levels could be beneficial for innate immune defense and preventing respiratory infections.
The concentrations of testosterone (T) and cortisol (C) are influenced by prolonged and high-intensity training [Citation13] while being markers of anabolic/catabolic status, which could be used to monitor athletes’ training responses and recovery status [Citation14]. A study found that footballers with smaller decrease in salivary T levels had better exercise performance [Citation15]; additionally, an increase in salivary C was observed in basketball players during intense training and competition period [Citation16]. The ratio of T/C has been shown to be a sensitive marker for overtraining and widely used in the sports field [Citation17,Citation18]. A study investigated the changes of T/C ratio in professional basketball players before and after a 4-month training season showed that the team experiencing two-fold training volume resulted in a decreased T/C ratio [Citation19]. Another study indicated that the greatest performance enhancement of male swimmers was associated with higher T level, lower C level and higher T/C ratio [Citation20]. Accordingly, athletes’ hormone responses during training and competition could serve as predictors of their recovery status, leading in turn to potential improvement in performance.
L-glutamine, the most abundant amino acid in circulation, is an important fuel for the immune system and serves as an essential nutrient for cytokine production, immune cell proliferation and functions [Citation21]. The deficiency of L-glutamine occurred during periods of high-intensity and prolonged exercise [Citation22]. Reduced glutamine levels are responsible for the immune suppression associated with increased infection rates observed in over-trained athletes [Citation23], where plasma L-glutamine level was seen to decrease by approximately 20% after 1 h of marathon running [Citation24,Citation25]. A previous study showed that chronic L-glutamine supplementation has beneficial effects on immune markers such as CD4+ T, CD8+ T cells and cytokines like IL-6 [Citation26]; moreover, L-glutamine plays several other biological functions such as energy production, glycogenesis and ammonia buffering [Citation27]. Consequently, L-glutamine has attracted the interest of scientists in sport nutrition because of its beneficial effects on the immune system and recovery of athletes.
Saliva analysis is a noninvasive option and has been established as one of the useful and less complex ways to assess the immunological and endocrinological status associated with exercise and training [Citation28]. So far, a supplement like probiotics has increased the total SIgA of pre-school children [Citation29]. L-citrulline, either synthetic or naturally present in watermelon, increased NO synthesis [Citation30], while supplementation of betaine resulted in lower post-exercise C level, higher T level and T/C ratio in handball players [Citation31]. However, till now, no study has reported the effects of L-glutamine on salivary immune markers and hormonal status; as a result, this study aimed to assess whether three-week L-glutamine supplementation after intensive training could benefit the mucosal immune and hormonal status and rate of URTI as well as the feelings of well-being of combat-sport athletes.
2. Methods
2.1. Participants
The estimated sample size was nine participants based on an alpha level of 0.05 and a beta level of 0.8 to identify differences in mucosal immunity after exercise [Citation32]. The characteristics of the participants are presented in . Twenty-one national-level combat-sport athletes (13 taekwondo athletes and 8 boxing athletes) from the National Taiwan University of Sport were recruited for this study with athletes who were injured or unable to participate in regular training being excluded. Each participant was fully informed of all potential risks and experimental procedures, after which written informed consent was obtained. All experimental procedures and protocols were approved by the Institutional Human Ethics Committee (#108–85) of Jen-Ai Hospital, Taichung, Taiwan.
Table 1. Physical characteristics of the participants (n = 21).
2.2. Study design
This study was conducted in a randomized crossover design. The participants adhered to their regular training regimen during the study, which involved warm-up exercises, specific drills, strength and conditioning, and finally concluding with sparring. This training schedule consisted of a 2-h session each day 5 days a week, with an intensity reaching 80% of their maximum heart rate. After training, the participants were randomly divided into two groups: one group consumed a powder form of L-glutamine at 0.3 g/kg body weight (b.w.) (GLU group) and the other consumed maltodextrin (PLA group). These treatments were done in a randomized order, and they carried out this regimen at the same time every day for 3 weeks. The dose of L-glutamine supplementation was selected by referring to previous studies [Citation33,Citation34] while participants were advised to maintain consistent food intake and were prohibited from taking any supplements or drugs throughout the study. Before the start of the experiment and the last training session of three weeks, 2 ml of saliva samples of the participants were collected into sterile plastic containers, and they were asked to complete the Hooper’s index questionnaire. The sample collections were conducted at the same time of the day and on the same day of the week, and the participants were instructed not to eat and drink before the assessment, although participants were allowed to drink water ad libitum during the training session. The saliva samples were stored at −80 °C until the assay was performed.
