ABSTRACT
Olive oil by-products have an important fertilizing value due to their high organic matter and nutrient content; however, they also possess a phytotoxic effect. Therefore, it is necessary to study the reaction of plants and, more specifically, the roots of olive trees to the presence of husks used as amendment in olive orchards. In this experimental work olive plants were grown in split-pots in which the roots were divided in four sectors and grown in different substrates. Husks, hay, and a mixture of the two was added to a soil substrate to simulate growing conditions in an olive orchard with green mulching. Results showed that olive roots performed differently in the different substrates. Husk substrates were avoided if a husk-free section was present. If not, the plants stopped growing and half of them perished. If hay was added with husks the toxic effect was reduced. It can be argued that in the field, olive plants, thanks to their great transmigration capacity, may be able to avoid the patches amended with husks, but repeated treatments with husks could cause shoot growth problems because it would be more difficult for roots to find optimal substrate to continue their growth.
INTRODUCTION
Plant roots live in a peculiar environment and in continuous interaction with the physical components of soil system, in addition to its biota. Soils have a solid, liquid, and gaseous phase and the characteristics of each phase vary in space and time (CitationDoussan et al., 2003; CitationForde and Lorenzo, 2001), forcing plant roots to adapt to this variability (CitationForde and Lorenzo, 2001). The ability to adapt root structure and physiology to the environment is known as plasticity (CitationForde and Lorenzo, 2001; CitationFransen et al., 1999) and it provides many advantages to plant adaptation in a soil system (CitationBingham and Bengough, 2003). In fact plants collect signals and information from the environment and develop specialized roots that can optimally exploit the substrate (CitationDeak and Malamy, 2005; CitationHodge, 2006). In this way, otherwise immobile plants can place roots in the favorable soil volumes for water and nutrient uptake (CitationDoussan et al., 2003). Moreover plant root structure can be adjusted to the changing soil conditions; old roots modify their physiology in order to maintain optimal absorption despite unavoidable environmental changes (CitationFransen et al., 1998).
Nevertheless plasticity has a cost for the plant, as energy is consumed to adapt to environmental changes, while causing plants to reduce shoot activity (CitationMalamy, 2005). Depending on the entity of the change or the physiological conditions of the plant, adaptation can cause growth reduction, defoliation, or even plant death. Also, nutrient and organic matter content of the soil constitute important factors that influence root plasticity and plant growth (CitationZucconi et al., 1981), making an adequate level of these two soil attributes essential. To do so agricultural by-product residues are often used as amendments. However, organic residues in the soil are degraded by soil microorganisms and during this decomposition some metabolic compounds may become toxic for plants (CitationFransen et al., 1998; CitationHodge, 2004; CitationLeyser and Fitter, 1998; CitationLopez-Bucìo et al., 2003). The degree and duration of the toxic effect depend on the nature of the original matter (composition and physical properties).
In all the Mediterranean countries the disposal of olive oil by-products (olive husks) poses a major environmental problem (CitationCegarra et al., 1996; CitationMarques, 2001; CitationTomati et al., 1996). A possible solution consists of using olive husks as a soil amendment, because of their high fertilizer value when applied to the soil, due to their high organic matter and nutrient content (CitationCalvet et al., 1985; CitationParedes et al., 1999; CitationTomati et al., 1996). However, concerns about the use of olive husks as soil amendments remain linked to their phytotoxic properties (CitationTagliavini and Marangoni, 1992; CitationZucconi et al., 1981).
Thus, studying the reaction of olive roots to the presence of olive husks in the soil is an important field of investigation because the toxic effect on the roots may affect the aerial growth and productivity of the plant. The purpose of this study was to evaluate the plasticity response of olive roots to the presence of husks in limited sectors of the substrate in which they were growing. Since organic matter composition may influence degradation rate and hence toxicity effect, olive husks were evaluated alone and mixed to hay to simulate olive orchard conditions with green mulching, typical of central Italy.
MATERIALS AND METHODS
Split-Root Pots
Cylindrical pots (1.7 liter) were divided in four sectors to allow a split-root experiment as proposed by CitationBingham and Bengough (2003).
