ABSTRACT
Background
This study aimed to determine the optimal time point, either 30 or 60 minutes, at which muscle reactivity to caffeine administration is highest. Unlike previous studies that focused on the nervous system response, we employed tensiomyography (TMG) to directly assess the effects of caffeine on muscle fibers.
Methods
TMG measurements were performed on the gastrocnemius medialis muscle of 42 male athletes who regularly consumed caffeine. Participants received a dose of 6 mg/kg body weight and TMG measurements were taken prior to caffeine intake, as well as 30 and 60 minutes afterward.
Results
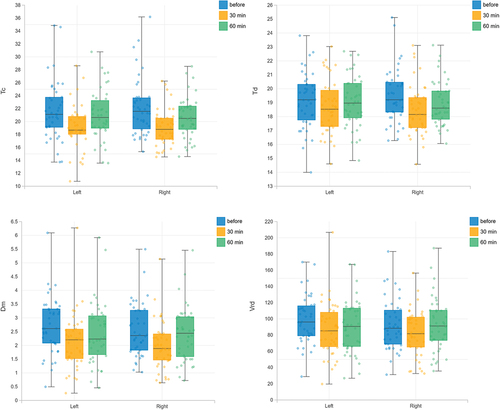
Analysis of TMG parameters including time to contraction (Tc), time delay (Td), and maximal displacement (Dm) revealed that muscles exhibited faster contractions and greater stiffness at the 30-minute mark compared to both pre-caffeine intake and the 60-minute time point. Time exerted a significant main effect on Tc (F(2, 246) = 12.09, p < .001, ή2p = 0.09), Td (F(2, 246) = 3.39, p = .035, ή2p = 0.03), and Dm (F(2, 246) = 6.83, p = .001, ή2p = 0.05), while no significant effect of body side was observed.
Conclusions
The findings indicate that muscle contraction time (Tc) and delay time (Td) are influenced by the time elapsed since caffeine ingestion, with the fastest responses occurring after 30 minutes. Additionally, a systemic effect of caffeine was observed, as there were no discernible differences in measurements between the two sides of the body. TMG proves to be an effective noninvasive method for assessing muscle responses following caffeine administration.
1. Introduction
Caffeine (CAF) is one of the most commonly consumed stimulants in the world. It has the ability to improve sports performance and cognitive function [Citation1]. However, it is difficult to determine the exact ergogenic mechanism of caffeine on athletic performance. The most important mechanism of caffeine is the blockade of adenosine receptors, which contributes to increased alertness and a possible increase in muscle fiber recruitment [Citation2]. In addition, caffeine has the ability to inhibit phosphodiesterase, resulting in increased cyclic adenosine monophosphate concentration, increased catecholamine release [Citation3], and inhibition of γ-aminobutyric acid receptors [Citation4]. The presence of caffeine can also open ion channels, particularly in muscle myocytes [Citation5], and release a calcium reserve in the sarcoplasmic reticulum, resulting in improved muscle speed and strength [Citation6]. However, increased mobilization of calcium ions under the influence of caffeine leads to a slowing of muscle relaxation [Citation7]. In addition, muscles exposed to caffeine showed a lower ability to restore homeostasis. In experiments in the isolated sarcoplasmic reticulum, caffeine administration resulted in an immediate release of calcium and thus a greater ability of muscles to function under electrical stimulation [Citation8]. The ability of caffeine to increase adrenaline release, release calcium ions, and decrease pain perception appears to be directly related to improved athletic performance, however, there are conflicting reports on the optimal caffeine dose and timing of ingestion to achieve maximum benefit [Citation9]. In the literature, the most effective caffeine doses typically ranged from 3 to 6 mg/kg of body weight, most commonly recommended to be taken 60 minutes before physical activity [Citation10].