2.3. Incidence of URTI
The incidence and duration of URTI were recorded during the entire study period. The participants were asked to fill out a health checklist every Friday before training. URTI was recorded when participants reported two or more flu symptoms, including fever, headache, sore throat, sneezing, stuffy nose, nasal discharge, cough, malaise, and chilliness, for at least two consecutive days [Citation2].
2.4. Evaluation of muscle soreness, mood, sleep quality and stress
Hooper’s index questionnaire was adapted to assess participant’s fatigue and wellbeing before and after three weeks of supplementation [Citation35,Citation36]. Subjective ratings of general muscle soreness, mood and quality of sleep during the previous night and the stress level were assessed by a 5-point Likert scale.
2.5. Analysis of IgA, testosterone, cortisol and NO in saliva
The saliva concentration of bovine serum albumin was determined using the BioRAD protein assay kit (Bio-RAD, Hercules, CA, USA) and was represented as salivary total protein (TP) levels. Salivary concentrations of IgA, testosterone and cortisol were measured using enzyme-linked immunosorbent assays (ELISA, Calbiochem, Darmstadt, Germany), following the manufacturer’s instructions while NO production was assessed according to the Griess reagent. Briefly, 100 μl of saliva was mixed with 100 μl of Griess reagent [equal volumes of 1% (w/v) sulfanilamide in 5% (v/v) phosphoric acid and 0.1% (w/v) naphthylethylenediamine-HCl], incubated at room temperature for 10 min, and then the absorbance was measured at 540 nm in a spectrophotometer. All samples were measured in triplicates. The inter-assay coefficients of variation were 2.5% for IgA, 3.4% for NO and 1.5% for both testosterone and cortisol measurements. The intra-assay coefficients of variation for IgA, testosterone, cortisol, and NO measurements were 3%, 4%, 4% and 1.7% respectively, with the percentage changes of testosterone, cortisol, and T/C ratio being calculated individually by (POST-PRE)/PRE x100%.
2.6. Statistical analyses
The results were expressed as means ± standard deviations (SD). The Shapiro – Wilk test was used to analyze the distribution of datasets; statistical comparisons in each group were analyzed using paired t-tests; while Student’s t-test was used to compare the percentage changes between two groups. The relationship between supplementation and the incidence of URTI was determined by using Chi-Square test and the scores for Hooper’s index questionnaire were analyzed using Wilcoxon signed-rank test. Significant differences were set at *p < 0.05, **p < 0.01 and ***p < 0.001.
3. Results
3.1. Effects of L-glutamine on the concentrations of IgA, TP, NO in saliva and the incidence of URTI
After 3-week supplementation of L-glutamine, the concentrations of IgA, TP and NO in saliva were significantly increased as was the ratio of IgA/TP. The incidence of URTI was significantly lower in GLU group (p < 0.01, ).
Table 2. Concentrations of salivary IgA, TP, NO and incidence of URTI.
3.2. Effects of L-glutamine on the concentrations of testosterone (T) and cortisol (C) in saliva
The results showed that the concentration of testosterone significantly decreased after L-glutamine supplementation, whereas that of cortisol significantly increased in the PLA group, but there were no significant changes of T and C concentrations in the GLU group. In addition, the T/C ratio showed a significant decrease in the PLA group, whereas there was no significant change in the GLU group (). Moreover, the percentage changes in testosterone and T/C ratio exhibited significant differences between the two groups. The decreased percentage of T level in the GLU group was significantly smaller than that in the PLA group (−0.49% in GLU; −31.24% in PLA, p < 0.01, ). In addition, the increased percentages of T/C ratio in the GLU group was significantly higher than that in the PLA group (+84.86% in GLU; −6.36% in PLA, p < 0.05, ). However, the percentage change of cortisol showed no significant difference () between the two groups.