The following substrates were tested:
| • | Basic (B): commercial compost (composted horse manure, pH 7.8, C/N 31.8), vermiculite, and sand (1:1:1 in volume); | ||||
| • | Olive husks (O): basic substrate amended with 10% by volume of olive husks powder (oven dried husks, ground and sieved at 1 mm); | ||||
| • | Hay (H): basic substrate amended with 10% by volume of mixed hay (ground); | ||||
| • | Hay and olive husks (OH): basic substrate amended with 10% by volume of ground hay and 2% husks powder. | ||||
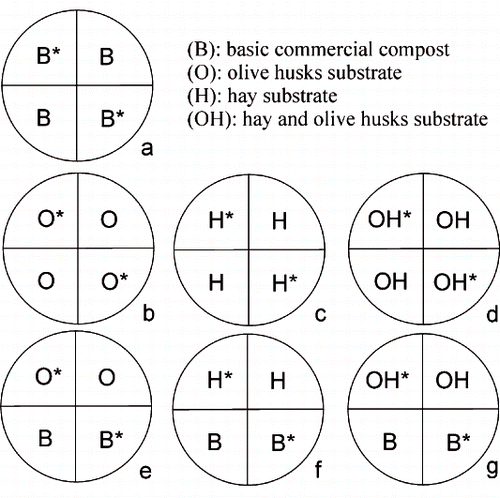
The single substrates were placed in all sectors of a pot to have a “whole-pot distribution,” or in only two adjacent sectors (while the other two were filled with the basic substrate) to have a “half-pot distribution” in which two kinds of substrate were present in a single pot. The complete set of treatments is reported in . Eight replicates per treatment were tested for a total of 56 plants.
FIGURE 1. Split-root pots have been filled to obtain: control = basic substrate (B) in all the sectors, whole-O = olive-husk-amended substrate (O) in all the sectors (whole-pot distribution), whole-H = hay-amended substrate (H) in all the sectors (whole-pot distribution), whole-OH = hay-husk-amended substrate (OH) in all the sectors (whole-pot distribution), half-O = basic substrate in two sectors and husk-amended substrate (O) in the other two (half-pot distribution), half-H = basic substrate in two sectors and hay-amended substrate (H) in the other two (half-pot distribution), half-OH = basic substrate in two sectors and hay-husk-amended substrate (OH) in the other two (half-pot distribution). The treatments had eight replicates each. The asterisk (*) indicates pot sectors occupied by root system at planting.
Plant Material
Micropropagated plants of Olea europaea L. (cv. Arbequina), well-acclimated ex-vitro, were transplanted in the center of the cylindrical pot, when the shoots averaged 12 cm in height. The existing root system was cut to 5 cm in length and split between two opposite sectors. The sectors with the preformed root system are indicated with an asterisk (*) in and are considered as “occupied” sectors in the text. The sectors free from roots at planting are called “new” sectors. The pots were kept at 25°C (light) and 20°C (dark). White incandescent lights were lit for 16h per day (250 mmol/m2s). Watering was provided twice a week with tap water (once with a solution 0.3 × Murashige and Skoog salts).
Measurements
Lengths of the primary and lateral shoots were measured monthly. Relative growth rate (RGR) was also calculated as the ratio between shoot growth per month (difference between two measurements) and shoot length in the previous month. At the end of the experiment (140 days) roots from different sectors were separated and washed from soil. Roots and shoots fresh and dry weights were recorded and shoot-to-root ratio calculated (using dry weights). Root distribution in each sector was calculated as the percentage of root total dry weight. Plant death was recorded and plant mortality was calculated for each treatment (dead plants/total plants). Data from the dead plants were considered as missing data in the analysis.
All data were compared using analysis of variance (ANOVA) for all the parameters. The data were also checked for deviations from normality and homogeneity of variances. A posteriori comparisons were carried out with Duncan's multiple comparison test or with t-tests where appropriate.
