ABSTRACT
Position statement
The International Society of Sports Nutrition (ISSN) provides an objective and critical review of the use of a ketogenic diet in healthy exercising adults, with a focus on exercise performance and body composition. However, this review does not address the use of exogenous ketone supplements. The following points summarize the position of the ISSN:
1. A ketogenic diet induces a state of nutritional ketosis, which is generally defined as serum ketone levels above 0.5 mM. While many factors can impact what amount of daily carbohydrate intake will result in these levels, a broad guideline is a daily dietary carbohydrate intake of less than 50 grams per day.
2. Nutritional ketosis achieved through carbohydrate restriction and a high dietary fat intake is not intrinsically harmful and should not be confused with ketoacidosis, a life-threatening condition most commonly seen in clinical populations and metabolic dysregulation.
3. A ketogenic diet has largely neutral or detrimental effects on athletic performance compared to a diet higher in carbohydrates and lower in fat, despite achieving significantly elevated levels of fat oxidation during exercise (~1.5 g/min).
4. The endurance effects of a ketogenic diet may be influenced by both training status and duration of the dietary intervention, but further research is necessary to elucidate these possibilities. All studies involving elite athletes showed a performance decrement from a ketogenic diet, all lasting six weeks or less. Of the two studies lasting more than six weeks, only one reported a statistically significant benefit of a ketogenic diet.
5. A ketogenic diet tends to have similar effects on maximal strength or strength gains from a resistance training program compared to a diet higher in carbohydrates. However, a minority of studies show superior effects of non-ketogenic comparators.
6. When compared to a diet higher in carbohydrates and lower in fat, a ketogenic diet may cause greater losses in body weight, fat mass, and fat-free mass, but may also heighten losses of lean tissue. However, this is likely due to differences in calorie and protein intake, as well as shifts in fluid balance.
7. There is insufficient evidence to determine if a ketogenic diet affects males and females differently. However, there is a strong mechanistic basis for sex differences to exist in response to a ketogenic diet.
1. Background
The use of ketogenic diets for enhancing sports performance and body composition has become increasingly popular due to a combination of layman books and articles, social media platforms, and academic investigations. The International Society of Sports Nutrition (ISSN) has published several position stands addressing nutrient requirements for optimizing training adaptations and sports performance; toward this end the ISSN recommends higher carbohydrate intakes of ~ 5–12 grams per kilogram body weight per day (g/kg/d) for endurance athletes [Citation1,Citation2], and at least 3–5 g/kg for general fitness, including strength athletes [Citation2]. Additionally, the ISSN recommends that a wide range of dietary approaches, from high-carbohydrate to ketogenic, can be effective for improving body composition [Citation3].
The current paper represents the first ISSN Position Stand on the use of ketogenic diets in sports. The impact of ketogenic diets on athletic performance, muscular strength, resistance training adaptations, and body composition will be discussed.
2. Ketogenesis
Ketogenesis is a process that occurs primarily in the liver, by which three types of ketone bodies – acetoacetate, beta-hydroxybutyrate (βHB), and acetone (ketones) – are produced. Since these compounds are water-soluble, they can easily travel throughout the body to provide an alternative energy source to glucose. All cells that contain mitochondria are capable of using ketone bodies to produce energy; the exception is liver cells, which lack the necessary enzyme β-ketoacyl-CoA transferase. The brain is the organ best known for ketone use, as it can generate two-thirds of its energy requirements from ketones after several weeks of fasting [Citation4]. Under normal conditions of sufficient intake of energy and carbohydrates, most energy-producing molecules (glucose, fatty acids, and some amino acids) are broken down into acetyl-CoA, which then binds to oxaloacetate (OAA) within the tricarboxylic acid (TCA) cycle to form citrate [Citation5]. Several other reactions then occur as the TCA cycle continues, producing electron-carrying molecules (NADH and FADH2) that enter the electron transport chain to produce adenosine triphosphate (ATP) – the general energy currency of the cell.
Ketogenesis occurs when there is a relative abundance of acetyl-CoA that cannot enter the TCA cycle due to a shortage of OAA (). Although OAA is recycled with each turn of the TCA cycle, and some OAA can be made from glucose and amino acids, it is also diverted from the TCA cycle within the liver to be used as a substrate for gluconeogenesis during times of glucose deprivation (e.g. fasting or a low-carbohydrate diet). This is why ketogenesis occurs primarily in the liver – OAA is diverted toward gluconeogenesis, leading to a “build-up” of acetyl-CoA within the mitochondria. This is also why other tissues can use ketone bodies as a source of energy despite the requirement that they be turned back to acetyl-CoA and enter the TCA cycle for energy production – a relative deficit of OAA does not occur within these peripheral tissues since none is diverted toward gluconeogenesis.
Figure 1. Ketone synthesis and metabolism. When acetyl-CoA cannot enter the TCA cycle within the liver, due to a lack of OAA, they condense into the ketone molecule acetoacetate, which can be further transformed into β-hydroxybutyrate (βHB) or spontaneously decarboxylated into acetone. Acetoacetate and βHB enter circulation and travel to peripheral tissues (e.g. muscle, brain), where the process reverses and acetyl-CoA is made available to enter the TCA cycle of these tissues.
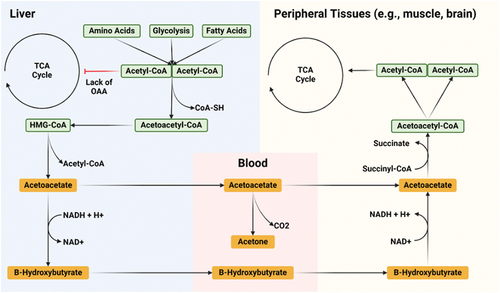
The buildup of acetyl-CoA within the hepatic mitochondria leads to its condensation into acetoacetate, which can be further converted to βHB. Both molecules are released into circulation and taken up by peripheral tissues, where the process reverses (βHB to acetoacetate to acetyl-CoA) to allow for ATP production. Acetone is a ketone produced primarily through the spontaneous decarboxylation of acetoacetate but is mostly excreted through urine or exhaled (some can be recycled into acetyl-CoA and OAA) [Citation6,Citation7].
From a macronutrient standpoint, carbohydrates are anti-ketogenic because they replenish OAA and stimulate insulin, which blunts lipolysis and thereby limits fatty acid availability for the liver (a primary source of acetyl-CoA). Dietary fat is ketogenic because it provides mostly acetyl-CoA from the beta-oxidation of fatty acids, though the glycerol backbone can be used in gluconeogenesis and thereby marginally alleviate the need for OAA. Dietary protein is mostly anti-ketogenic because most amino acids, with the exceptions of leucine and lysine which are predominately ketogenic, can replenish OAA or alleviate its use in gluconeogenesis.
Many amino acids enter the TCA cycle to replenish intermediates through a process called anaplerosis, making more OAA available to bind with incoming acetyl-CoA. This reduces ketogenesis, as more acetyl-CoA goes through the TCA cycle instead of “backing up” and leading to ketone formation. Depending on the body’s needs, many amino acids can be used to generate acetyl-CoA or ketone precursors. Importantly, while there are glucogenic amino acids, anaplerosis is the mechanism through which higher protein diets likely exert anti-ketogenic effects. Gluconeogenesis and ketogenesis have evolved to operate simultaneously during prolonged fasting; therefore, it is illogical that increases in gluconeogenesis impair ketogenesis.
Exercise may also reduce carbohydrate availability and stimulate ketogenesis depending on its duration and intensity, especially during the post-exercise period [Citation8]. Prolonged and sufficiently intense aerobic exercise creates a catabolic state within the body akin to fasting, driven by elevations in cellular AMP/ADP to ATP ratios and an increase in circulating glucagon concentrations relative to insulin. Intense and/or prolonged exercise can also simultaneously deplete liver glycogen and liberate fatty acids from adipose tissue with both of these actions being the result of supplying peripheral tissues with energy to support the demands of exercise. The flood of fatty acids reaching the liver are oxidized into acetyl-CoA which cannot enter the TCA cycle due to a shortage of OAA and are instead converted to ketones as previously described to support the energy requirements of peripheral tissues. Post-exercise ketosis describes the accumulation of ketones in the blood following the cessation of exercise due to the sudden reduction in ketone utilization by peripheral tissues.
These processes were exemplified in a study on ultra-endurance runners [Citation9]. Despite consuming a non-ketogenic diet containing 486 grams of carbohydrate per day, serum ketone concentrations rose from nearly undetectable concentrations prior to exercise to 0.5 mM throughout a 3-hour submaximal run at 65% of VO2max. Moreover, ketone concentrations continued to rise to 0.7 mM in the 30 minutes following exercise cessation, dropping to 0.3 mM by 2 hours post-exercise. Serum fatty acids also rose sharply during exercise, reaching a 4-fold elevation above baseline by the end of the exercise session and dropping back to baseline during the 2-hour recovery window.
3. Ketosis and ketogenic diets
Ketosis is a metabolic state in which ketone bodies, particularly βHB, reach appreciable concentrations in the blood (medically referred to as hyperketonemia). Under normal mixed-diet conditions, blood ketone concentrations are usually 0.2 mM or less. A state of ketosis is generally defined as serum ketone levels greater than 0.5 mM [Citation10,Citation11], although serum ketone levels greater than 0.2 mM have also been suggested [Citation12]. The extent of ketosis achieved with similar dietary protocols varies between individuals, given that blood ketone concentrations are a dynamic reflection of both ketogenesis and ketone uptake by peripheral tissues. In other words, an increase in blood ketone levels could be due to an increase in ketone production or a reduction in ketone utilization, and vice versa.
Ketone production tends to increase concurrently with its utilization. This matching of production and utilization occurs until ketone levels reach 2–3 mM [Citation12]. Blood ketone concentrations can reach 8 mM after 3–4 weeks of fasting, but the rates of ketogenesis do not increase from the rates seen in the first several weeks when levels are only 2–3 mM [Citation13,Citation14]. This excessive hyperketonemia found with prolonged fasting is largely the result of the conservation of ketone bodies by the kidney (i.e. less excretion) [Citation15] and decreased uptake of ketone bodies by skeletal muscle (in favor of fatty acids) [Citation16].
Importantly, ketosis should not be confused with ketoacidosis, a life-threatening condition in which blood ketone levels exceed 12–15 mM. Under normal circumstances, ketogenesis operates under a negative feedback loop – a “fail-safe” system to ensure decreased synthesis of ketone bodies when concentrations increase excessively. As ketone concentrations increase, they inhibit the release of fat from fat cells, stimulate insulin secretion, and sensitize fat cells to the effects of insulin, all of which act to reduce the amount of fatty acids that reach the liver to be oxidized into acetyl-CoA to be converted into ketones [Citation17,Citation18]. Ketoacidosis is a potential risk only when these feedback mechanisms do not function, such as with a lack of insulin signaling in type I diabetes.
Generally, ketogenic diets contain less than 50 grams of carbohydrates per day and promote a relatively constant state of ketosis [Citation19]. However, there is a natural variation in the magnitude of ketosis achieved due to factors such as protein intake and activity level [Citation20]. For sustaining ketosis, it has traditionally been recommended to avoid high protein intakes, due to purported glucogenic/anti-ketogenic effects (colloquially, getting “kicked out” of ketosis). However, in athletic populations, physical activity levels may permit higher protein intakes by (1) enhancing lipolysis and the accumulation of acetyl-CoA within the liver and (2) diverting amino acids toward biosynthetic purposes rather than anaplerosis. For example, Burke et al. reported a blood ketone concentration of 1.8 mM in the ketogenic diet group despite a daily protein intake of 2.2 g/kg [Citation21], and Wilson et al. reported a blood ketone level of 1.0 mM despite a daily protein intake of 1.7 g/kg [Citation22]. The marked difference in ketone levels between these two studies could be explained by the substantially higher activity levels of the elite race walkers of Burke et al. compared to the more modest levels of resistance-trained men in Wilson et al.