Figure 1. The percentage changes of salivary testosterone (T), cortisol (C) and T/C ratio. Values are mean ± SD. *p < 0.05, **p < 0.01, reflecting significant difference compared to the PLA group. GLU, L-glutamine group; PLA, placebo group.
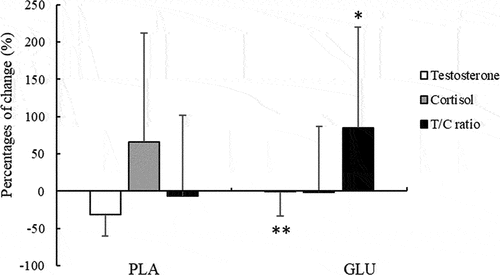
Table 3. Concentrations and the ratios of testosterone and cortisol.
3.3. Effect of L-glutamine on the scores of Hooper’s index questionnaire
The results showed that the scores for mood in the PLA group were significantly higher than those in the GLU group after 3 weeks of L-glutamine supplementation (). The scores of general muscle soreness, sleep quality and stress levels showed no significant differences between the two groups.
Table 4. Scores of Hooper’s index questionnaire.
4. Discussion
To our knowledge, this is the first study reporting that 3-week supplementation of L-glutamine after combat training significantly increased the IgA, NO and T/C ratio in saliva, decreased the incidence of URTI, and led to better mood status. These findings suggest that inveterate or intense supplementation of L-glutamine has the potential to prevent anabolic/catabolic hormonal disturbances caused by prolonged and intensive training, thereby helping athletes maintain their health and improve sports performance.
A previous study collected unstimulated saliva samples from 38 elite America’s Cup yacht-racing athletes over a period of 50 weeks of training and resting and found that IgA determined a substantial proportion of the variability in weekly URTI incidence [Citation8]. Another study analyzed the saliva of 19 male elite rugby union players biweekly over a 10-week training period and their symptoms of URTI were documented in an illness log with results revealing the likelihood of suffering from increased URTI when IgA was decreased; for example, players were reported as being at greater risk of URTI within the subsequent 2 weeks when SIgA decreased by 65% or more [Citation37]. Furthermore, another study indicated that L-glutamine might affect intestinal secretory IgA production through intestinal microbiota [Citation38,[Citation39]]. When elderly participants were supplemented with 0.3 g/kg b.w./day of L-glutamine for 30 consecutive days, the SIgA levels increased [Citation34]. On the other hand, TP in saliva has been shown to be a sensitive noninvasive marker of overall body hydration status and is strongly associated with the changes in body mass during progressive acute dehydration. It is worth mentioning that the TP level was not changed by L-glutamine supplementation in our study, which suggests that the increased concentration of SIgA was not associated with hydration status [Citation40], so the present study revealed that L-glutamine supplementation increased SIgA level and consequently led to decreased incidence of URTI.
In NO synthesis, L-Glutamine plays a crucial role as an essential amino acid, serving as a precursor of arginine [Citation41]. An in vitro study exposed cells to various concentrations of arginine before stimulation with lipopolysaccharide (LPS), where the cells precultured with lower concentrations of arginine produced less NO than those with higher concentrations of arginine, indicating a positive correlation between arginine availability and NO production following LPS stimulation [Citation42]. It has been validated that the effects of arginine supplementation on immunity and anti-infection in humans are via increasing NO synthesis [Citation43]. A six-month supplementation of L-arginine improved the NO production in hypertensive patients with type 2 diabetes [Citation44]. Our study showed a significant increase of NO concentration after 3-week L-glutamine supplementation, which could be explained by its role as arginine precursor. This suggests that salivary NO assessment could serve as a simple and fast method to monitor the immunity of athletes during training and competition.