Studies on the effects of caffeine are mostly based on the assessment of the concentration of caffeine and its metabolites in plasma [Citation11], heart rate or heart rate variability [Citation12], improvement in cognition [Citation13], and assessment of improvement in athletic performance [Citation14]. Researchers have used various methods to evaluate the effects of caffeine on athlete performance, although there are still important considerations about how caffeine actually affects muscles. For this reason, direct muscle diagnostics, such as tensiomyography (TMG), may be a useful tool to optimize caffeine intake. TMG is a practical, time- and cost-effective method for assessing changes in muscle function and preventing injury [Citation15].
TMG is considered a reliable measurement tool, and its results have good to excellent reliability parameters [Citation16]. TMG measures the contraction of the superficial muscles and does not require effort from the subject. It also provides fast and accurate information without affecting the subject’s body, so does not cause any direct physical harm, discomfort, or alteration to the subject’s body during the measurement process. [Citation17]. TMG is unique because it tests the direct response of muscle fibers rather than the response of the nervous system, which has also been found with the administration of caffeine [Citation9]. This is important because it allows for a direct understanding of how caffeine affects the muscle fibers themselves, without the potential secondary effects resulting from interaction with the nervous system. In addition, TMG examines the effect of the intervention on the muscles studied by measuring the speed and muscle stiffness of individual muscles [Citation17–22]. In TMG, the individual muscle is analyzed in isolation from the central nervous system [Citation23], by electrically stimulating the muscle contraction. The stimulation passes through the nerve fiber and finally reaches the muscle fiber. The stimulus can act on a motor nerve [Citation24] or on a motor point identified on the muscle surface [Citation25]. Thus, the effect of caffeine influence on muscle can be confirmed in this way.
In the previous study, a significant effect of caffeine on the neuromuscular system was demonstrated, as after 60 minutes of caffeine administration, a change in contraction time and muscle belly displacement was demonstrated by TMG [Citation9]. Considering the findings of Domaszewski et al., the present study aims to delve further into the temporal aspect of caffeine’s effects on muscle reactivity. Specifically, the focus is on determining whether there exists a critical time point within the first hour post-caffeine intake that leads to the maximum enhancement of muscle performance. Based on the previous study’s indication of changes at the 60-minute mark, the present study explores the possibility of an earlier, more pronounced effect at the 30-minute mark.
Building upon the findings of the previous study by Domaszewski et al. and pilot study, we hypothesize that muscle contraction time and delay time will exhibit the most significant changes 30 minutes after caffeine ingestion compared to both pre-caffeine intake and the 60-minute mark. Furthermore, we expect to observe a systemic effect of caffeine, indicating no discernible differences in measurements between the two sides of the body.
2. Materials and methods
2.1. Experimental approach to the problem
In order to determine the time response of the muscle under the influence of a dose of caffeine a repeated measures design was used. TMG was used as a tool to measure the mechanical response of the muscle. The tests were conducted on male athletes during their competitive period. Participants were asked not to change their caffeine consumption habits before the day of the experiment. On the day of the study, the athletes were asked to refrain from products containing caffeine. The exact body composition was determined using the SECA mBCA 515 analyzer (Seca GmbH&Co. KG, Hamburg, Germany). The caffeine dose of 6 mg/kg calculated for body weight was administered in transparent cellulose capsules (hydroxypropyl methylcellulose 100%), which were washed with purified water. Caffeine was administered after the first TMG test, and the TMG test was repeated twice more during the experiment: 30 and 60 min after caffeine administration. Fresh injuries and high caffeine sensitivity were exclusion criteria.
2.2. Subjects
Forty-two male athletes playing football at national level (age = 24.8 ± 4.4, body mass = 81.5 ± 8.4 kg, BMI = 24.4 ± 3.6) were included in the study. The athletes were classified as high habitual caffeine consumers, based on the Food Frequency Questionnaire (FFQ). All participants of the study signed an informed consent form. The study was approved by the Bioethics Committee of the Medical University of Poznań (No. 108/22), registered in the Australian New Zealand Clinical Trials Registry (No. 12622000823774), and conducted in accordance with the guidelines described in the Helsinki Declaration for research involving humans.