4. Exercise bioenergetics
To understand the effects of a ketogenic diet on athletic performance, it is critical to understand the fundamentals of energy metabolism during exercise. Skeletal muscles require ATP for contraction. While some ATP is stored within skeletal muscle fibers, it is a finite amount that can sustain no more than a few seconds of contractions. Skeletal muscle relies on three complementary energy systems to generate ATP: mitochondrial respiration (a.k.a., the aerobic energy system, oxidative phosphorylation, etc.), anaerobic glycolysis (a.k.a., fast glycolysis, the lactic acid energy system, etc.), and the phosphagen system (a.k.a., the ATP-PCr energy system).
Mitochondrial respiration utilizes oxygen to produce ATP from glucose, fatty acids, amino acids, and ketones. The requirement for oxygen is why mitochondrial respiration is also known as aerobic or oxidative metabolism. This is the primary method of energy production within the body and produces most of the energy used by working muscles during prolonged exercise [Citation23,Citation24]. However, the rate of energy production is inherently limited by inefficiencies in transporting intermediates such as pyruvate and lactate into mitochondria and metabolizing them, making aerobic metabolism incapable of meeting energy demands during high-intensity efforts or at the beginning of exercise regardless of the exercise intensity ().
Figure 2. The relative contribution of the three energy systems to the total energy supply during 90 seconds of all-out cycle exercise. Adapted from gastin 2001 [Citation23].
![Figure 2. The relative contribution of the three energy systems to the total energy supply during 90 seconds of all-out cycle exercise. Adapted from gastin 2001 [Citation23].](/cms/asset/a4c2110a-f177-4eb9-8ef6-5175cf5dfda2/rssn_a_2368167_f0002_oc.jpg)
When energy demands exceed the ability of aerobic metabolism to supply energy, whether at the beginning of exercise or during a high-intensity bout, the production of ATP via anaerobic metabolism compensates for the deficit. This is called the ventilatory anaerobic threshold (VAT) [Citation25]. As the name implies, no oxygen is required for anaerobic metabolism. Although heart and respiration rates increase in an attempt to supply the working muscles with more oxygen as exercise intensity progresses, aerobic metabolism can contribute only a portion of energy requirements during exercise that rapidly exceeds VAT, such as sprinting [Citation23,Citation24].
Glycolysis involves the generation of lactate via glucose catabolism. When glucose is catabolized to generate ATP, it is broken down into pyruvate via glycolysis. During aerobic metabolism, pyruvate enters the mitochondria, is converted to acetyl-CoA, and then enters the TCA cycle. During anaerobic metabolism, pyruvate is converted to lactate. While the conversion of pyruvate to lactate does not generate ATP, the conversion enables the muscle to sustain a high rate of ATP regeneration from glycolysis by preventing product inhibition (i.e. pyruvate inhibiting glycolysis). Lactate may also be used as an energy substrate itself or converted into glucose in the liver via the Cori cycle [Citation26–28].
The phosphagen system, also known as the ATP-PCr energy system, is the shortest-lived and most rapid method of energy production and simply involves the transfer of a phosphate group from creatine phosphate (CP; also called phosphocreatine or PCr) to adenosine diphosphate (ADP) via the creatine kinase reaction. The phosphagen system acts to buffer cellular ATP concentrations during the first several seconds of exercise. For instance, several studies have demonstrated that intramuscular ATP concentrations remain relatively constant during various exercises as CP concentrations quickly drop by 75% to 85% within 10 seconds [Citation23,Citation24]. This is why creatine supplementation, which increases the amount of CP within muscle tissue, helps to maintain ATP concentrations during all-out exercise and thereby increases performance in events requiring all-out exertion (e.g. sprinting, weight lifting) [Citation29].
5. Methods
This position stand is a review of the literature on the effects of ketogenic diets on endurance exercise performance, muscular strength, and body composition in recreationally active adults and competitive athletes. A comprehensive literature search was performed using the Medline database of the US National Library of Medicine of the National Institutes of Health (“PubMed”). The search strategy involved entering “ketogenic diet” and “endurance,” “strength,” “power,” and “body composition,” as well as searching the reference list of relevant papers.
To be eligible for inclusion and discussion, studies had to be controlled trials comparing a ketogenic diet – defined as containing < 50 grams of carbohydrate per day or resulting in blood ketone values ≥0.5 mM or resulting in the presence of urinary ketones – to a non-ketogenic control diet in adults undergoing an exercise regimen. Studies involving cyclic ketogenic diets were excluded.
ISSN position stands are invited reviews of topics the Journal of the ISSN (JISSN) editors and Research Committee identify as being of interest to the sports nutrition community. JISSN Editors and/or the Research Committee identify a lead author or team of authors to perform a comprehensive literature review. The draft is then sent to leading scholars for review and comment. The paper is then revised as a consensus statement and reviewed and approved by the Research Committee and JISSN Editors as the official position of the ISSN.
6. Exercise performance
Interest in the use of ketogenic diets to benefit athletic performance began in the 1980s [Citation30] and has been reignited several times among the scientific community [Citation31,Citation32], more recently with a 2015 paper titled “Rethinking fat as a fuel for endurance exercise” [Citation10]. At the time of that paper’s publication, there had been a growing number of endurance athletes experimenting with ketogenic diets as a means of performance enhancement, but clinical research testing this hypothesis was lacking.
Relying instead on inferences drawn from mechanistic reasoning, the authors proposed the idea of “keto-adaptation,” in which there is a pronounced shift in fuel utilization toward fat oxidation during submaximal exercise that can preserve muscle glycogen and enhance endurance exercise performance [Citation10]. It was noted that fat oxidation rates of male elite keto-adapted cyclists were extraordinarily high (average of 1.5 g/min after 4 weeks on a ketogenic diet) [Citation30] compared to the fat oxidation rates of 300 male and female adults with diverse levels of cardiorespiratory fitness (average of 0.46 g/min) [Citation33], and the lowest fat oxidation rate of the keto-adapted cyclists (1.4 g/min) was still notably higher than the highest value of the 300 adults (1.0 g/min) [Citation10].
Although keto-adaptation may allow for higher rates of fat oxidation, other metabolic effects must also be considered, such as changes in enzyme activity and movement economy. Such changes collectively impact exercise bioenergetics and are, therefore, essential for understanding how a ketogenic diet impacts exercise performance.
As of this writing, 16 controlled trials have investigated the effects of a ketogenic diet on athletic performance () [Citation21,Citation30,Citation34–47]. Of these studies, none exclusively involved females and most involved only males (11 trials); participants included either recreationally active adults (11 trials) or elite athletes (5 trials). The exercise intervention was to maintain usual exercise habits (9 trials) or follow a prescribed routine (7 trials), and the diet was either monitored via food logs (11 trials) or provided by the research staff (5 trials).
Table 1. The effects of a ketogenic diet on endurance exercise performance.
Seven controlled trials were excluded from our analysis because they were performed in adolescents [Citation48], failed to achieve a state of ketosis in the ketogenic diet group [Citation49,Citation50], reported similar blood ketone concentrations between the ketogenic diet and control diet groups [Citation51,Citation52], exceeded the carbohydrate threshold for ketogenic diets without an objective measurement of ketosis [Citation53], and used exclusively descriptive statistics [Citation54]. Another five trials were excluded due to being case studies without a control group [Citation55–59].
A variety of testing modalities have been used to assess exercise performance, either via cycling, running, or race walking, including submaximal or graded time to exhaustion, time trials, Wingate sprints, and an intermittent yo-yo test. The results of these studies are summarized in .
Figure 3. An overview of differences in performance outcomes between the ketogenic diet and control diet groups, in relation to the study duration from the controlled trials, is shown in . Higher values favor the ketogenic diet group, and lower values favor the control diet group, with the signs adjusted based on whether a higher or lower value is desirable (i.e. time-trial vs. time-to-exhaustion). Differences were calculated in two ways depending on the available data being reported and are depicted by different shapes; squares were calculated as the percentage change between a single value for each group (keto vs. control), circles were calculated as the difference in percent change scores (pre vs. post intervention) for each group. Points in red indicate statistically significant differences between groups (p < 0.05). It should be noted that despite 11 performance outcomes, only two studies longer than 10 weeks have been conducted.
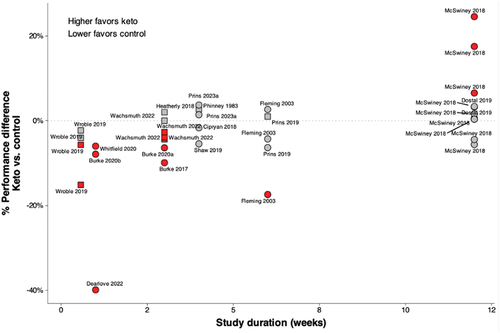
Only one of the 16 controlled trials reported a significant improvement in performance for the ketogenic diet group compared to the control group [Citation46] (). This study involved 20 recreationally-trained male endurance athletes who consumed a ketogenic diet (i.e. 41 g carbohydrate, 259 g fat, 131 g protein per day) or a control diet (400 g carbohydrate, 55 g fat, 91 g protein per day) for 12 weeks alongside a standardized exercise routine. It is important to note calories and protein were not matched between groups. Before and after the intervention, participants completed three exercise tests back-to-back on a cycle ergometer: a 6-second sprint test, a 100-km time trial, and a 3-minute critical power test. Both groups similarly improved their performance on the 100-km time trial (1–3%), while the ketogenic diet group also improved their peak power output on the sprint (6%) and critical power (17%) tests. However, the power output metrics were normalized to body weight. On average, the ketogenic diet group was 10 kg heavier at baseline and lost 7-fold more body weight, entirely from fat mass, than the control group. In absolute terms, peak and average power output declined in the ketogenic diet group for the 6-second sprint (−3%), and average (but not peak) power output also declined (−4%) during the 3-minute critical power test. Relative power is a better predictor of cycling performance than absolute power [Citation60], and relative power development appears to be driven by lean body mass rather than body weight [Citation61]. Therefore, it is notable that the ketogenic diet group experienced a decline in power output relative to the control group when power output was expressed relative to lean body mass.
Compared to one study reporting an ergogenic benefit with a ketogenic diet, eight studies reported that a ketogenic diet impairs athletic performance. Four of these studies involved elite race walkers under strict researcher-controlled diets and exercise routines [Citation21,Citation37–39], while the others involved recreationally active adults maintaining their habitual exercise and tracking their food intake with food logs [Citation40,Citation41,Citation44,Citation47]. The ketogenic diet groups in these studies had significantly worse performance on a 10-km time trial [Citation21,Citation37–39] and an incremental test to fatigue [Citation40,Citation41]; less work output on a 45-minute time trial [Citation44]; lower Wingate peak and average power [Citation47]; and less distance covered in a yo-yo intermittent recovery test to exhaustion [Citation47].
The remaining seven studies reported no statistically significant differences between the effects of a ketogenic diet and a control diet on exercise performance. The outcomes included submaximal time to exhaustion [Citation34,Citation62, graded time to exhaustion [Citation35,Citation36,Citation43], and 1.6-km and 5-km time trials [Citation36,Citation42,Citation45]. One of these studies involved competitive cyclists [Citation30], while all remaining studies involved recreationally active adults.
Importantly, time to exhaustion tests generally have low reliability for estimating athletic performance [Citation63,Citation64]. Moreover, the submaximal efforts often utilized (e.g. ~60–80% of VO2max) are not realistic for real-world training and competition intensities observed in elite and recreational athletes. For example, professional cyclists train and compete for long periods (>30 mins) at or above 90% of VO2max [Citation65,Citation66]; elite marathon competitors run at speeds (19–21 km/hr) at or above 90% of VO2max for extended periods [Citation67,Citation68]; recreational marathon runners race at intensities of ~ 85% of VO2max [Citation69]; and the average 20-km pace for elite race walkers is 85–90% of VO2max [Citation21].