Our results also indicated that following three weeks of training, the PLA group exhibited significant reduction in salivary T level and a corresponding increase of C level, leading to a decrease of the T/C ratio. It is essential for athletes to maintain an appropriate anabolic/catabolic hormone balance, because a decline in T level and sustained elevation of C level resulting from physiological and/or psychological stress during training can negatively impact an athlete’s performance and overall health [Citation15,Citation45]. The intense match schedule (7 matches played in 7 days) might affect the youth soccer players’ hormonal status and induce a decrease in T concentration [Citation46]. When 11 elite male weightlifters were trained before a national competition, the salivary T/C ratio was significantly higher in the training stages than in the recovery stage [Citation47]. A study including 30 professional soccer players from the National Soccer League reported a significant decrease of T/C ratio by more than 30% at the end of the competition phase [Citation48]. A previous study has indicated that supplementation of 2 g/day of betaine for 14 weeks increased the T level and T/C ratio in youth professional soccer players during a competition season [Citation49], while another study showed that supplementation of 3 g/day of citrulline and 2.1 g/day of nitrate-rich beetroot extract for 9 weeks improved the recovery status through preventing the increase of C level and increasing the T/C ratio in trained male triathletes [Citation50]. Likewise, the present study found that 3-week supplementation of L-glutamine at 0.3 g/kg b.w./day attenuated both the decrease of T level and the increase of C level in saliva, and further increased the percentage change of T/C ratio in combat-sport athletes after three weeks of training. We suggest that the use of L-glutamine might potentially lead to better recovery after training and prevent the occurring harmful effects of overtraining.
It is well known that in the field of sports, mood state is affected by psychophysiological responses and mood state variation has been associated with performance achievement or failure [Citation51]. A previous study evaluating elite soccer players during an intense competition period (playing 10 games over 6 weeks) showed a significant increase in total mood disturbance along with a significant decrease in the T/C ratio and sports performance [Citation52]. A clinical study providing 40 g of L-glutamine to patients undergoing transplantation reported an improvement of mood [Citation53], while another study of the elderly showed that 0.3 g/kg b.w./day of L-glutamine supplementation for 30 days increased anti-inflammation and anti-oxidation abilities [Citation54]. Furthermore, a review article suggested that glutamine supplementation could attenuate mood- and cognitive-worsening in hypoxia while reducing the inflammatory mediators released during injury and stress conditions [Citation55]. The role of L-glutamine to reduce inflammation mediated by a leaky gut during exercise can contribute to the changes in the central nervous system [Citation56,Citation57]. In our study, we found a significant improvement of the scores for mood in the GLU group, whereas there were no changes in the scores for general muscle soreness, sleep quality and stress levels. Additionally, the average rate of perceived exertion for athletes did not change during the training period in both groups (data not shown). Although we did not assess inflammatory markers in this study, we speculate that the improvement in mood of combat-sport athletes was caused by the potentially anti-inflammatory properties of L-glutamine. Future studies could investigate the effects of L-glutamine on athletes’ mood state and the relationship with their overall well-being and performance.
5. Conclusions
This study demonstrated that 3-week supplementation of L-glutamine after training led to increased concentrations of IgA and NO in saliva and a reduction in the incidence of URTI. In addition, the decreased percentage of T level was attenuated and the T/C ratio was increased, resulting in better anabolic/catabolic status; moreover, the subjective mood status of the athletes was improved. Accordingly, L-glutamine supplementation has the potential to enhance performance and recovery status of combat-sport athletes.