RESULTS
Shoot Length
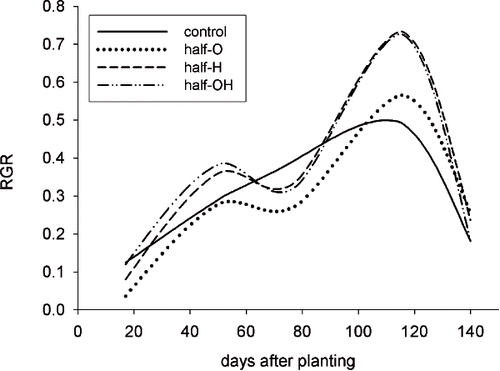
Total growth (primary + lateral shoots) showed no significant differences in the half-pot distribution (). However, the plants with H and OH substrates showed a higher RGR at the end of the experiment, when compared to the control ().
FIGURE 2. Total shoots length (primary + laterals) of plants in pots with a half-pot distribution. The treatments were: Control, Olive husks (half-O), Hay (half-H), Olive husks and Hay (half-OH). The bar indicates standard error of the mean (SEM; n = 64) of all treatments values recorded at the end of the experiment.
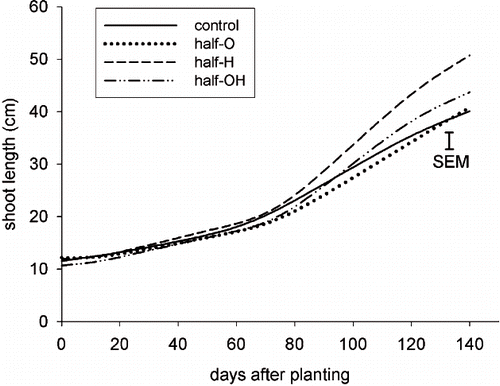
FIGURE 3. Relative Growth Rate (RGR) of shoots (primary + lateral) of plants in pots with a half-pot distribution. RGR was measured as the shoot growth per month, divided for the shoot length measured in the previous month.
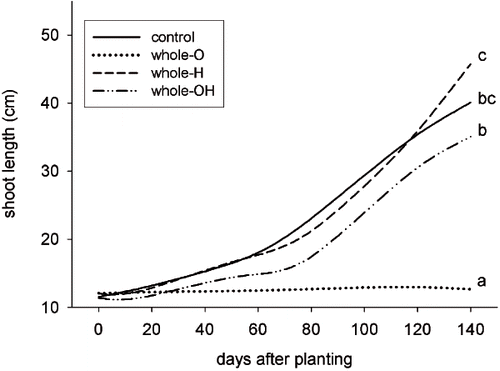
In the whole-pot distribution, the presence of husks in the compost soil (O) inhibited shoot growth. Hay and husks substrate (OH) determined a more prominent growth of shoots when compared to husks only (O). Hay substrate (H) induced longer shoots when compared to hay and husks (OH). Basic substrate yielded an intermediate growth between H and OH treatments ().
FIGURE 4. Total shoots length (primary + laterals) of plants in pots with a whole-pot distribution (all sectors with treatment). The treatments were: Control, Olive husks (whole-O), Hay (whole-H), Olive husks and Hay (whole-OH). Different letters indicate significant differences between the values of the last measurement (Duncan's test P < 0.05).
Root Weight
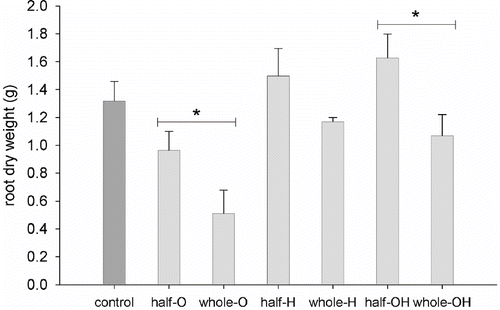
The total weight of the roots per pot was higher in the half-pot distribution compared to the whole pot in all the treatments but H. In comparison with the control, husks treatment (O) showed a lower growth of the root system, slightly lower for the half-pot distribution and greatly lower for the whole pot. H and OH treatments showed similar growth. Root weight was comparable to the control in the whole-pot distribution and higher than the control in the half pot ().