Study duration is another important consideration when evaluating studies using a ketogenic diet (). Among the eight studies that showed performance detriments for the ketogenic diet group, four lasted one week or less [Citation38–40,Citation47]. All other studies lasted 3 to 12 weeks, including those that showed no performance difference between diet groups. Although restoration of peak aerobic power and sub-maximal endurance exercise performance (60–75% of VO2max) has been observed 2–3 weeks after initiating a ketogenic diet [Citation10,Citation70,Citation71], it is possible that longer periods of adaptation beyond what is needed to show changes in substrate oxidation may be important for athletes following a ketogenic diet to enhance exercise performance [Citation38]. Further research is necessary to investigate how dietary duration of following a ketogenic diet affects exercise performance.
Collectively, controlled trials demonstrate that ketogenic diets tend to impair athletic performance in elite athletes and have either no effect or a negative effect on the performance of recreational athletes. However, the most relevant metric to real-world performance (time trials) was limited mostly to short durations that involved intense effort. It is possible that endurance athletes could benefit from a ketogenic diet if they compete in events featuring prolonged, submaximal efforts where it may be difficult to consume adequate carbohydrates due to logistical constraints and/or gastrointestinal distress resulting from high carbohydrate intakes, such as open water swimming (>10 km), ultramarathons (>80 km), and multi-day endurance races (e.g. Race Across America or Atlantic Rowing Race). However, there is currently insufficient evidence to support the use of a ketogenic diet in this population [Citation72], and there is a need for field research investigating how a ketogenic diet may impact race performance in these situations.
7. Movement economy
Movement economy refers to the rate of energy expenditure relative to the speed of movement during exercise and is considered a key factor influencing endurance sports performance [Citation73]. Effectively, having a higher movement economy means that the working muscles require less oxygen and energy to generate a given movement speed (i.e. they are more efficient at producing work under energy constraints).
A decrease in movement economy has been consistently observed following ketogenic dietary interventions in both elite [Citation21,Citation37–39] and recreational athletes [Citation34,Citation45], which has been accompanied by decrements in performance [Citation21]. It has long been established that the oxygen cost of energy production is greater for fat than for glucose [Citation74], and the lower movement economy may, therefore, be a product of increased fat oxidation on a ketogenic diet. Shaw et al. [Citation34] reported impaired exercise efficiency only at higher (>70% VO2max) but not lower (<60% VO2max) exercise intensities, suggesting that the impact of a ketogenic diet may be intensity-dependent. In support, other studies showing an impairment in movement economy have used exercise intensities of 64–90% VO2max [Citation21,Citation37–39,Citation45].
While there may be a theoretical advantage to the metabolic cost of energy production from ketones [Citation75], it appears unlikely that ketone bodies are a major source of energy production in skeletal muscle when other substrates like fatty acids are available [Citation76]. More research is needed in cyclists, where the mechanical cost of exercise can be more clearly determined by measuring watts produced on a cycle ergometer to better understand how much of the observed changes in oxygen cost are due to true changes in efficiency as opposed to reflecting changes in substrate use.
8. Enzyme activity and substrate oxidation
The ketogenic diet is well-established as a method for increasing fat oxidation capacity during steady-state exercise (). Compared to a control diet, a ketogenic diet has been found to increase fat oxidation rates from 37% to 500%, with greater differences observed in elite athletes than in recreationally active adults. In a 12-week crossover study, male athletes on a ketogenic diet demonstrated greater fat oxidation rates than those on a high-carbohydrate diet (1.6 vs. 0.7 g/min, respectively) and could work at a higher exercise intensity before reaching maximal rates of fat oxidation (86% vs. 80% of VO2max) [Citation42].
The process of keto-adaptation occurs relatively rapidly. Daily fat oxidation measured via indirect calorimetry with 24-hour measurements of fuel selection demonstrates that adipose lipolysis and hepatic ketogenesis reach their maximal diet-induced values within a week in sedentary adults [Citation77]. Recent studies have reported high rates of fat oxidation in elite race-walkers following ketogenic diet interventions lasting 3–4 weeks [Citation21,Citation37], as well as 5–6 days [Citation38,Citation39]. Following the re-introduction of carbohydrates for 24 hours to keto-adapted athletes, fat oxidation was lower but continued to be elevated above baseline values, while carbohydrate oxidation remained lower than baseline values [Citation38]. However, substrate utilization returned to baseline values after 5–6 days of a high-carbohydrate diet.
Along with an increase in fat oxidation on a ketogenic diet, there is a concomitant decrease in carbohydrate oxidation [Citation38], likely related to changes in mitochondrial enzyme activity. The conversion of pyruvate to acetyl-CoA requires the enzyme complex pyruvate dehydrogenase (PDH). The activity of PDH increases with exercise intensity [Citation78], but low-carbohydrate, high-fat diets inhibit this increase [Citation79], even with carbohydrate loading and glycogen restoration before exercise [Citation80]
The inhibition of glucose oxidation is of real-world relevance considering that most competitive athletes’ training and competition events are performed at intensities above the ventilatory anaerobic threshold [Citation21,Citation65,Citation66,Citation81]. Indeed, running at a competitive half-marathon pace is not inhibited by chemically suppressing mobilization and utilization of fatty acids as an energy source [Citation82], indicating carbohydrate dependence at real-world race intensities. Further, it has been suggested that a world-class marathon pace is predominantly fueled by carbohydrate oxidation, which provides ~ 80% of the energy needed during competitive marathon and half-marathon paces [Citation83]. The reliance on glucose as an energy source at competitive exercise intensities occurs due to both the ability of carbohydrate oxidation to generate ATP more rapidly than fat oxidation and the inhibition of fat metabolism during higher exercise intensities. To this point, when free fatty acids (FFA) are oxidized, the maximum rate of ATP resynthesis is ~0.40 moL/min; in contrast, aerobic or anaerobic breakdown of glycogen yields 1.0 to 2.0 moL of ATP/min [Citation49].
It has also been reported that elite male endurance athletes following a long-term ketogenic diet (>9 months; 82 g carbohydrate, 226 g fat, 2.1 g/kg protein per day) had similar pre-exercise muscle glycogen concentrations (140 mmol/kg w.w.) to athletes following a high-carbohydrate diet (486 g carbohydrate, 91 g fat, 1.7 g/kg protein), as well as similar rates of glycogen use during exercise (66% and 62% depletion, respectively) and glycogen resynthesis (38% and 34% depletion, respectively) following exercise [Citation9]. However, the accuracy of these findings has been questioned [Citation84], and a separate study showed a 14% decrease in muscle glycogen occurs in both males and females following a 12-week ketogenic diet and training intervention [Citation85]. It could also be that the higher protein intake of the first study (2.1 g/kg) helped maintain glycogen concentrations, given that the opposing study stated a goal of moderating protein intake (actual intake was not reported). Future research is needed to determine if longer-term adherence to a ketogenic diet can influence the breakdown and storage of muscle glycogen.
9. Exercise performance summary
A series of well-controlled studies, and some of the strongest evidence demonstrating the effects ketogenic diets have on endurance performance under real-world conditions, have been conducted in elite race walkers, who were strictly monitored by the research staff for 1–4 weeks within a professional training camp where both diet and physical activity could be controlled [Citation21,Citation37–39]. Athletes eating a ketogenic diet consistently demonstrated impaired performance on a standardized 10-km race walk compared to those eating a higher carbohydrate diet. Moreover, an abundance of rigorously collected measures of whole‐body metabolism at rest and during exercise suggests that this impairment was primarily due to reduced movement economy and an increased oxygen requirement for exercise.
In contrast to these consistent findings in elite athletes, the performance effect of a ketogenic diet on recreational athletes is equivocal. Although one study reported benefits of a ketogenic diet for sprint and critical peak power output [Citation46], other studies reported detriments for Wingate power output [Citation47], yo-yo intermittent recovery test performance [Citation47], an incremental test to fatigue [Citation40,Citation41], and total work output in a 45-minute time trial [Citation44]. Most studies reported no effect of a ketogenic diet on performance outcomes, including Wingate power output [Citation44], shuttle run performance [Citation35], time to exhaustion protocols [Citation34–36,Citation43], or timed cycling trials for 5 km [Citation36,Citation45] and 100 km [Citation46]. It is plausible that recreational athletes have more leeway in their dietary choices than elite athletes do, given that they are still far from their peak potential for endurance-related changes in physiology and metabolism. It is also possible that longer study periods, which can be more easily achieved with non-elite athletes, are needed to better examine the implications of a ketogenic diet on athletic performance. At this time, the effect of a ketogenic diet on exercise performance in recreational athletes cannot be conclusively stated, although the current evidence points toward either no effect or a small detriment.
While it is clear that ketogenic diets and keto-adaptation increase rates of fat oxidation [Citation9,Citation21,Citation30], this appears to be of little relevance at real-world training and competition intensities. Indeed, the collective evidence demonstrates that ketogenic diets either impair (in mostly elite athletes) or do not affect (in mostly recreational athletes) exercise performance. However, there is an absence of research investigating how a ketogenic diet affects endurance exercise performance for long-duration events performed at intensities less than 70% of VO2max, such as ultramarathons or ultra-distance triathlons, which could hypothetically benefit from keto-adaptation.
10. Muscular strength
Although the use of a ketogenic diet for enhancing muscular strength and weightlifting performance has not reached a level of interest similar to its use for enhancing endurance exercise performance, there are strength athletes and bodybuilders who train while consuming ketogenic diets. It is, therefore, important to assess how ketogenic diets affect strength training performance.
To date, 10 controlled trials have investigated the effects of a ketogenic diet on strength training performance () [Citation22,Citation71,Citation86–93]. Of these, only one exclusively involved women, while four included both men and women; participants were either untrained before the intervention (1 trial), recreationally active (6 trials), or involved in a competitive sport (3 trials); the exercise intervention was to maintain usual exercise habits (4 trials) or follow a prescribed routine (6 trials); and dietary monitoring was either via food logs (9 trials) or not described (1 trial).
Table 2. The effects of a ketogenic diet on strength training performance.
Three controlled trials were excluded from our analysis due to not assessing baseline strength [Citation94], exceeding the carbohydrate threshold for ketogenic diets without an objective measurement of ketosis [Citation95], and not reporting on dietary intake or objective measures of ketosis [Citation96]. Another trial was excluded due to being a case study without a control group [Citation97].
11. Strength training adaptations
A variety of exercise tests were used to assess strength training adaptations, including maximal and submaximal bench press, back squat, and leg press exercises, Olympic lifts, vertical and countermovement jumps, and calisthenics.
All but one study reported no significant differences in strength training performance between the ketogenic diet and control diet groups. Only one study with female participants reported significantly different performance outcomes between ketogenic and control diet groups [Citation91]. In this 8-week intervention, participants followed the same strength training routine while eating either a ketogenic diet (40 grams of carbohydrate per day) or a control diet (280 grams of carbohydrate per day). Despite no differences in strength measures at baseline, only the control diet group experienced significant gains in maximal bench press (4.8 vs 1.5 kg) and back squat (15.6 vs 5.6 kg) strength, and comparisons with the ketogenic diet group yielded statistically significant differences.
12. Muscle glycogen and anaerobic metabolism
Similar to high-intensity endurance exercise, strength training relies heavily on anaerobic metabolism to produce the energy required to lift weights. Therefore, any dietary approach that influences anaerobic energy production could affect strength training performance. Analysis of the contribution of each energy system during 10 repetitions with a 10-repetition maximum (RM) weight on the leg press suggests that anaerobic glycolysis contributes 64–71% of the necessary energy and the phosphagen system contributes 29–36% [Citation98]. The contribution from anaerobic glycolysis increased from 46–54% during the first five reps to 81–85% during the last five reps, indicating that energy system requirements may vary depending on the training schemes.
Even when lifting very light weights, anaerobic glycolysis is operative. Studies examining blood lactate concentrations as an indication of anaerobic metabolism report that blood lactate levels rise from 1 mM to 3 mM after performing 30 repetitions (25% of 1-RM) on the half squat [Citation99–101]. Other evidence shows that blood lactate levels exhibit a similar increase after 6 sets of 20 leg press repetitions (30% of 1-RM) and a further increase to 4 mM after 15 sets, each set separated by 1 minute of passive recovery [Citation102]. The reliance on anaerobic glycolysis increases further as lifting intensity increases. Performing 5 repetitions with a 10-RM load on the leg press has been shown to increase blood lactate levels to 7 mM [Citation103]. When taken to failure (i.e. all 10 repetitions performed with the 10-RM weight), blood lactate levels reached 17 mM. After 5 sets of 10 repetitions and 2 minutes of passive rest between sets, blood lactate levels were further elevated to 25 mM [Citation103].