2.3. Procedures
The selected muscles for TMG measurement were the medial gastrocnemius muscles of the right and left leg, starting with the right one. The participants lay on a supine position on a study couch. The correct angle of the knee joint to relax the muscles under study, about 150°, was ensured using a roller placed under the ankle joints. The test was performed according to the guidelines and recommendations of the device manufacturers (GK 40 Panoptik doo, Ljubljana, Slovenia). Two self-adhesive electrodes (Axelgaard, Pulse) that stimulated the muscle were placed perpendicularly along the course of the muscle fibers. Rectangular electrodes 5 cm × 5 cm were placed 5 cm from one another. The electrodes were placed so that they did not affect the tendons and isolated the contraction of the muscle in question or to avoid simultaneous activation of nearby muscles. The sensor location was manually selected to locate the thickest and most deformable part of the muscle [Citation19]. If necessary, the sensor placement was adjusted during the test to obtain the best mechanical response of the muscle. The sensor was applied to the skin halfway between the electrodes.
The electrodes received a 1-ms single-phase rectangular pulse from an electrostimulator (TMG-S1, Furlan & Co. ltd., Ljubljana, Slovenia) to induce transcutaneous muscle contraction. The pulse amplitude was gradually increased by 10 mA until reaching the stimulator’s maximal output (110 mA). To minimize the effects of fatigue, 10-second intervals were taken between pulses. The digital TMG signal was received directly from the Matlab Compiler Toolbox at a sampling rate of 1 kHz. The TMG signal was recorded and stored on a portable PC. Maximum muscle response was recorded for future analysis. Only the curve with the highest Dm value was considered for further analysis.
The variables assessed in this study were the maximum radial displacement of the muscle belly (Dm), contraction time (Tc), delay time (Td), and radial displacement velocity (Vrd). Dm was defined as the peak amplitude in the displacement – time curve of the tensiomyographical twitch response; Tc was obtained by determining the time interval from 10 to 90% of Dm; Td was defined as the time between the electrical stimulus and 10% of Dm; and Vrd was calculated from the formula [(0.8*DM)/TC]*1000.
All measurements were carried out by the same evaluator who had extensive experience in the use of TMG. Relative and absolute reliability of Dm, Tc, and Td measurement was calculated according to previous studies: Dm .92 (.80–.97), cv 6.5%, SEM 7.35%; Tc .92 (.80–.96), cv 4.4%, SEM 4.37%; Td .93 (.84–.97), cv 3.4%, SEM 2.89% [Citation19].
2.4. Statistical analyses
To assess the significance of differences between the main effects of SIDE (left vs. right) and TIME (before vs. 30 min vs. 60 min) and individual variables (Tc, Td, Dm, Vrd), an ANOVA analysis was employed. To detect specific differences in the examined main effects, the Post-hoc Tukey HSD test was used. The calculations were conducted using StatsCloud software (https://statscloud.app/). The sample size of 42 participants is sensitive enough to detect with GPower effect size f = 0.19, power 80% and a 5% significance level.
3. Results
In the conducted 2 × 3factorial ANOVA, the effects of the factors Side and Time on the variables Tc, Td, Dm, and Vrd were examined. The analysis of the Tc parameter revealed a significant effect of Time, F(2, 246) = 12.09, p < .001, ή2p = 0.09. No significant effect was found for the Side factor and the interaction between Side and Time (). In the analysis of the effect of Side and Time on Td, a significant effect of Time was found, F(2, 246) = 3.39, p = .035, ή2p = 0.03. No significant effect was found for the Side factor and the interaction between Side and Time. In the study examining the effect of Side and Time on Dm, a statistically significant effect of Time was found, F(2, 246) = 6.83, p = .001, ή2p = 0.05. No significant effect was found for the Side factor and the interaction between Side and Time. In the analysis of the effect of Side and Time on Vrd, no significant effect was found for each factor.