Author contributions
Study Design: TLL and SHF; Data Collection: ACZ, CCL, and JYW; Data Analysis: TLL, ACZ, JYW, and SHF; Manuscript Writing and Editing: TLL, KS, and SHF; Final Manuscript Review and Approval: TLL, KS, and SHF
Acknowledgments
All authors would like to thank coaches and athletes of the taekwondo and boxing teams in the National Taiwan University of Sport for assisting in the study process and contributing to this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Agha-Alinejad H, Ahmadi Hekmatikar AH, Ruhee RT, et al. A guide to different intensities of exercise, vaccination, and sports nutrition in the course of preparing elite athletes for the management of upper respiratory infections during the COVID-19 pandemic: a narrative review. Int J Environ Res Public Health. 2022;19(3):1888. doi: 10.3390/ijerph19031888
- Tsai ML, Chou KM, Chang CK, et al. Changes of mucosal immunity and antioxidation activity in elite male Taiwanese taekwondo athletes associated with intensive training and rapid weight loss. Br J Sports Med. 2011;45(9):729–53. doi: 10.1136/bjsm.2009.062497
- Tsai ML, Ko MH, Chang CK, et al. Impact of intense training and rapid weight changes on salivary parameters in elite female taekwondo athletes. Scand J Med Sci Sports. 2011;21(6):758–764. doi:10.1111/j.1600-0838.2010.01099.x
- Kordi R, Ziaee V, Rostami M, et al. Sports injuries and health problems among wrestlers in Tehran. J Pak Med Assoc. 2012;62(3):204–208. doi:10.1186/1758-2555-3-4
- Hanson LA, Ahlstedt S, Andersson B, et al. Mucosal immunity. Ann N Y Acad Sci. 1983;409:1–21. doi:10.1111/j.1749-6632.1983.tb26855.x
- Li Y, Jin L, Chen T. The effects of secretory IgA in the mucosal immune system. Biomed Res Int. 2020;2020:2032057. doi:10.1155/2020/2032057
- Moreira A, Mortatti AL, Arruda AF, et al. Salivary IgA response and upper respiratory tract infection symptoms during a 21-week competitive season in young soccer players. J Strength Cond Res. 2014;28(2):467–473. doi:10.1519/JSC.0b013e31829b5512
- Neville V, Gleeson M, Folland JP. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med Sci Sports Exerc. 2008;40(7):1228–1236. doi:10.1249/MSS.0b013e31816be9c3
- Taghavi-Zonuz A, Jamali Z, Pourzare-Mehrbani S, et al. Effect of mobile phone waves and Wi-Fi on electrolytes and oxidative stress indices of saliva. World J Dent. 2017;8:370–373. doi:10.5005/jp-journals-10015-1467
- Kasapović J, Pejić S, Stojiljković V, et al. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages after chemotherapy with 5-fluorouracil, doxorubicin and cyclophosphamide. Clin Biochem. 2010;43(16–17):1287–1293. doi:10.1016/j.clinbiochem.2010.08.009
- Proud D. Nitric oxide and the common cold. Curr Opin Allergy Clin Immunol. 2005;5(1):37–42. doi:10.1097/00130832-200502000-00008
- Ritz T, Trueba AF, Vogel PD, et al. Exhaled nitric oxide and vascular endothelial growth factor as predictors of cold symptoms after stress. Biol Psychol. 2018;132:116–124. doi:10.1016/j.biopsycho.2017.11.006
- Kamarauskas P, Conte D. Changes in salivary markers during basketball long-term and short-term training periods: a systematic review. Biol Sport. 2022;39(3):673–693. doi:10.5114/biolsport.2022.107018
- Crewther BT, Lowe TE, Ingram J, et al. Validating the salivary testosterone and cortisol concentration measures in response to short high-intensity exercise. J Sports Med Phys Fitness. 2010;50(1):85–92.
- Penailillo L, Maya L, Nino G, et al. Salivary hormones and IgA in relation to physical performance in football. J Sports Sci. 2015;33(20):2080–2087. doi:10.1080/02640414.2015.1064151
- He CS, Tsai ML, Ko MH, et al. Relationships among salivary immunoglobulin A, lactoferrin and cortisol in basketball players during a basketball season. Eur J Appl Physiol. 2010;110(5):989–995. doi:10.1007/s00421-010-1574-8
- Grandys M, Majerczak J, Frolow M, et al. Age-related decrease in serum dihydrotestosterone concentration is accompanied by impaired vascular status. Exp Gerontol. 2023;173:112104. doi: 10.1016/j.exger.2023.112104.
- Li CY, Hsu GS, Suzuki K, et al. Salivary immuno factors, cortisol and testosterone responses in athletes of acompetitive 5,000mrace. Chin JPhysiol. 2015;58(4):263–269.
- González-Bono E, Moya-Albiol L, Martínez-Sanchis S, et al. Salivary testosterone and cortisol responses to cycle ergometry in basketball players with different training vol. J Psychophysiol. 2002;16(3):158–166.
- Majumdar P, Srividhya S Jr. Monitoring training load in Indian male swimmers. Int J Exerc Sci. 2010;3(3):102–107.