Despite its common association with endurance exercise, muscle glycogen provides an important fuel source for strength training. A reliance on anaerobic glycolysis during resistance training has been echoed in studies that measured glycogen concentrations in muscle tissue after a weightlifting session. In bodybuilders, an exercise session consisting of four sets of 6–10 repetitions of front squat, back squat, leg press, and leg extension taken to momentary muscular failure (16 sets in all) reduces quadriceps muscle glycogen by 26% [Citation104]. Glycogen depletion in other muscles contributing to the effort (such as the hamstrings and glutes) was not measured. Similarly, in untrained adults, eight sets of 10 repetitions of leg press and leg extension exercises (16 sets in all) depleted quadriceps muscle glycogen by 33%, particularly in type II fibers that are less dependent on aerobic metabolism [Citation105]. The relative rate of muscle glycogen reduction per set can be more rapid in smaller muscles or muscle groups. For example, even just a single to-failure set of 12 repetitions of one-arm biceps curls can reduce muscle glycogen by 12%, while 3 sets reduce glycogen by 24% [Citation106]. The collective evidence indicates that a typical resistance training session can reduce muscle glycogen stores by approximately 25–40% [Citation107].
In highly trained endurance athletes, 4 weeks on a eucaloric ketogenic diet (85% fat) resulted in a resting/pre-exercise muscle glycogen storage level that was 46.8% less than that of the eucaloric high-carbohydrate control condition (33% fat) [Citation30]. Although dietary carbohydrates are the major source of muscle glycogen, it is possible that a long-term ketogenic diet can still allow normal levels of muscle glycogen. The FASTER study reported that long-term ketogenic diet athletes (20 months, on average) had similar resting levels of glycogen as their high-carbohydrate diet counterparts [Citation9]. Unfortunately, exercise performance, which is ultimately what matters, was not reported in this trial. Other studies have suggested that under conditions of restricted or even absent exogenous carbohydrate provision, the body is capable of producing glucose to replenish glycogen from amino acids, lactate, and glycerol [Citation108,Citation109]. There is currently a lack of data on the duration of ketogenic diets required to allow similar muscle glycogen storage levels to traditional high-carbohydrate diets in athletic populations. To date, the FASTER study remains anomalous and unreplicated in this regard.
13. Muscular strength summary
Ketogenic diets do not appear to affect strength training performance differently from control diets, which are higher in carbohydrates. Notably, this includes both acute performance and strength gains that occur over 4–12 weeks of resistance training. Caution is warranted since a minority of studies indeed show superior effects of the higher-carbohydrate comparator for improving or retaining strength performance. Furthermore, there is a lack of trials exceeding 12 weeks involving highly trained strength athletes. Cautious monitoring of individual response is recommended for strength athletes choosing a ketogenic diet, due to its potential to suboptimize training adaptations in the long-term.
There is limited evidence of sex differences in the strength response to eating a ketogenic diet, given that the only study to report a difference between groups exclusively involved females. As will be discussed in the “Sex Differences” section of this paper, it is possible that females are more sensitive to glycogen availability to fuel high-intensity exercise, such as weight training. Further research is required to verify potential sex differences in metabolic pathways implicated in strength training and how they may be affected by ketogenic diets.
14. Body composition
One of the most popular uses of the ketogenic diet is to promote fat loss. Both within the scientific and popular communities, it has been proposed that excessive carbohydrate intakes, coupled with the insulin responses evoked, promote fat storage, and a ketogenic diet is superior to higher carbohydrate diets for reducing fat mass [Citation110]. This idea is not without controversy [Citation111], and these concepts are primarily discussed within the context of obesity and type 2 diabetes which is beyond the scope of this position stand. However, there are body composition studies of healthy active adults utilizing a ketogenic diet.
To date, 19 controlled trials have investigated the effects of a ketogenic diet on body composition () [Citation22,Citation35,Citation36,Citation41–43,Citation46,Citation71,Citation86–93,Citation112–114]. Of these, only two involved exclusively females, while 11 involved exclusively males; participants were either untrained before the intervention (2 trials), recreationally active (14 trials), or involved in a competitive sport (3 trials); the exercise intervention was to maintain usual exercise habits (9 trials) or follow a prescribed routine (10 trials); and dietary monitoring was either via food logs (17 trials) or not described (2 trials). Body composition was assessed using dual x-ray absorptiometry (DXA; 10 trials), bioelectrical impedance analysis (BIA; 8 trials), and skinfold measurements (1 trial). Studies lasted for 3–12 weeks (median of 8 weeks).
Table 3. The effects of a ketogenic diet on body composition.
Seven trials were excluded from our analysis due to being performed in adolescents [Citation48], failing to achieve a state of ketosis in the ketogenic diet group [Citation49], exceeding the carbohydrate threshold for ketogenic diets without an objective measurement of ketosis [Citation95,Citation115], not reporting on dietary intake or objective measures of ketosis [Citation96], or not assessing baseline body composition [Citation45,Citation94]. Another five trials were excluded due to being case studies without a control group [Citation55–58,Citation97].
15. Body composition changes
An overview of body composition changes in the ketogenic and control groups is shown in . All but two studies reported that a ketogenic diet reduced body weight by 0.3–7.8 kg (3.2 kg average) relative to the control diet, with statistical significance achieved in eight studies. The outlier studies reported that the ketogenic diet intervention led to a non-significant 0.1 and 0.4 kg greater weight gain than control diets [Citation36,Citation41].
Figure 4. Overview of changes in body composition during controlled trials for ketogenic diet and control diet groups. Lines represent the mean values from each unique study. It can be seen that changes in fat-free mass are inconsistent across studies (B), whereas changes in body fat percent show a more similar pattern (D). Lines in red indicate statistically significant differences between groups (p < 0.05).
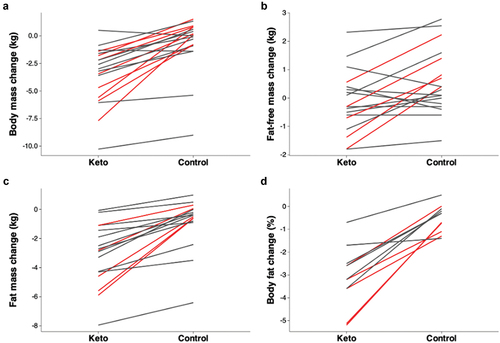
All studies reported that a ketogenic diet reduced fat mass by 0.3–5.3 kg (2.3 kg average) relative to the control diet, although statistical significance was achieved in only five studies. Of the 11 studies reporting body fat percentage, all reported reductions of 0.3–4.5% (2.6% average) with a ketogenic diet relative to the control diet, with statistical significance observed in five studies. All but four studies reported that a ketogenic diet reduced fat-free mass by 0.2–2.3 kg (0.9 kg average) relative to the control diet, with statistical significance achieved in five studies. The four other studies reported increases in fat-free mass of 0.2 [Citation46], 0.4 [Citation41], 0.7 [Citation112], and 0.8 kg [Citation36] with a ketogenic diet relative to a control diet, despite these studies’ also reporting reductions in body weight and fat mass in the ketogenic diet group relative to the control group. Overall, the ketogenic diet seems superior to the control diet for reducing body fat in exercising individuals. Although fat-free mass tends to be reduced on a ketogenic diet, the reductions in fat mass are larger and more consistent in both male and female populations, leading to a reduction in body fat percentage. However, disparate study designs make it difficult to draw specific conclusions from the available data.
Importantly, no study discussed in this section implemented strict dietary controls (e.g. live-in studies and/or providing food for participants), and all but two studies [Citation90,Citation114] relied on food logs to assess dietary intake (those two studies did not report dietary information). Ketosis was confirmed in all but two studies [Citation71,Citation91] with blood (0.2–1.5 mM) or urinary ketone assessments. Differences between groups for self-reported calorie intake varied widely among studies, with five studies reporting the ketogenic diet group consumed over 100 kcal/d more than the control group [Citation35,Citation36,Citation41,Citation46,Citation112] and six reporting eating over 100 kcal/d less [Citation43,Citation71,Citation87,Citation88,Citation91,Citation113]. On average, the ketogenic diet group consumed 75 fewer kcal per day. Collectively, protein intake was about 0.5 g/kg higher in the ketogenic diet groups (1.8 vs. 1.3 g/kg), which is not a trivial amount. Only one study reported greater protein intake in the control group (by 0.4 g/kg) [Citation88].
Although average calorie intake was similar between groups when considering all studies that reported dietary information, the energy intake of athletes tended to be underestimated in food logs by an average of 19% (range: 0.4–36%) [Citation116]. A ketogenic diet, especially one high in protein [Citation117], is particularly satiating [Citation118]. Higher-protein diets generally reduce appetite and food intake [Citation119]. Given the lack of dietary control among all included studies, it is plausible that the differences in body composition between the ketogenic and control diets were due to differences in energy and protein intakes between groups. Although differences in energy intake were modest between the ketogenic and control diets, a meta-regression has suggested that an energy deficit of at least 500 kcal per day prevents gains in lean mass from resistance training [Citation120].
Higher protein intakes in the ketogenic diet group and effects on lean mass changes warrant additional discussion. Two large meta-analyses investigating the effects of protein supplementation on muscle mass and strength suggested that gains in fat-free mass are maximally realized when protein intake is at least 1.6 g/kg/d [Citation121,Citation122]. The ISSN recommends that athletes and recreationally active adults consume 1.4–2.2 g/kg of protein to optimize performance and recovery [Citation123].To this point, only six studies in this paper reported a protein intake in the control group within the ISSN’s recommended range, and only two reported an intake above 1.6 g/kg. Comparatively, 12 studies reported a protein intake in the ketogenic diet group within the ISSN’s recommended range, with 10 studies reporting a daily protein intake above 1.6 g/kg. It is, therefore, notable that all but three studies reported a loss of lean mass in the ketogenic diet group compared to the control group, despite the ketogenic diet groups’ consuming more protein. The higher self-reported protein intakes in ketogenic diet groups in these studies may have been responsible for preserving more lean mass than would have occurred otherwise.
Another limitation of the available data is the use of DXA to assess body composition while participants were on their respective diets. Each gram of liver glycogen is paired with about 2.4 grams of water [Citation124], and each gram of skeletal muscle glycogen is paired with at least 3 grams of water [Citation125]. When glycogen stores are depleted during a low-carbohydrate diet and resistance training, fluid loss from lean tissue may occur. Ten studies assessed body composition with DXA, but glycogen depletion has been shown to reduce DXA-determined fat-free mass, while repletion increases total body water content and fat-free mass [Citation126]. Moreover, a 5% variation in fat-free mass hydration can change DXA-determined body fat percentage by nearly 3% (absolute value) [Citation127]. It is possible that glycogen levels on a ketogenic diet return to normal after a period of time (20 months in the FASTER study) [Citation9], but this has not been confirmed. Thus, the measurement of fat-free mass with DXA following a ketogenic diet is a potential limitation, and values may be artificially lowered due to changes in muscle glycogen.
These concepts are illustrated by one of the studies by Wilson et al. [Citation22] included in this review, who assessed body composition at baseline, after a 10-week intervention on a ketogenic or control diet, and again after one additional week of increased carbohydrate intake (3 g/kg) in the ketogenic diet group. After 10 weeks, both groups showed a similar increased fat-free mass, while the ketogenic diet group experienced a significantly greater reduction in fat mass. After 11 weeks, reductions in fat mass were reported to be similar between groups, while the ketogenic diet group experienced a significantly greater increase in fat-free mass. Within the ketogenic diet group, comparing changes between weeks 10 and 11 demonstrated that consuming a higher-carbohydrate diet for one week increased both fat mass and fat-free mass as measured by DXA.