Table 1. Analysis of the influence of caffeine on muscle by Anova for TMG parameters: Tc, td, dm, Vrd.
For TMG parameters (Tc, Td, Dm, Vrd), the results change significantly between the measurement before caffeine administration and the measurement 30 minutes after caffeine administration (). The post-hoc analysis revealed a statistically significant difference for the Tc parameter between the assessment before caffeine use and 30 minutes after (p < .001), as well as between 30 and 60 minutes after caffeine consumption (p = 0.008). Statistically significant differences were also observed between the assessment before caffeine use and 30 minutes after for the Td (p = 0.027) and Dm (p < .001) parameters. For the calculated parameter Vrd, no significant differences between the measurements were found. Muscle contraction is faster after 30 minutes (parameters Tc, Td) than before and 60 minutes after caffeine administration: Tc (before = 21.94 ± 4.5 ms, 30 min = 19.09 ± 3.1 ms, 60 min = 20.84 ± 3.5 ms), Td (before = 19.35 ± 2.0 ms, 30 min = 18.6 ± 1.9 ms, 60 min = 19.01 ± 1.7 ms). In addition, the muscle showed more stiffness (parameter Dm) 30 minutes after caffeine administration than before and 60 minutes after caffeine administration Dm (before = 2.64 ± 1.0 mm, 30 min = 2.07 ± 0.9 mm, 60 min = 2.47 ± 1.1 mm). In the Vrd parameter, the results before caffeine administration were at the level of 95.91 ± 3.2 mm/ms, at 30 minutes after caffeine administration at the level of 85.74 ± 1.5 mm/ms, and at 60 minutes they reached the level of 91.73 ± 1.1 mm/ms.
4. Discussion
TMG was used to investigate the effects of caffeine ingestion on changes in muscle biological signals. The main achievement of this study is the fact that the measurement of muscles 30 minutes after caffeine administration was associated with a shorter contraction time and a lower maximum displacement of the muscle belly than the test performed before and 60 minutes after the administration of caffeine, with no significant effect of side of the body.
In our previous study, a single dose of caffeine was associated with a significant decrease in Tc and Dm 60 minutes after ingestion [Citation9]. In the present study, TMG measurement was performed 30 and 60 minutes after caffeine administration to determine the exact time of caffeine action. The results showed that caffeine had a greater effect on muscle 30 minutes after caffeine ingestion than 60 minutes after. However, it is crucial to note a discrepancy in our findings compared to a previous study by Domaszewski et al. [Citation9], where changes in muscle parameters were reported to persist 60 minutes after caffeine ingestion. In our study, the duration of enhanced muscle reactivity appears to be shorter, with effects diminishing by the 60-minute timepoint. This disparity suggests that the optimal time window for maximal muscle reactivity to caffeine may vary among individuals or under different conditions.
The effect of caffeine on human performance continues to be a popular area of research with efforts to better understand its effects and more precise recommendations for use. This is particularly true in sports, as caffeine consumption improves physical performance, i.e. muscle endurance, muscle strength, anaerobic power, and aerobic endurance, which is supported by moderate to high quality ratings and moderate quality evidence [Citation1]. The results of this study indicate a similar trend, as the effect of caffeine on changing parameters toward improved muscle dynamics was found in all subjects. This is also confirmed in muscle studies performed outside the body on isolated muscle fibers, where caffeine significantly improved the speed and force of contractions triggered by an electrical impulse [Citation26].