FIGURE 5. Dry weight per plant of whole root systems. All treatments are represented: Control, Olive husks (half-O and whole-O), Hay (half-H and whole-H), Olive husks and Hay (half-OH and whole-OH). Error bars represent standard error (SE; n = 8). Asterisks indicate significant differences (t-test between different distributions, P<0.01).
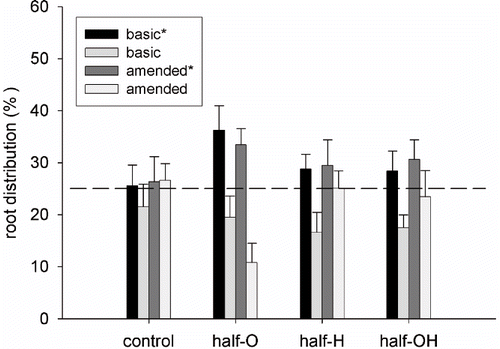
The weight of the roots in each sector at the end of the experiment proved a selective placement of roots, depending on the substrate composition. shows root dry weight distribution between the four sectors as a percentage of the total dry weight of the root system for the half-pot distribution. In the sectors that were occupied by roots at the beginning of the experiment (indicated by *), no significant differences were found between the basic and amended substrates. In the new sectors with H and OH substrates, at the end of the experiment, there were a higher percentage of roots than in the basic sectors. While the sectors with O substrate showed a lower root percentage than the basic.
FIGURE 6. Dry weight distribution of olive root systems grown in split-root pots. Control, Olive husks (half-O), Hay (half-H), Olive husks and Hay (half-OH) are represented. The dashed line represents the average root distribution per sector in the control pot. Error bars represent SE (n = 8).
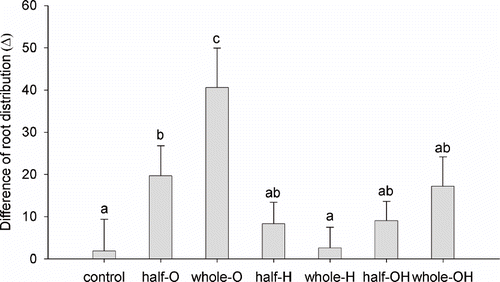
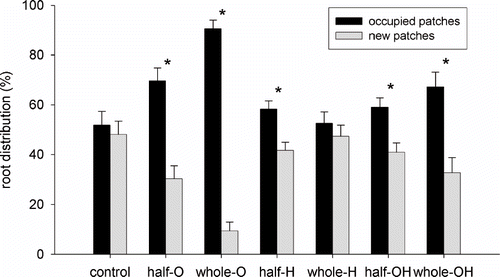
The root distribution between occupied (*) and new sectors is shown in . Control and H (whole-pot distribution) showed a uniform distribution of the root system between the sectors. In all the other treatments the percentage of root weight present in the occupied sectors was significantly higher than in the new ones. The rate of new sectors occupation was calculated as the difference between root percentage in new and occupied sectors (). The larger the difference the less new sectors were occupied by roots. The greater difference was observed in the O treatments, followed by OH (whole-pot), OH (half-pot), and H (half-pot); whereas H (whole-pot) showed an equilibrated root distribution, similar to the control.
FIGURE 7. Percent distribution of root systems among the sectors: “occupied sectors,” where roots were present at the beginning of the experiment, and “new sectors” that were free from roots at the beginning of the experiment. Error bars represent SE (n = 8). Asterisks indicate significant differences between new and occupied sectors distribution (Duncan's test; P < 0.05).
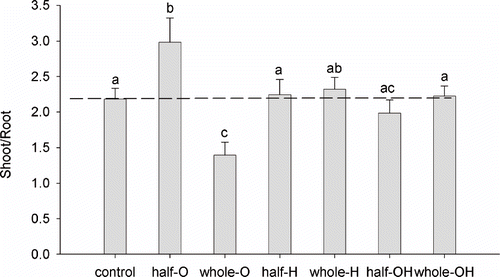
Shoot/Root Ratio
The ratio between shoot (S) and root (R) dry weight was similar to the control for the plants grown on H and OH substrates. Plants grown on O substrate showed an S/R ratio higher than the control in the half-pot distribution, and lower than the control in the whole-pot distribution ().