The eight studies employing BIA assessment for body composition analysis also demonstrate similar limitations regarding the influence of hydration status on body composition. A study of recreational endurance athletes reported that a 3% change in hydration status altered body fat percentage by 2% [Citation128]. Being more dehydrated, which could be due to fluid shifts during a ketogenic diet, can be expected to result in a lower body fat percentage. Therefore, BIA assessment in people who are following a ketogenic diet when hydration status is not known or well-controlled may not be an appropriate means of determining body fat
16. Body composition summary
A ketogenic diet reduces body weight, body fat, and fat-free mass more than control diets higher in carbohydrate among active adults and athletes. Changes in fat-free mass are more disparate with a similar number of studies indicating greater losses of fat-free mass when following a ketogenic diet as the number of studies that show negligible changes in fat-free mass. However, as discussed in this section, these results may be influenced by alterations in body fluid, increases in protein intake, and reductions in calorie intake with ketogenic diets. Further research in which there is strict control over the diet is needed to disentangle how a ketogenic diet impacts the body composition of active adults and athletes.
17. Sex differences
The overarching theory behind using a ketogenic diet to improve athletic performance is that it enhances fat oxidation and spares glycogen use during exercise. It is well documented that sex differences exist in metabolism at rest and during exercise. Specifically, females metabolize a higher percentage of total energy from fat than males, independent of intensity or mode of exercise [Citation129], and utilize different proportions of carbohydrate and fat sources for fuel [Citation130].
Ovarian hormones play a role in sparing hepatic and skeletal muscle glycogen with greater fat oxidation. Progesterone antagonizes the estrogen-mediated effect on contraction-stimulated glucose uptake and increases the activity of β-oxidation in the skeletal muscle during exercise [Citation131]. Estrogen, specifically 17-β estradiol (E2), increases expression of the respiratory complexes, antioxidant molecules, and antiapoptotic factors that directly impact mitochondrial structure and function [Citation132–134], and circulating E2 exerts a significant metabolic effect by increasing the maximal activity of key enzymes in the fat oxidative pathway of skeletal muscle [Citation135]. Moreover, females have significantly higher protein content for trifunctional protein alpha (TFPα), very long-chain acyl-CoA dehydrogenase (VLCAD), and medium-chain acyl-CoA dehydrogenase, the primary enzymes involved in long- and medium-chain fatty acid utilization [Citation136,Citation137].
Recently, it has been documented that the skeletal muscle of females has: 1) higher inhibition of mitochondrial fatty acid metabolism by malonyl-CoA, 2) greater abundance of the fatty acid transporter CD36, and 3) lower ADP sensitivity of mitochondrial respiration (thus lesser ATP production). All of these effects mechanistically contribute to sex differences in lipid metabolism during exercise and enhanced mitochondrial resilience to exercise-induced damage [Citation137–139]. When examining the ketogenic diet-induced alterations in mitochondrial function, the mechanisms include increased mitochondrial respiratory control and expression of fatty acid transporters, as well as greater ATP production [Citation85].
In both sexes, energy homeostasis is controlled by various hormones secreted from the gut, pancreas, adipose tissue, and gonads. Sex hormones contribute to sex-related differences in ingestive behavior [Citation140]. Estrogen, a primarily female hormone, reduces food intake and body weight and exerts a profound effect on meal size [Citation141]. Estrogen therefore decreases energy intake, acting in conjunction with other circulating factors such as leptin and ghrelin to exert tonic inhibition [Citation142]. Moreover, nutrient-sensing pathways are also sensitive to circulating estrogen, including the regulation of hypothalamic kisspeptin neurons and the metabolic regulation of kisspeptin 1 (Kiss1) gene expression and secretion. Kisspeptin is a neuropeptide involved in the tight regulation of reproductive function and plays a significant role in the regulation of glucose homeostasis, feeding behavior, and body composition. The threshold for downregulation of kisspeptin signaling in GnRH neurons is lower in females than in males. This is primarily due to sex differences in the density of kisspeptinARC and kisspeptinAVPV/PeN neurons whereby kisspeptinAVPV/PeN neurons are almost exclusive to the female brain [Citation143]. Estrogen increases the expression of Kiss1 in kisspeptinAVPV/PeN and downregulates the expression in kisspeptinARC promoting a decrease in energy intake. Importantly, when energy deficits occur through insufficient energy intake or excessive energy expenditure, the Kiss1 gene is downregulated, with a subsequent repression of the GnRH neurons and downregulation of the reproductive axis [Citation144,Citation145]. Thus, substantial reductions in energy availability and associated low energy availability (LEA), which may or may not be accompanied by reductions in body weight, may negatively impact mental, hormonal, and bone health, as well as recovery time and general exercise performance [Citation146]. Understanding sex differences and ovarian hormone effects on lipid metabolism and energy balance should be considered before following a ketogenic diet that may otherwise cause enhanced satiety among females who are not attempting to lose weight [Citation147].
These biological differences in metabolism may lead to sex differences in the performance effects of ketogenic diets since females present a higher capacity for fatty acid oxidation. Notably, Venables et al. [Citation33] reported that females had a significantly higher rate of absolute fat oxidation than males and the exercise intensity eliciting maximal fat oxidation was significantly higher for females (52% of VO2max vs 45% in males). Additionally, Durkalec-Michalski et al. investigated a between-sex comparison of metabolic shifts in submaximal, intermittent exercise and reported there were more evident changes in fat and CHO utilization after KD in males at exercise intensities up to 80% of VO2max [Citation148]. Moreover, an increase in the area under the curve (AUC) for fat utilization was seen in males but not in females at up to ≤ 65% VO2max. Further research investigating how ketogenic diets impact submaximal exercise activity and metabolism in females specifically is needed.
As discussed in this review, the ketogenic diet appears to be superior to a control diet at reducing body mass and fat mass in both males and females. However, males seem to experience larger benefits than females in terms of weight loss and body composition changes [Citation149], although these differences may be attenuated after menopause due to changes in sex hormonal profile and hormonal control of appetite [Citation147]. Major limitations in the existing literature include measurements of appetite and energy balance, both of which have broad implications for body composition and subsequent health and performance outcomes.
18. Sex differences summary
In recent years, there has been an increased interest in using ketogenic diets to improve endurance exercise capacity through a shift toward the primary use of fatty acids and ketones as a fuel source; however, the viability of this diet in female athletes for long-term performance and health has yet to be established. From the few studies that have been conducted, it appears the ketogenic diet has a relatively greater benefit in males with regard to metabolic shifts and body composition changes. Further research in females is needed to determine the full effect of a ketogenic diet on performance, body composition, appetite regulation, and overall health outcomes.
19. Summary and conclusions
The research to date indicates that a ketogenic diet has largely neutral or detrimental effects on athletic performance. For endurance events, a ketogenic diet interferes with the body’s ability to generate energy from glucose, a necessity when performing at high intensities observed in real-world competitions. Even when one is “keto-adapted,” performance under real-world race conditions is impaired in endurance athletes. Special consideration is needed for female athletes, as sex differences in metabolic pathways, mitochondrial function, and the effects of ovarian hormones may nullify many desirable adaptations from ketogenic diets that are observed in male participants. Additionally, potential negative effects on certain endocrine feedback mechanisms are possible and should be further researched in female endurance athletes. Ketogenic diets seem to lead to similar strength and associated performance outcomes as diets higher in carbohydrates. Despite a substantial contribution from muscle glycogen during resistance training, low-carbohydrate diets do not appear to meaningfully impair performance. However, this conclusion is less certain since some studies suggest that higher-carbohydrate diets are superior for strength gains or maintenance. Studies beyond 12 weeks in highly trained strength athletes are also lacking.
For body composition, ketogenic diets appear to be superior to higher carbohydrate diets for reducing body weight and fat mass, but they are suboptimal for increasing fat-free mass. However, no study has used a diet-controlled design, meaning that differences in body composition are likely due to differences in energy and protein intake. Additionally, the true effects of a ketogenic diet on body composition may be skewed by fluid alterations that affect the analysis of fat-free mass and fat mass using commonly used methodologies (i.e. DXA and BIA).
20. Position of the International Society of Sports Nutrition (ISSN)
A ketogenic diet induces a state of nutritional ketosis, which is generally defined as serum ketone levels above 0.5 mM. While many factors can impact what amount of daily carbohydrate intake will result in these levels, a broad guideline is a daily dietary carbohydrate intake of less than 50 grams per day.
Nutritional ketosis achieved through carbohydrate restriction and a high dietary fat intake is not intrinsically harmful and should not be confused with ketoacidosis, a life-threatening condition most commonly seen in clinical populations and metabolic dysregulation.
A ketogenic diet has largely neutral or detrimental effects on athletic performance compared to a diet higher in carbohydrates and lower in fat, despite achieving significantly elevated levels of fat oxidation during exercise (~1.5 g/min).
The endurance effects of a ketogenic diet may be influenced by both training status and duration of the dietary intervention, but further research is necessary to elucidate these possibilities. All studies involving elite athletes showed a performance decrement from a ketogenic diet, all lasting six weeks or less. Of the two studies lasting more than six weeks, only one reported a statistically significant benefit of a ketogenic diet.
A ketogenic diet tends to have similar effects on maximal strength or strength gains from a resistance training program compared to a diet higher in carbohydrates. However, a minority of studies show superior effects of non-ketogenic comparators.
When compared to a diet higher in carbohydrates and lower in fat, a ketogenic diet may cause greater losses in body weight, fat mass, and fat-free mass, but may also heighten losses of lean tissue. However, this is likely due to differences in calorie and protein intake, as well as shifts in fluid balance.
There is insufficient evidence to determine if a ketogenic diet affects males and females differently. However, there is a strong mechanistic basis for sex differences to exist in response to a ketogenic diet.
21. List of abbreviations
Authors’ contributions
AL, JAR, TS, SS, and CM were responsible for drafting the manuscript and incorporating critical feedback and revisions suggested by the ISSN Research Committee coauthors. All coauthors were equally responsible for reviewing, editing, and providing feedback for submission of the final draft. All authors read and approved the final manuscript.
Availability of data and materials
Raw data and R code used to make are publicly available at https://osf.io/mfa2q/
Ethics approval and consent to participate
This paper was reviewed by the International Society of Sports Nutrition Research Committee and represents the official position of the Society.
Supplemental Material
Download Zip (33.6 KB)Acknowledgments
The authors would like to thank Chris Masterjohn and Victoria LaFont for their feedback on the draft manuscript.
Disclosure statement
All authors declare that they have no competing interests concerning this Position Paper.
BIC has received grants and contracts to conduct research on dietary supplements; has served as a paid consultant for industry; has received honoraria for speaking at conferences and writing lay articles about sports nutrition ingredients and topics; is a member of the International Protein Board that disseminates knowledge on protein and protein products; has served as an expert witness on behalf of the plaintiff and defense in cases involving dietary supplements; and receives compensation for writing and providing educational services related to exercise and nutrition-related topics. AL has served as a paid consultant in the supplement industry and receives compensation for writing educational materials related to general health and nutrition. TMS and CJM are shareholders in and are on the scientific advisory board of Outplay Inc., which researches transdermal drug and nutrient delivery. JAR is on the scientific advisory board of Applied Behavior Systems Ltd. STS has served as a paid scientific expert in the supplement industry but has no COIs regarding this paper; she has no financial conflict of interest with ketogenic diets or ketone-related products. SMA has received grants to research dietary supplements, served as a paid consultant for industry, and received honoraria for speaking at conferences about sports nutrition ingredients and topics. DD is an inventor on USF patents related to applications of therapeutic ketosis, advisor to Levels Health, and co-owner of Ketone Technologies LLC, which does research, consulting and public speaking events. JRS has received grants and contracts to research dietary supplements, served as a paid consultant for industry, and received honoraria for speaking at conferences and writing lay articles about sports nutrition ingredients and topics. CMK has received funding from nutrition studies to conduct scientific studies related to exercise and nutrition and currently serves in a consulting and advisory capacity for different dietary supplement brands or manufacturers that sell products related to items discussed in this paper. He has not received any funding from these companies as part of this work. RBK has conducted grant and contract-funded research on nutritional supplements awarded to the universities he has been affiliated with, received an honorarium for making scientific presentations, and served as a paid scientific expert. He has no financial conflict of interest with ketogenic diets or ketone-related products. MJO has received grants and contracts from companies that manufacture dietary supplements and has been appointed to the scientific advisory board of some exercise and nutrition companies. JA is the CEO of the International Society of Sports Nutrition, a 501c3 nonprofit, which receives grant support from a variety of companies in the sports nutrition category.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15502783.2024.2368167.