These studies on the effect of the timing of caffeine administration on the quality of muscle contractions were carried out using TMG, because it images biological signals from the muscles. It also measures the displacement of the muscle belly in millimeters and the duration of the muscle contraction in milliseconds. As many studies have shown, the most reliable and analyzed parameters of TMG are Tc and Dm. Reduced Tc values should be treated with caution as it is a measure that is subject to biasing influences from changes in Dm. Indeed, our findings indicate that the Tc and the Dm changed in the same direction [Citation27]). A lower Tc could be related to a progressive recruitment of type II fibers and a more efficient excitation-contraction muscle coupling [Citation28]. Therefore, the hypothesis is that this dose of caffeine improves a more efficient excitation-contraction coupling of the Gastrocnemius medialis. A reduced Dm value indicates higher muscle stiffness [Citation22]. Both of these parameters can ideally indicate changes in muscle dynamics, which is expected with caffeine administration. The present study confirmed the greatest usefulness of measuring these two parameters. Furthermore, based on previous studies, the reliability of Tc and Dm was good to excellent, which cannot be said about the other parameters [Citation9], whose results in the present study were also unclear. Interestingly, Vrd parameter is independent of Dm and it has been considered a sensitive parameter to identify neuromuscular fatigue [Citation29], but surprisingly no change after a caffeine administration.
Studies indicated that caffeine caused a decrease in the Tc parameter, what confirmed that caffeine changed the neuromuscular profile and that a person could temporarily act similarly as after training muscles in consequence of dynamic or strength training [Citation18–20,Citation22,Citation30]. Furthermore, the Tc parameter is correlated with the proportion of myosin heavy chain Type I [Citation31], or with the speed of sprinters [Citation32]. Caffeine stimulation also decreased the Dm parameter, the maximum radial displacement, which is related to the absolute spatial transverse deformation of the muscle. When Dm is reduced, it is interpreted as an increase in muscle stiffness [Citation33], and indicates a good predisposition to tasks such as sprints or jumps [Citation20]. Such changes in muscle response are not the result of a change in muscle fiber structure, but a short-term response to caffeine stimulation. Changes in the structural aspects of muscle can be influenced by genetic programs, hormonal influences, or fatigue. The number of striated muscle fibers cannot change; the only thing that can change within skeletal muscle is the percentage of muscle fibers that contract rapidly to slow down [Citation34].
Coffee consumed about 60 minutes before exercise seems to improve athletic performance in most people [Citation35]. This is confirmed by most studies that recommend caffeine intake 60 minutes before exercise [Citation1,Citation36,Citation37]. However, some studies suggest the administration of caffeine 30 minutes before physical activity [Citation38]. Therefore, it is not yet clear what effects of caffeine are observed with a shorter/longer waiting time between caffeine intake and physical activity. In the present study, we tried to prove that the time 30 minutes after caffeine ingestion has a greater effect on muscle dynamics. However, when TMG parameters were measured after 60 minutes, an upward trend has already been shown. Sometimes the values in this measurement increased above the first measurement, which may be the reason for the lack of caffeine’s effect on the muscles at that time.
It should be noted that some studies also suggest that the body response after caffeine ingestion may be influenced by varying doses of caffeine [Citation39]. However, caffeine at a dose of 6 mg/kg b.m., has a direct effect on the mechanical activity of skeletal muscle by reducing the parameters of TMG: Tc, Td and Dm, which occur independently of the induction of changes in the central nervous system. The study indicates that caffeine may have a shortening effect on movement speed. However, it should be noted that TMG measurements determine the muscle response to an external stimulus and not the stimulus produced directly in the brain [Citation6].
Our research clearly shows an increased positive effect of caffeine on muscles 30 minutes after administration than after 60 minutes. However, to fully understand the caffeine mechanisms on muscles activity, it is useful to study the different doses of caffeine when studying athletes to determine the caffeine dose that works best for muscles. It would also be worth considering studying participants who are not habitual caffeine consumers, which could likely yield interesting conclusions and thus point to some future research needs.
One limitation of this study could be the lack of a control group. However, a nonintervention group would be of limited value as the results of subsequent studies would not change without prior intervention. In addition, the value of the study could be limited by the fact that only one muscle was studied. However, in the context of caffeine research, this is the last muscle to be supplied with blood and the effects measured here apply to the whole body. The limitation is also that the study involved only male athletes, so the results should not be generalized. In practice, the results of this study can be related to the caffeine supplementation. It turns out that muscles respond to caffeine and are faster after 30 minutes than after 60 minutes.