- Hall JC, Heel K, McCauley R. Glutamine. Br J Surg. 2005;83(3):305–312. doi:10.1002/bjs.1800830306
- Cruzat V, Macedo Rogero M, Noel Keane K, et al. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10(11):1564. doi: 10.3390/nu10111564
- Master PBZ, Macedo RCO. Effects of dietary supplementation in sport and exercise: a review of evidence on milk proteins and amino acids. Crit Rev Food Sci Nutr. 2021;61(7):1225–1239. doi:10.1080/10408398.2020.1756216
- Castell LM, Newsholme EA. The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition. 1997;13(7–8):738–742. doi: 10.1016/S0899-9007(97)83036-5.
- Castell LM, Newsholme EA. Glutamine and the effects of exhaustive exercise upon the immune response. Can JPhysiol Pharmacol. 1998;76(5):524–532.
- Alipanah-Moghadam R, Molazadeh L, Jafari-Suha Z, et al. Glutamine supplementation can reduce some atherosclerosis markers after exhaustive exercise in young healthy males. Nutrition. 2022;94:111506. doi:10.1016/j.nut.2021.111506
- Coqueiro AY, Rogero MM, Tirapegui J. Glutamine as an anti-fatigue amino acid in sports nutrition. Nutrients. 2019;11(4):863. doi: 10.3390/nu11040863
- Papacosta E, Nassis GP. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J Sci Med Sport. 2011;14(5):424–434. doi:10.1016/j.jsams.2011.03.004
- Surono IS, Koestomo FP, Novitasari N, et al. Novel probiotic enterococcus faecium IS-27526 supplementation increased total salivary sIgA level and bodyweight of pre-school children: a pilot study. Anaerobe. 2011;17(6):496–500. doi: 10.1016/j.anaerobe.2011.06.003
- Figueroa A, Wong A, Jaime SJ, et al. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr Opin Clin Nutr Metab Care. 2017;20(1):92–98. doi:10.1097/MCO.0000000000000340
- Arazi H, Aboutalebi S, Taati B, et al. Effects of short-term betaine supplementation on muscle endurance and indices of endocrine function following acute high-intensity resistance exercise in young athletes. J Int Soc Sports Nutr. 2022;19(1):1–16. doi:10.1080/15502783.2022.2041988
- Gillum T, Kuennen M, Miller T, et al. The effects of exercise, sex, and menstrual phase on salivary antimicrobial proteins. Exerc Immunol Rev. 2014;20:23–38.
- Ogden HB, Child RB, Fallowfield JL, et al. Gastrointestinal tolerance of low, medium and high dose acute oral l-glutamine supplementation in healthy adults: a pilot study. Nutrients. 2020;12(10):2953. doi:10.3390/nu12102953
- Paixão V, Almeida EB, Amaral JB, et al. Elderly subjects supplemented with L-glutamine shows an improvement of mucosal immunity in the upper airways in response to influenza virus vaccination. Vaccines. 2021;9(2):107. doi:10.3390/vaccines9020107
- Reiner S, Smith G, Davis R, et al. Exercise participation and subjective well-being of collegiate athletes during COVID-19 pandemic. J Human Sport Exercise. 2021;17. doi: 10.14198/jhse.2022.173.16.
- Hooper SL, Mackinnon LT, Howard ALF, et al. Markers for monitoring overtraining and recovery. Med Sci Sports Exerc. 1995;27(1).
- Tiernan C, Lyons M, Comyns T, et al. Salivary IgA as a predictor of upper respiratory tract infections and relationship to training load in elite rugby union players. J Strength Cond Res. 2020;34(3):782–790. doi:10.1519/JSC.0000000000003019
- Wu M, Xiao H, Liu G, et al. Glutamine promotes intestinal SIgA secretion through intestinal microbiota and IL-13. Mol Nutr Food Res. 2016;60(7):1637–1648. doi: 10.1002/mnfr.201600026.
- Ren W, Wang K, Yin J, et al. Glutamine-induced secretion of intestinal secretory immunoglobulin A: amechanistic perspective. Front Immunol. 2016;7:503.