Additional information
Funding
References
- Kerksick CM, Wilborn CD, Roberts MD, et al. ISSN exercise & sports nutrition review update: research & recommendations. J Int Soc Sports Nutr [Internet]. 2018;15(1):38. doi: 10.1186/s12970-018-0242-y
- Kerksick CM, Arent S, Schoenfeld BJ, et al. International society of sports nutrition position stand: nutrient timing. J Int Soc Sports Nutr [Internet]. 2017;14(1):33. doi: 10.1186/s12970-017-0189-4
- Aragon AA, Schoenfeld BJ, Wildman R, et al. International society of sports nutrition position stand: diets and body composition. J Int Soc Sports Nutr [Internet]. 2017;14(1):16. doi: 10.1186/s12970-017-0174-y
- Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr [Internet]. 2006;26:1–40. doi: 10.1146/annurev.nutr.26.061505.111258
- Shi L, Tu BP. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr Opin Cell Biol [Internet]. 2015;33:125–131. doi: 10.1016/j.ceb.2015.02.003
- Kalapos MP. On the mammalian acetone metabolism: from chemistry to clinical implications. Biochim Biophys Acta [Internet]. 2003;1621(2):122–139. Available from doi: 10.1016/s0304-4165(03)00051-5
- Musa-Veloso K, Likhodii SS, Cunnane SC. Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals. Am J Clin Nutr [Internet]. 2002;76(1):65–70. doi: 10.1093/ajcn/76.1.65
- Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol [Internet]. 2017;595(9):2857–2871. doi: 10.1113/JP273185
- Volek JS, Freidenreich DJ, Saenz C, et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism [Internet]. 2016;65(3):100–110. doi: 10.1016/j.metabol.2015.10.028
- Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. EJSS [Internet]. 2015;15(1):13–20. doi: 10.1080/17461391.2014.959564
- Shaw DM, Merien F, Braakhuis A, et al. Exogenous ketone supplementation and keto-adaptation for endurance performance: disentangling the effects of two distinct metabolic states. Sports Med [Internet]. 2020;50(4):641–656. doi: 10.1007/s40279-019-01246-y
- Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev [Internet]. 1980;60(1):143–187. Available from http://www.physiology.org/doi/10.1152/physrev.1980.60.1.143
- Owen OE, Felig P, Morgan AP, et al. Liver and kidney metabolism during prolonged starvation. J Clin Invest [Internet]. 1969;48:574–583. doi: 10.1172/JCI106016
- Reichard GA Jr, Owen OE, Haff AC, et al. Ketone-body production and oxidation in fasting obese humans. J Clin Invest [Internet]. 1974;53:508–515. doi: 10.1172/JCI107584
- Sapir DG, Owen OE. Renal conservation of ketone bodies during starvation. Metabolism [Internet]. 1975;24(1):23–33. doi: 10.1016/0026-0495(75)90004-9
- Owen OE, Reichard GA. Human forearm metabolism during progressive starvation. J Clin Invest [Internet]. 1971;50:1536–1545. doi: 10.1172/JCI106639
- Balasse EO, Féry F. Ketone body production and disposal: Effects of fasting, diabetes, and exercise. Diabetes/Metabolism Rev [Internet]. 1989;5(3):247–270. doi: 10.1002/dmr.5610050304
- Taggart AKP, Kero J, Gan X, et al. (d)-β-Hydroxybutyrate inhibits adipocyte lipolysis via the Nicotinic Acid Receptor PUMA-G. J Biol Chem [Internet]. 2005;280(29):26649–26652. doi: 10.1074/jbc.C500213200
- Westman EC, Feinman RD, Mavropoulos JC, et al. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr [Internet]. 2007;86(2):276–284. doi: 10.1093/ajcn/86.2.276
- Sumithran P, Proietto J. Ketogenic diets for weight loss: a review of their principles, safety and efficacy. Obes Res Clin Pract [Internet]. 2008;2(1):1–13. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1871403X07000816
- Burke LM, Ross ML, Garvican-Lewis LA, et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol [Internet]. 2017;595(9):2785–2807. doi: 10.1113/JP273230
- Wilson JM, Lowery RP, Roberts MD, et al. Effects of ketogenic dieting on body composition, strength, power, and hormonal profiles in resistance training men. J Strength Cond Res [Internet]. 2020;34. Available from 12):3463–3474. doi: 10.1519/JSC.0000000000001935
- Gastin PB. Energy system interaction and relative contribution during maximal exercise. Sports Med [Internet]. 2001;31(10):725–741. doi: 10.2165/00007256-200131100-00003
- Baker JS, McCormick MC, Robergs RA. Interaction among skeletal muscle metabolic energy systems during intense exercise. J Nutr Metab [Internet]. 2010;2010:1–13. doi: 10.1155/2010/905612
- Kominami K, Nishijima H, Imahashi K, et al. Gas exchange threshold to guide exercise training intensity of older individuals during cardiac rehabilitation. Medicine [Internet]. 2021;100(42):e27540. doi: 10.1097/MD.0000000000027540
- Ferguson BS, Rogatzki MJ, Goodwin ML, et al. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol [Internet]. 2018;118(4):691–728. doi: 10.1007/s00421-017-3795-6
- Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol [Internet]. 2004;558(1):5–30. doi: 10.1113/jphysiol.2003.058701
- Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol [Internet]. 2004;287(3):R502–16. doi: 10.1152/ajpregu.00114.2004
- Kreider RB, Kalman DS, Antonio J, et al. International society of sports nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr [Internet]. 2017;14(1):18. doi: 10.1186/s12970-017-0173-z
- Phinney SD, Bistrian BR, Evans WJ, et al. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism [Internet]. 1983;32(8):769–776. doi: 10.1016/0026-0495(83)90106-3
- Burke LM, Hawley JA. Effects of short-term fat adaptation on metabolism and performance of prolonged exercise. Med Sci Sports Exerc [Internet]. 2002;34(9):1492–1498. doi: 10.1097/00005768-200209000-00015
- Yeo WK, Carey AL, Burke L, et al. Fat adaptation in well-trained athletes: effects on cell metabolism. Appl Physiol Nutr Metab [Internet]. 2011;36(1):12–22. doi: 10.1139/H10-089
- Venables MC, Achten J, Jeukendrup AE. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. J Appl Physiol [Internet]. 2005;98(1):160–167. doi: 10.1152/japplphysiol.00662.2003
- Shaw DM, Merien F, Braakhuis A, et al. Effect of a ketogenic diet on submaximal exercise capacity and efficiency in runners. Med Sci Sports Exerc [Internet]. 2019; Available from 51(10):2135–2146. doi: 10.1249/MSS.0000000000002008
- Dostal T, Plews DJ, Hofmann P, et al. Effects of a 12-week very-low carbohydrate high-fat diet on maximal aerobic capacity, high-intensity intermittent exercise, and cardiac autonomic regulation: non-randomized parallel-group study. Front Physiol [Internet]. 2019;10:912. doi: 10.3389/fphys.2019.00912
- Prins PJ, Noakes TD, Welton GL, et al. High rates of fat oxidation induced by a low-carbohydrate, high-fat diet, do not impair 5-km running performance in competitive recreational athletes. J Sports Sci Med [Internet]. 2019;18(4):738–750. Available from https://www.ncbi.nlm.nih.gov/pubmed/31827359
- Burke LM, Sharma AP, Heikura IA, et al. Crisis of confidence averted: impairment of exercise economy and performance in elite race walkers by ketogenic low carbohydrate, high fat (LCHF) diet is reproducible. PLoS One [Internet]. 2020;15(6):e0234027. doi: 10.1371/journal.pone.0234027
- Burke LM, Whitfield J, Heikura IA, et al. Adaptation to a low carbohydrate high fat diet is rapid but impairs endurance exercise metabolism and performance despite enhanced glycogen availability. J Physiol [Internet]. 2021;599(3):771–790. doi: 10.1113/JP280221
- Whitfield J, Burke LM, McKay AKA, et al. Acute ketogenic diet and ketone ester supplementation impairs race walk performance. Med Sci Sports Exerc [Internet]. 2021;53(4):776–784. doi: 10.1249/MSS.0000000000002517
- Dearlove DJ, Soto Mota A, Hauton D, et al. The effects of endogenously- and exogenously-induced hyperketonemia on exercise performance and adaptation. Physiol Rep [Internet]. 2022;10(10):e15309. doi: 10.14814/phy2.15309
- Wachsmuth NB, Aberer F, Haupt S, et al. The impact of a high-carbohydrate/low fat vs. low-carbohydrate diet on performance and body composition in physically active adults: a cross-over controlled trial. Nutrients [Internet]. 2022;14(3):423. doi: 10.3390/nu14030423
- Prins PJ, Noakes TD, Buga A, et al. Low and high carbohydrate isocaloric diets on performance, fat oxidation, glucose and cardiometabolic health in middle age males. Front Nutr [Internet]. 2023;10:1084021. doi: 10.3389/fnut.2023.1084021
- Cipryan L, Plews DJ, Ferretti A, et al. Effects of a 4-week very low-carbohydrate diet on high-intensity interval training responses. J Sports Sci Med [Internet]. 2018;17(2):259–268. https://www.ncbi.nlm.nih.gov/pubmed/29769827
- Fleming J, Sharman MJ, Avery NG, et al. Endurance capacity and high-intensity exercise performance responses to a high fat diet. Int J Sport Nutr Exerc Metab [Internet]. 2003;13(4):466–478. Available from: https://www.ncbi.nlm.nih.gov/pubmed/14967870
- Heatherly AJ, Killen LG, Smith AF, et al. Effects of ad libitum low-carbohydrate high-fat dieting in middle-age male runners. Med Sci Sports Exerc [Internet]. 2018;50(3):570–579. Available from doi: 10.1249/MSS.0000000000001477
- McSwiney FT, Wardrop B, Hyde PN, et al. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism [Internet]. 2018;81:25–34. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0026049517302986
- Wroble KA, Trott MN, Schweitzer GG, et al. Low-carbohydrate, ketogenic diet impairs anaerobic exercise performance in exercise-trained women and men: a randomized-sequence crossover trial. J Sports Med Phys Fitness [Internet]. 2019;59(4):600–607. doi: 10.23736/S0022-4707.18.08318-4
- Rhyu H-S, Cho S-Y. The effect of weight loss by ketogenic diet on the body composition, performance-related physical fitness factors and cytokines of taekwondo athletes. J Exerc Rehabil [Internet]. 2014;10(5):326–331. doi: 10.12965/jer.140160
- Zajac A, Poprzecki S, Maszczyk A, et al. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Nutrients [Internet]. 2014;6(7):2493–2508. doi: 10.3390/nu6072493
- Michalczyk MM, Chycki J, Zajac A, et al. Anaerobic performance after a Low-Carbohydrate Diet (LCD) Followed by 7 days of carbohydrate loading in male basketball players. Nutrients [Internet]. 2019;11(4):11. doi: 10.3390/nu11040778
- Lambert EV, Speechly DP, Dennis SC, et al. Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur J Appl Physiol Occup Physiol [Internet]. 1994;69(4):287–293. doi: 10.1007/BF00392032
- Goedecke JH, Christie C, Wilson G, et al. Metabolic adaptations to a high-fat diet in endurance cyclists. Metabolism [Internet]. 1999;48(12):1509–1517. doi: 10.1016/S0026-0495(99)90238-X
- O’Keeffe KA, Keith RE, Wilson GD, et al. Dietary carbohydrate intake and endurance exercise performance of trained female cyclists. Nutr Res [Internet]. 1989;9(8):819–830. doi: 10.1016/S0271-5317(89)80027-2
- Chiarello N, Leger B, De Riedmatten M, et al. Effect of a four-week isocaloric ketogenic diet on physical performance at very high-altitude: a pilot study. BMC Sports Sci Med Rehabil [Internet]. 2023;15(1):37. doi: 10.1186/s13102-023-00649-9