With 6 mg/kg/body weight caffeine administration, muscles diagnosed with the TMG device have a decrease contraction time (Tc) and delay time (Td) at 30 minutes than 60 minutes after caffeine administration. There is also a similar trend in the Dm parameter, so muscles at 30 minutes could have higher excitation-contraction muscle coupling, and muscle stiffness than at 60 minutes. TMG is an effective tool for noninvasive imaging of biomedical signals from muscles after caffeine administration.
These findings suggest that individuals who require enhanced muscle performance, such as athletes or individuals engaging in physical activities, may benefit from consuming caffeine approximately 30 minutes before their performance. By doing so, they may experience faster muscle contraction and reduced delay time, potentially leading to improved athletic performance.
Additionally, the use of TMG enables noninvasive monitoring of muscle responses after caffeine administration, facilitating further research and practical applications in the field of sports performance and physical rehabilitation.
Author contributions
Conceptualization, PP, MK and ES; methodology, PP, MK and MG; soft-ware, MK and MG.; validation, PP, OG and ES; formal analysis, PP, MK and TD.; investigation, PP, MK; re-sources, MK and TD; data curation, PP, MK, PD, TD, and TD; writing – original draft preparation, PP, OG and ES; writing – review and editing, PP, PD, MK, TD and ES; visualization, PP, OG and ES; supervision, PP, MK and ES; project administration, PP, OG and ES; funding acquisition, PP and ES. All authors have read and agreed to the published version of the manuscript.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data sets generated and/or analyzed during the current study are not publicly available. However, the data are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Grgic, J, Grgic, I, Pickering, C, et al. Wake up and smell the coffee: caffeine supplementation and exercise performance—an umbrella review of 21 published meta-analyses. Br J Sports Med. 2020;54(11):681–165. doi: https://doi.org/10.1136/bjsports-2018-100278
- Bazzucchi, I, Felici, F, Montini, M, et al. Caffeine improves neuromuscular function during maximal dynamic exercise. Muscle Nerve. 2011;43(6):839–844. doi: 10.1002/mus.21995
- Davis, JK, Green, JM. Caffeine and anaerobic performance: Ergogenic value and mechanisms of action. Sports Med. 2009;39(10):813–832. doi: 10.2165/11317770-000000000-00000
- Alasmari, F. Caffeine induces neurobehavioral effects through modulating neurotransmitters. Saudi Pharm J. 2020;28(4):445–451. doi: https://doi.org/10.1016/j.jsps.2020.02.005.
- Orbán, C, Vásárhelyi, Z, Bajnok, A, et al. Effects of caffeine and phosphodiesterase inhibitors on activation of neonatal T lymphocytes. Immunobiology. 2018;223(11):627–633. doi: 10.1016/j.imbio.2018.07.008
- Grgic, J, Trexler, ET, Lazinica, B, et al. Effects of caffeine intake on muscle strength and power: a systematic review and meta-analysis. J Int Soc Sports Nutr. 2018;15(1). doi: https://doi.org/10.1186/s12970-018-0216-0
- Allen, DG, Westerblad, H. The effects of caffeine on intracellular calcium, force and the rate of relaxation of mouse skeletal muscle. Journal Of Physiology. 1995;487(2):331–342. doi: 10.1113/jphysiol.1995.sp020883
- Tallis, J, James, RS, Cox, VM, et al. The effect of a physiological concentration of caffeine on the endurance of maximally and submaximally stimulated mouse soleus muscle. J Physiol Sci. 2013;63(2):125–132. doi: 10.1007/s12576-012-0247-2
- Domaszewski, P, Pakosz, P, Konieczny, M, et al. Caffeine-induced effects on human skeletal muscle contraction time and maximal displacement measured by tensiomyography. Nutrients. 2021;13(3):815–819. doi: https://doi.org/10.3390/nu13030815
- Guest, NS, VanDusseldorp, TA, Nelson, MT, et al. International society of sports nutrition position stand: caffeine and exercise performance. J Int Soc Sports Nutr. 2021;18(1):1–15. doi: 10.1186/s12970-020-00383-4
- Nehlig, A, Alexander, SPH. Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol Rev. 2018;70(2):384–411. doi: 10.1124/pr.117.014407.