- Walsh NP, Montague JC, Callow N, et al. Saliva flow rate, total protein concentration and osmolality as potential markers of whole body hydration status during progressive acute dehydration in humans. Arch Oral Biol. 2004;49(2):149–154. doi:10.1016/j.archoralbio.2003.08.001
- Bellows CF, Jaffe BM. Glutamine is essential for nitric oxide synthesis by murine macrophages. J Surg Res. 1999;86(2):213–219. doi:10.1006/jsre.1999.5713
- Bryk J, Ochoa JB, Correia MI, et al. Effect of citrulline and glutamine on nitric oxide production in RAW 264.7 cells in an arginine-depleted environment. JPEN J Parenter Enteral Nutr. 2008;32(4):377–383.
- Wu G, Meininger CJ, McNeal CJ, et al. Role of L-arginine in nitric oxide synthesis and health in humans. Adv Exp Med Biol. 2021;1332:167–187.
- Martina V, Masha A, Gigliardi VR, et al. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diab Care. 2008;31(5):940–944. doi:10.2337/dc07-2251
- Freitas CG, Aoki MS, Franciscon CA, et al. Psychophysiological responses to overloading and tapering phases in elite young soccer players. Pediatr Exerc Sci. 2014;26(2):195–202. doi:10.1123/pes.2013-0094
- Moreira A, Bradley P, Carling C, et al. Effect of a congested match schedule on immune-endocrine responses, technical performance and session-RPE in elite youth soccer players. J Sports Sci. 2016;34(24):2255–2261. doi:10.1080/02640414.2016.1205753
- Tsai ML, Li TL, Chou LW, et al. Resting salivary levels of IgA and cortisol are significantly affected during intensive resistance training periods in elite male weightlifters. J Strength Cond Res. 2012;26(8):2202–2208. doi:10.1519/JSC.0b013e31823a4246
- Handziski Z, Maleska V, Petrovska S, et al. The changes of ACTH, cortisol, testosterone and testosterone/cortisol ratio in professional soccer players during a competition half-season. Bratisl Lek Listy. 2006;107(6–7):259–263.
- Nobari H, Kargarfard M, Minasian V, et al. The effects of 14-week betaine supplementation on endocrine markers, body composition and anthropometrics in professional youth soccer players: a double blind, randomized, placebo-controlled trial. J Int Soc Sports Nutr. 2021;18(1):20. doi:10.1186/s12970-021-00417-5
- Burgos J, Viribay A, Calleja-Gonzalez J, et al. Long-term combined effects of citrulline and nitrate-rich beetroot extract supplementation on recovery status in trained male triathletes: a randomized, double-blind, placebo-controlled trial. Biology. 2022;11(1):75. doi: 10.3390/biology11010075
- Selmi O, Ouergui I, Muscella A, et al. Monitoring mood state to improve performance in soccer players: a brief review. Front Psychol. 2023;14:1095238. doi:10.3389/fpsyg.2023.1095238
- Saidi K, Ben Abderrahman A, Boullosa D, et al. The interplay between plasma hormonal concentrations, physical fitness, workload and mood state changes to periods of congested match play in professional soccer players. Front Physiol. 2020;11:835. doi:10.3389/fphys.2020.00835
- Young LS, Bye R, Scheltinga M, et al. Patients receiving glutamine-supplemented intravenous feedings report an improvement in mood. JPEN J Parenter Enteral Nutr. 1993;17(5):422–427. doi:10.1177/0148607193017005422
- Almeida EB, Santos JMB, Paixao V, et al. L-glutamine supplementation improves the benefits of combined-exercise training on oral redox balance and inflammatory status in elderly individuals. Oxid Med Cell Longev. 2020;2020:2852181. doi:10.1155/2020/2852181
- Dos Santos Quaresma M, Souza W, Lemos VA, et al. The possible importance of glutamine supplementation to mood and cognition in hypoxia from high altitude. Nutrients. 2020;12(12):3627. doi: 10.3390/nu12123627
- Clapp M, Aurora N, Herrera L, et al. Gut microbiota’s effect on mental health: the gut-brain axis. Clin Pract. 2017;7(4):987. doi: 10.4081/cp.2017.987.
- Chantler S, Griffiths A, Matu J, et al. A systematic review: role of dietary supplements on markers of exercise-associated gut damage and permeability. PLoS One. 2022;17(4):e0266379.