- Klement RJ, Frobel T, Albers T, et al. A pilot case study on the impact of a self-prescribed ketogenic diet on biochemical parameters and running performance in healthy and physically active individuals. Nutr And Med [Internet]. 2013 [cited 2021 Sep 10];1:10–37. Available from: https://www.researchgate.net/publication/248398348_A_pilot_case_study_on_the_impact_of_a_self-prescribed_ketogenic_diet_on_biochemical_parameters_and_running_performance_in_healthy_and_physically_active_individuals
- Waldman HS, Krings BM, Basham SA, et al. Effects of a 15-day low carbohydrate, high-fat diet in resistance-trained men. J Strength Cond Res [Internet]. 2018;32(11):3103–3111. doi: 10.1519/JSC.0000000000002282
- Zinn C, Wood M, Williden M, et al. Ketogenic diet benefits body composition and well-being but not performance in a pilot case study of New Zealand endurance athletes. J Int Soc Sports Nutr [Internet]. 2017;14(1):22. doi: 10.1186/s12970-017-0180-0
- McSwiney FT, Fusco B, McCabe L, et al. Changes in body composition and substrate utilization after a short-term ketogenic diet in endurance-trained males. Biol Sport [Internet]. 2021;38(1):145–152. Available from doi: 10.5114/biolsport.2020.98448
- Durkalec-Michalski K, Nowaczyk PM, Główka N, et al. Is a four-week ketogenic diet an effective nutritional strategy in CrossFit-trained female and male athletes? Nutrients [Internet]. 2021;13. Available from (3):864. doi: 10.3390/nu13030864
- Gregory J, Johns DP, Walls JT. Relative vs. absolute physiological measures as predictors of mountain bike cross-country race performance. Journal Of Strength And Conditioning Research [Internet]. 2007;21(1):17–22. Available from doi: 10.1519/R-17635.1
- Maciejczyk M, Wiecek M, Szymura J, et al. Influence of increased body mass and body composition on cycling anaerobic power. J Strength Cond Res [Internet]. 2015;29(1):58–65. Available from doi: 10.1519/JSC.0000000000000727
- Phinney SD, Bistrian BR, Evans WJ et al, The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism [Internet]. 1983;32:769–776. Available from https://www.ncbi.nlm.nih.gov/pubmed/6865776
- McLellan TM, Cheung SS, Jacobs I. Variability of time to exhaustion during submaximal exercise. Can J Appl Physiol [Internet]. 1995;20(1):39–51. Available from doi: 10.1139/h95-003
- Faude O, Hecksteden A, Hammes D, et al. Reliability of time-to-exhaustion and selected psycho-physiological variables during constant-load cycling at the maximal lactate steady-state. Appl Physiol Nutr Metab [Internet]. 2017;42(2):142–147. Available from doi: 10.1139/apnm-2016-0375
- Lucía A, Hoyos J, Pérez M, et al. Heart rate and performance parameters in elite cyclists: a longitudinal study. Medicine & Science In Sports & Exercise [Internet]. 2000;32(10):1777–1782. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11039652
- Fernández-García B, Pérez-Landaluce J, Rodríguez-Alonso M, et al. Intensity of exercise during road race pro-cycling competition. Med Sci Sports Exerc [Internet]. 2000;32:1002–1006. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10795793
- Jones AM, Kirby BS, Clark IE, et al. Physiological demands of running at 2-hour marathon race pace. J Appl Physiol [Internet]. 2021;130(2):369–379. Available from doi: 10.1152/japplphysiol.00647.2020
- Molinari CA, Edwards J, Billat V. Maximal time spent at VO2max from sprint to the marathon. Int J Environ Res Public Health [Internet]. 2020;17. Available from 24):9250. doi: 10.3390/ijerph17249250
- Gordon D, Wightman S, Basevitch I, et al. Physiological and training characteristics of recreational marathon runners. Open Access J Sports Med [Internet]. 2017;8:231–241. Available from doi: 10.2147/OAJSM.S141657
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab [Internet]. 2004;1(1):2. Available from doi: 10.1186/1743-7075-1-2
- Paoli A, Grimaldi K, D’Agostino D, et al. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J Int Soc Sports Nutr [Internet]. 2012;9(1):34. Available from http://jissn.biomedcentral.com/articles/10.1186/1550-2783-9-34
- Tiller NB, Roberts JD, Beasley L, et al. International society of sports nutrition position stand: nutritional considerations for single-stage ultra-marathon training and racing. J Int Soc Sports Nutr [Internet]. 2019;16(1):50. doi: 10.1186/s12970-019-0312-9
- Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol [Internet]. 2008;586(1):35–44. Available from doi: 10.1113/jphysiol.2007.143834
- Krogh A, Lindhard J. The relative value of fat and carbohydrate as sources of muscular energy: with appendices on the correlation between standard metabolism and the respiratory quotient during rest and work. Biochem J [Internet]. 1920;14(3–4):290–363. Available from doi: 10.1042/bj0140290
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol [Internet]. 1983;55(2):628–634. Available from doi: 10.1152/jappl.1983.55.2.628
- Petrick HL, Brunetta HS, Pignanelli C, et al. In vitro ketone-supported mitochondrial respiration is minimal when other substrates are readily available in cardiac and skeletal muscle. J Physiol [Internet]. 2020;598(21):4869–4885. Available from doi: 10.1113/JP280032
- Hall KD, Chen KY, Guo J, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr [Internet]. 2016;104(2):324–333. Available from doi: 10.3945/ajcn.116.133561
- Spriet LL, Heigenhauser GJF. Regulation of pyruvate dehydrogenase (PDH) activity in human skeletal muscle during exercise. Exerc Sport Sci Rev [Internet]. 2002;30(2):91–95. Available from doi: 10.1097/00003677-200204000-00009
- Peters SJ, Harris RA, Wu P, et al. Human skeletal muscle PDH kinase activity and isoform expression during a 3-day high-fat/low-carbohydrate diet. Am J Physiol Endocrinol Metab [Internet]. 2001;281(6):E1151–8. Available from doi: 10.1152/ajpendo.2001.281.6.E1151
- Stellingwerff T, Spriet LL, Watt MJ, et al. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am J Physiol Endocrinol Metab [Internet]. 2006;290(2):E380–8. Available from doi: 10.1152/ajpendo.00268.2005
- Billat VL, Demarle A, Slawinski J, et al. Physical and training characteristics of top-class marathon runners. Med Sci Sports Exerc [Internet]. 2001;33(12):2089–2097. Available from doi: 10.1097/00005768-200112000-00018
- Leckey JJ, Burke LM, Morton JP, et al. Altering fatty acid availability does not impair prolonged, continuous running to fatigue: evidence for carbohydrate dependence. J Appl Physiol [Internet]. 2016;120(2):107–113. Available from doi: 10.1152/japplphysiol.00855.2015
- Hawley JA, Leckey JJ. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med [Internet]. 2015;45 Suppl 1):S5–12. Available from doi: 10.1007/s40279-015-0400-1
- Burke LM. Ketogenic low-CHO, high-fat diet: the future of elite endurance sport? J Physiol [Internet]. 2021;599(3):819–843. Available from doi: 10.1113/JP278928
- Miller VJ, LaFountain RA, Barnhart E, et al. A ketogenic diet combined with exercise alters mitochondrial function in human skeletal muscle while improving metabolic health. Am J Physiol Endocrinol Metab [Internet]. 2020;319(6):E995–1007. Available from doi: 10.1152/ajpendo.00305.2020
- Wood RJ, Gregory SM, Sawyer J, et al. Preservation of fat-free mass after two distinct weight loss diets with and without progressive resistance exercise. Metab Syndr Relat Disord [Internet]. 2012;10(3):167–174. Available from http://online.liebertpub.com/doi/abs/10.1089/met.2011.0104
- Gregory RM. A low-carbohydrate ketogenic diet combined with 6-weeks of crossfit training improves body composition and performance. Int J Sports Exerc Med [Internet]. 2017;3. Available from 2). doi: 10.23937/2469-5718/1510054
- Kephart W, Pledge C, Roberson P, et al. The three-month effects of a ketogenic diet on body composition, blood parameters, and performance metrics in CrossFit trainees: a pilot study. Sportscience [Internet]. 2018;6(1):1. Available from http://www.mdpi.com/2075-4663/6/1/1
- Greene DA, Varley BJ, Hartwig TB, et al. A low-carbohydrate ketogenic diet reduces body mass without compromising performance in powerlifting and olympic weightlifting athletes. J Strength Cond Res [Internet]. 2018;32(12):3373–3382. Available from doi: 10.1519/JSC.0000000000002904
- LaFountain RA, Miller VJ, Barnhart EC, et al. Extended ketogenic diet and physical training intervention in military personnel. Mil Med [Internet]. 2019; Available from 184(9–10):e538–e547. doi: 10.1093/milmed/usz046
- Vargas-Molina S, Petro JL, Romance R, et al. Effects of a ketogenic diet on body composition and strength in trained women. J Int Soc Sports Nutr [Internet]. 2020;17(1):19. Available from doi: 10.1186/s12970-020-00348-7
- Paoli A, Cenci L, Pompei P, et al. Effects of two months of very low carbohydrate ketogenic diet on body composition, muscle strength, muscle area, and blood parameters in competitive natural body builders. Nutrients [Internet]. 2021;13. Available from 2):374. doi: 10.3390/nu13020374
- Vidić V, Ilić V, Toskić L, et al. Effects of calorie restricted low carbohydrate high fat ketogenic vs. non-ketogenic diet on strength, body-composition, hormonal and lipid profile in trained middle-aged men. Clin Nutr [Internet]. 2021;40(4):1495–1502. Available from doi: 10.1016/j.clnu.2021.02.028
- Sawyer JC, Wood RJ, Davidson PW, et al. Effects of a short-term carbohydrate-restricted diet on strength and power performance. J Strength Cond Res [Internet]. 2013;27(8):2255–2262. Available from doi: 10.1519/JSC.0b013e31827da314
- Meirelles CM, Gomes PSC. Effects of short-term carbohydrate restrictive and conventional hypoenergetic diets and resistance training on strength gains and muscle thickness. J Sports Sci Med [Internet]. 2016;15(4):578–584. Available from https://www.ncbi.nlm.nih.gov/pubmed/27928202
- Kruszewski M, Kruszewski A, Tabęcki R, et al. Effectiveness of high-fat and high-carbohydrate diets on body composition and maximal strength after 15 weeks of resistance training. Adv Med Sci [Internet]. 2024;69(1):139–146. Available from doi: 10.1016/j.advms.2024.02.008
- Chatterton S, Helms ER, Zinn C, et al. The effect of an 8-week low carbohydrate high fat (LCHF) diet in sub-elite Olympic weightlifters and powerlifters on strength, body composition, mental state and adherence: a pilot case-study. J Australian Strength And Conditioning [Internet]. 2017 [cited 2021 Sep 14];25:6–13. Available from: https://www.researchgate.net/publication/316675957_The_effect_of_an_8-week_low_carbohydrate_high_fat_LCHF_diet_in_sub-elite_Olympic_weightlifters_and_powerlifters_on_strength_body_composition_mental_state_and_adherence_a_pilot_case-study
- Gorostiaga EM, Navarro-Amézqueta I, Cusso R, et al. Anaerobic energy expenditure and mechanical efficiency during exhaustive leg press exercise. PLoS One [Internet]. 2010;5(10):e13486. Available from doi: 10.1371/journal.pone.0013486
- Garnacho-Castaño MV, Dominguez R, Maté-Muñoz JL. Understanding the meaning of lactate threshold in resistance exercises. Int J Sports Med [Internet]. 2015;36(5):371–377. Available from doi: 10.1055/s-0034-1398495
- Garnacho-Castaño MV, Albesa-Albiol L, Serra-Payá N, et al. The slow component of oxygen uptake and efficiency in resistance exercises: a comparison with endurance exercises. Front Physiol [Internet]. 2019;10:357. Available from doi: 10.3389/fphys.2019.00357