- Zimmermann-Viehoff, F, Thayer, J, Koenig, J, et al. Short-term effects of espresso coffee on heart rate variability and blood pressure in habitual and non-habitual coffee consumers – a randomized crossover study. Nutr Neurosci. 2016;19(4):169–175. doi: 10.1179/1476830515Y.0000000018
- Polito, MD, Grandolfi, K, de Souza, DB. Caffeine and resistance exercise: the effects of two caffeine doses and the influence of individual perception of caffeine. Eur J Sport Sci. 2019;19(10):1342–1348. doi: 10.1080/17461391.2019.1596166
- Wilk, M, Filip, A, Krzysztofik, M, et al. The acute effect of various doses of caffeine on power output and velocity during the bench press exercise among athletes habitually using caffeine. Nutrients. 2019;11(7):1465. doi: https://doi.org/10.3390/nu11071465
- Pakosz, P, Jakubowska-Lukanova, A, Gnoiński, M. Tmg as a prevention method of athletes muscles, ligaments and joints injuries. Polish J Sport Med. 2016;32(3):189–200. doi: https://doi.org/10.5604/1232406x.1227534.
- Martín-Rodríguez, S, Loturco, I, Hunter, AM, et al. Reliability and measurement error of tensiomyography to assess mechanical muscle function: a systematic review. J Strength Cond Res. 2017;31(12):3524–3536. doi: https://doi.org/10.1519/JSC.0000000000002250
- Gil, S, Loturco, I, Tricoli, V, et al. Tensiomyography parameters and jumping and sprinting performance in Brazilian elite soccer players. Sports Biomech. 2015;14(3):340–350. doi: 10.1080/14763141.2015.1062128
- Garcia-Garcia, O, Cancela-Carral, JM, Martínez-Trigo, R, et al. Differences in the contractile properties of the knee extensor and flexor muscles in professional road cyclists during the season. J Strength Cond Res. 2013;27(10):2760–2767. doi: 10.1519/JSC.0b013e31828155cd
- Pakosz, P, Lukanova-Jakubowska, A, Łuszczki, E, et al. Asymmetry and changes in the neuromuscular profile of short-track athletes as a result of strength training edited by E. Cè. PloS One. 2021;16(12):1–13. doi: 10.1371/journal.pone.0261265
- De Paula Simola, R, Harms, N, Raeder, C, et al. Assessment of neuromuscular function after different strength training protocols using tensiomyography. J Strength Cond Res. 2015;29(5):1339–1348. doi: 10.1519/JSC.0000000000000768
- Rusu, LD, Cosma, GG, Cernaianu, SM, et al. Tensiomyography method used for neuromuscular assessment of muscle training. J Neuroeng Rehabil. 2013;10(1):67–68. doi: https://doi.org/10.1186/1743-0003-10-67
- Zubac, D, Šimunič, B. Skeletal muscle contraction time and tone decrease after 8 weeks of plyometric training. J Strength Cond Res. 2017;31(6):1610–1619. doi: 10.1519/JSC.0000000000001626
- Macgregor, LJ, Hunter, AM, Orizio, C, et al. Assessment of skeletal muscle contractile properties by radial displacement: the case for Tensiomyography. Sports Med. 2018;48(7):1607–1620. doi: 10.1007/s40279-018-0912-6
- Regina Dias Da Silva, S, Neyroud, D, Maffiuletti, NA, et al. Twitch potentiation induced by two different modalities of neuromuscular electrical stimulation: implications for motor unit recruitment. Muscle Nerve. 2015;51(3):412–418. doi: 10.1002/mus.24315
- Orizio, C, Cogliati, M, Bissolotti, L, et al. The age related slow and fast contributions to the overall changes in tibialis anterior contractile features disclosed by maximal single twitch scan. Arch Gerontol Geriatr. 2016;66:1–6. doi: 10.1016/j.archger.2016.05.003