- Albesa-Albiol L, Serra-Payá N, Garnacho-Castaño MA, et al. Ventilatory efficiency during constant-load test at lactate threshold intensity: endurance versus resistance exercises. PLoS One [Internet]. 2019;14(5):e0216824. Available from doi: 10.1371/journal.pone.0216824
- de Sousa NMF, Magosso RF, Pereira GB, et al. Acute cardiorespiratory and metabolic responses during resistance exercise in the lactate threshold intensity. Int J Sports Med [Internet]. 2012;33:108–113. Available from doi: 10.1055/s-0031-1286315
- Gorostiaga EM, Navarro-Amézqueta I, Calbet JAL, et al. Energy metabolism during repeated sets of leg press exercise leading to failure or not. PLoS One [Internet]. 2012;7(7):e40621. Available from doi: 10.1371/journal.pone.0040621
- Tesch PA, Colliander EB, Kaiser P. Muscle metabolism during intense, heavy-resistance exercise. Eur J Appl Physiol Occup Physiol [Internet]. 1986;55(4):362–366. Available from doi: 10.1007/BF00422734
- Koopman R, Manders RJF, Jonkers RAM, et al. Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Eur J Appl Physiol [Internet]. 2006;96:525–534. Available from doi: 10.1007/s00421-005-0118-0
- Macdougall JD, Ray S, Sale DG, et al. Muscle substrate utilization and lactate production during weightlifting. Can J Appl Physiol [Internet]. 1999;24(3):209–215. Available from doi: 10.1139/h99-017
- Murray B, Rosenbloom C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr Rev [Internet]. 2018;76(4):243–259. Available from doi: 10.1093/nutrit/nuy001
- Fournier PA, Bräu L, Ferreira L-B, et al. Glycogen resynthesis in the absence of food ingestion during recovery from moderate or high intensity physical activity: novel insights from rat and human studies. Comparative Biochemistry And Physiology Part A: Molecular & Integrative Physiology [Internet]. 2002;133(3):755–763. Available from: https://www.ncbi.nlm.nih.gov/pubmed/12443931
- Fournier PA, Fairchild TJ, Ferreira LD, et al. Post-exercise muscle glycogen repletion in the extreme: effect of food absence and active recovery. J Sports Sci Med [Internet]. 2004;3(3):139–146. Available from https://www.ncbi.nlm.nih.gov/pubmed/24482591
- Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “calories in, calories out. JAMA Intern Med [Internet]. 2018;178(8):1098–1103. Available from doi: 10.1001/jamainternmed.2018.2933
- Hall KD, Guyenet SJ, Leibel RL. The carbohydrate-insulin model of obesity is difficult to reconcile with current evidence. JAMA Intern Med [Internet]. 2018;178(8):1103–1105. Available from doi: 10.1001/jamainternmed.2018.2920
- Volek JS, Sharman MJ, Love DM, et al. Body composition and hormonal responses to a carbohydrate-restricted diet. Metabolism [Internet]. 2002;51:864–870. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0026049502000100
- Jabekk PT, Moe IA, Meen HD, et al. Resistance training in overweight women on a ketogenic diet conserved lean body mass while reducing body fat. Nutr Metab [Internet]. 2010;7(1):17. Available from http://nutritionandmetabolism.biomedcentral.com/articles/10.1186/1743-7075-7-17
- Vargas S, Romance R, Petro JL, et al. Efficacy of ketogenic diet on body composition during resistance training in trained men: a randomized controlled trial. J Int Soc Sports Nutr [Internet]. 2018;15(1):31. Available from doi: 10.1186/s12970-018-0236-9
- Michalczyk M, Zajac A, Mikolajec K, et al. No modification in blood lipoprotein concentration but changes in body composition after 4 weeks of Low Carbohydrate Diet (LCD) followed by 7 days of carbohydrate loading in basketball players. J Hum Kinet [Internet]. 2018;65(1):125–137. Available from doi: 10.2478/hukin-2018-0102
- Capling L, Beck K, Gifford J, et al. Validity of dietary assessment in athletes: a systematic review. Nutrients [Internet]. 2017;9(12):1313. Available from http://www.mdpi.com/2072-6643/9/12/1313
- Johnstone AM, Horgan GW, Murison SD, et al. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr [Internet]. 2008;87(1):44–55. Available from doi: 10.1093/ajcn/87.1.44
- Paoli A, Bosco G, Camporesi EM, et al. Ketosis, ketogenic diet and food intake control: a complex relationship. Frontiers In Psychology [Internet]. 2015;6:27. Available from doi: 10.3389/fpsyg.2015.00027
- Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein – its role in satiety, energetics, weight loss and health. Br J Nutr [Internet]. 2012;108(S2):S105–12. Available from doi: 10.1017/S0007114512002589
- Murphy C, Koehler K. Energy deficiency impairs resistance training gains in lean mass but not strength: a meta-analysis and meta-regression. Scand J Med Sci Sports [Internet]. 2022;32(1):125–137. Available from doi: 10.1111/sms.14075
- Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med [Internet]. 2017;5:bjsports – 2017–097608. Available from http://bjsm.bmj.com/lookup/doi/10.1136/bjsports-2017-097608
- Nunes EA, Colenso-Semple L, McKellar SR, et al. Systematic review and meta-analysis of protein intake to support muscle mass and function in healthy adults. J Cachexia Sarcopenia Muscle [Internet]. 2022;13(2):795–810. Available from doi: 10.1002/jcsm.12922
- Jäger R, Kerksick CM, Campbell BI, et al. International society of sports nutrition position stand: protein and exercise. J Int Soc Sports Nutr [Internet]. 2017;14(1):65. Available from http://jissn.biomedcentral.com/articles/10.1186/s12970-017-0177-8
- Nilsson LH. Liver glycogen content in man in the postabsorptive state. Scand J Clin Lab Invest [Internet]. 1973;32(4):317–323. Available from doi: 10.3109/00365517309084354
- Fernández- ElíElíAs VE, Ortega JF, Nelson RK, et al. Relationship between muscle water and glycogen recovery after prolonged exercise in the heat in humans. Eur J Appl Physiol [Internet]. 2015;115(9):1919–1926. Available from doi: 10.1007/s00421-015-3175-z
- Bone JL, Ross ML, Tomcik KA, et al. Manipulation of muscle creatine and glycogen changes dual x-ray absorptiometry estimates of body composition. Med Sci Sports Exerc [Internet]. 2017;49(5):1029–1035. Available from doi: 10.1249/MSS.0000000000001174
- Prior BM, Cureton KJ, Modlesky CM, et al. In vivo validation of whole body composition estimates from dual-energy X-ray absorptiometry. J Appl Physiol [Internet]. 1997;83(2):623–630. Available from http://www.physiology.org/doi/10.1152/jappl.1997.83.2.623
- Saunders MJ, Blevins JE, Broeder CE. Effects of hydration changes on bioelectrical impedance in endurance trained individuals. Med Sci Sports Exerc [Internet]. 1998;30(6):885–892. Available from doi: 10.1097/00005768-199806000-00017
- Knechtle B, Müller G, Willmann F, et al. Fat oxidation in men and women endurance athletes in running and cycling. Int J Sports Med [Internet]. 2004;25:38–44. Available from doi: 10.1055/s-2003-45232
- Mittendorfer B, Horowitz JF, Klein S. Effect of gender on lipid kinetics during endurance exercise of moderate intensity in untrained subjects. Am J Physiol Endocrinol Metab [Internet]. 2002;283(1):E58–65. Available from doi: 10.1152/ajpendo.00504.2001
- Oosthuyse T, Bosch AN. The effect of the menstrual cycle on exercise metabolism: implications for exercise performance in eumenorrhoeic women. Sports Med [Internet]. 2010;40(3):207–227. Available from doi: 10.2165/11317090-000000000-00000
- Devries MC. Sex-based differences in endurance exercise muscle metabolism: impact on exercise and nutritional strategies to optimize health and performance in women. Exp Physiol [Internet]. 2016;101(2):243–249. Available from doi:10.1113/EP085369
- Chen J-Q, Yager JD, Russo J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim Biophys Acta [Internet]. 2005;1746(1):1–17. Available from doi: 10.1016/j.bbamcr.2005.08.001
- Sims ST, Heather AK. Myths and methodologies: reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp Physiol [Internet]. 2018;103(10):1309–1317. Available from doi: 10.1113/EP086797
- Tarnopolsky MA. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med Sci Sports Exerc [Internet]. 2008;40(4):648–654. Available from doi: 10.1249/MSS.0b013e31816212ff
- Maher AC, Akhtar M, Vockley J, et al. Women have higher protein content of β-oxidation enzymes in skeletal muscle than men. PLoS One [Internet]. 2010;5(8):e12025. Available from doi: 10.1371/journal.pone.0012025
- Kiens B, Roepstorff C, Glatz JFC, et al. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol [Internet]. 2004;97(4):1209–1218. Available from doi: 10.1152/japplphysiol.01278.2003
- Miotto PM, McGlory C, Holloway TM, et al. Sex differences in mitochondrial respiratory function in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol [Internet]. 2018;314(6):R909–15. Available from doi: 10.1152/ajpregu.00025.2018
- Roepstorff C, Thiele M, Hillig T, et al. Higher skeletal muscle α 2 AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol [Internet]. 2006;574(1):125–138. Available from doi: 10.1113/jphysiol.2006.108720
- Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav [Internet]. 2011;104(4):517–524. Available from doi: 10.1016/j.physbeh.2011.04.014
- Butera PC. Estradiol and the control of food intake. Physiol Behav [Internet]. 2010;99(2):175–180. Available from doi: 10.1016/j.physbeh.2009.06.010
- Mela V, Vargas A, Meza C, et al. Modulatory influences of estradiol and other anorexigenic hormones on metabotropic, Gi/o-coupled receptor function in the hypothalamic control of energy homeostasis. J Steroid Biochem Mol Biol [Internet]. 2016;160:15–26. Available from doi: 10.1016/j.jsbmb.2015.07.014
- Navarro VM. Metabolic regulation of kisspeptin — the link between energy balance and reproduction. Nat Rev Endocrinol [Internet]. 2020;16(8):407–420. Available from doi: 10.1038/s41574-020-0363-7
- Castellano JM, Tena-Sempere M. Metabolic regulation of kisspeptin. Adv Exp Med Biol [Internet]. 2013;784:363–383. Available from doi: 10.1007/978-1-4614-6199-9_17
- Toufexis D, Rivarola MA, Lara H, et al. Stress and the reproductive axis. J Neuroendocrinol [Internet]. 2014;26(9):573–586. Available from doi: 10.1111/jne.12179
- Mountjoy M, Sundgot-Borgen JK, Burke LM, et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br J Sports Med [Internet]. 2018;52(11):687–697. Available from doi: 10.1136/bjsports-2018-099193
- Sims ST, Kerksick CM, Smith-Ryan AE, et al. International society of sports nutrition position stand: nutritional concerns of the female athlete. J Int Soc Sports Nutr [Internet]. 2023;20(1):2204066. Available from doi: 10.1080/15502783.2023.2204066
- Durkalec-Michalski K, Nowaczyk PM, Siedzik K. Effect of a four-week ketogenic diet on exercise metabolism in CrossFit-trained athletes. J Int Soc Sports Nutr [Internet]. 2019;16(1):16. doi: 10.1186/s12970-019-0284-9
- Koerich ACC, Borszcz FK, Thives Mello A, et al. Effects of the ketogenic diet on performance and body composition in athletes and trained adults: a systematic review and bayesian multivariate multilevel meta-analysis and meta-regression. Crit Rev Food Sci Nutr [Internet]. 2023;63(32):11399–11424. doi: 10.1080/10408398.2022.2090894