- Tallis, J, Higgins, MF, Cox, VM, et al. Does a physiological concentration of taurine increase acute muscle power output, time to fatigue, and recovery in isolated mouse soleus (slow) muscle with or without the presence of caffeine? Can J Physiol Pharmacol. 2014;92(1):42–49. doi: https://doi.org/10.1139/cjpp-2013-0195
- Cuba-Dorado, A, Álvarez-Yates, T, Carballo-López, J, et al. Neuromuscular changes after a long distance triathlon world championship. Eur J Sport Sci. 2023;23(9):1838–1848. doi: https://doi.org/10.1080/17461391.2022.2134053
- Zubac, D, Ivančev, V, Valić, Z, et al. Long-lasting exercise involvement protects against decline in VO2max and VO2 kinetics in moderately active women. Appl Physiol Nutr Metab. 2021;46(2):108–116. doi: 10.1139/apnm-2020-0307
- Mesquita, RNO, Latella, C, Ruas, CV, et al. Contraction velocity of the elbow flexors assessed by Tensiomyography: a comparison between formulas. Journal Of Strength And Conditioning Research. 2023;37(10):1969–1977. doi: 10.1519/JSC.0000000000004495
- Završnik, J, Pišot, R, Šimunič, B, et al. Biomechanical characteristics of skeletal muscles and associations between running speed and contraction time in 8- to 13-year-old children. J Int Med Res. 2017;45(1):231–245. doi: 10.1177/0300060516687212
- Šimunič, B, DEGENS, H, RITTWEGER, J, et al. Noninvasive estimation of myosin heavy chain composition in human skeletal muscle. Med Sci Sports Exerc. 2011;43(9):1619–1625. doi: 10.1249/MSS.0b013e31821522d0
- Dahmane, R, Djordjevič, S, Smerdu, V. Adaptive potential of human biceps femoris muscle demonstrated by histochemical, immunohistochemical and mechanomyographical methods. Med Bio Eng Comput. 2006;44(11):999–1006. doi: https://doi.org/10.1007/s11517-006-0114-5.
- Pišot, R, Narici, MV, Šimunič, B, et al. Whole muscle contractile parameters and thickness loss during 35-day bed rest. Eur J Appl Physiol. 2008;104(2):409–414. doi: https://doi.org/10.1007/s00421-008-0698-6
- Lieber, RL. Skeletal muscle structure and function: implication for rehabilitation and sports medicine. Baltimore: Williams & Wilkins; 1992.
- Desbrow, B, Hughes, R, Leveritt, M, et al. An examination of consumer exposure to caffeine from retail coffee outlets. Food Chem Toxicol. 2007;45(9):1588–1592. doi: 10.1016/j.fct.2007.02.020
- Lago-Rodríguez, Á, JODRA, P, BAILEY, S, et al. Caffeine improves performance but not duration of the countermovement jump phases. J Sports Med Phys Fitness. 2021;61(2):199–204. doi: 10.23736/S0022-4707.20.11099-5
- Pickering, C, Kiely, J. Are the Current guidelines on caffeine use in sport optimal for everyone? inter-individual variation in Caffeine ergogenicity, and a move towards personalised sports nutrition. Sports Med. 2018;48(1):7–16. doi: 10.1007/s40279-017-0776-1
- Jacobson, BH, Hester, GM, Palmer, TB, et al. Effect of energy drink consumption on power and velocity of selected sport performance activities. J Strength Cond Res. 2018;32(6):1613–1618. doi: 10.1519/JSC.0000000000002026
- Talanian, JL, Spriet, LL. Low and moderate doses of caffeine late in exercise improve performance in trained cyclists. Appl Physiol Nutr Metab. 2016;41(8):850–855. doi: 10.1139/apnm-2016-0053