Plant Mortality
Mortality was higher when husks were uniformly distributed in the pot. Fifty percent of the plants of this treatment died. The other treatments had a maximum of 12.5% dead plants (1 out of 8).
DISCUSSION
In this work we considered the effect of olive husks used as an amendment while monitoring the growth of olive tree microcuttings (shoot and root). Basic substrate was compared to substrates amended with husks, hay, or a mix of hay and husks to simulate growth in olive orchards with green mulching, which is typical of our region (Marche, Italy).
Plant growth was influenced by the treatments. Husks reduced shoot growth, up to the complete arrest in the whole-pot treatment. The addition of hay to the husks significantly improved plant growth, mitigating the negative effect. Finally shoot growth was not significantly different from control in the hay and hay-husks treatments.
The shoot-root ratio showed in both distributions values similar to the control. The half-pot distribution led to a higher total root growth than the whole pot, independent of the treatment, showing a clear niche-effect (CitationMaina et al., 2002).
In the half-pot distribution treatments, the roots grew in the new sectors more in the H and OH substrate than in the basic ones, while they grew less in the O substrate (). These results show that a substrate containing husks is not a good environment for olive root growth. On the other end, an addition of hay produced an environment that enhanced olive root and, consequently, longer shoots. The addition of hay and husks led to intermediate results suggesting that the positive effect of hay compensated the negative one of the husks. Moreover, these results indicate that plants are able to exert a selective placement of roots depending on the physical-chemical soil characteristics of the sectors. Such a phenomenon is likely related to allelopathic effects of organic residues that release different compounds during microbial degradation that could be toxic for plants (CitationNeri et al., 2005). The response of the plants depend on the organic matter composition and on the plant species (CitationBonanomi et al., 2006; CitationZucconi et al., 1981).
Olive husks add organic matter and minerals to the soil, but the use of undecomposed husks in our experiments also induced toxicity. In our study roots had limited growth in the husks sectors of the split-root pot where olive microcuttings were planted. When the roots found sectors free from husks the growth shifted to these sectors. Therefore, the plants survived, without showing a significantly different shoot growth from the control. When the root could not find sectors free from olive husks, like in the case of the whole-pot distribution, then root and shoot growth diminished greatly or even stopped completely, causing plant death (50% of plant mortality in the whole-pot husk treatment) before any kind of adaptation could occur.
CONCLUSIONS
In Italy the olive oil extraction process produces 2 x 106 tons of by-products and part of this organic material is reintegrated in the field because Italian law permits the use of olive mill by-products as an amendment (CitationRana et al., 2003). But undecomposed olive mill wastes are toxic to plants and microorganisms (CitationBonanomi et al., 2006; CitationMartin et al., 2002).
In the present study we described the negative reaction of olive roots to the presence of husks in the substrate, the positive niche-effect related to free-from-husk sectors, and the mitigation due to the hay mixing. This is consistent with the knowledge that olive tree roots have a great transmigration capacity (CitationMorettini, 1972; CitationZucconi, 2003); so in the field, plants should be able to overcome the presence of husks and their toxic residues by finding new sectors free from husks. Nevertheless, our work makes farmers aware of the possibility that repeated treatments with undecomposed husks could inhibit plant growth because it is more challenging for roots to find optimal sectors, especially where trees are cultivated at high densities. Our work does not exclude the possibility of using husks as amendment but indicates the need for correct agronomic management of olive mill by-products, such as the combination of green mulching with husks amendment that diminishes toxicity effects caused by husks alone. This may indicate that an increase in biodiversity of the organic matter applied can mitigate toxic effects. However, for a better understanding of the effects of husks amendments on plant growth and fruit production, further studies on the expansion and activity of olive roots in substrates with husks are required. They should particularly focus on the quantitative amount of husks that could be tolerated, and on the time required for husk substrate to overcome the toxic phase (complete degradation and stabilization) and to be explored by roots.
Acknowledgments
We thank Prof. Franco Zucconi (Polytechnics University of Marche) for the very useful discussions on phytotoxicity of organic residues and Prof. Bruno Borsari (Winona State University, MN 55987, USA) for the article revision and useful advice.
Notes
Morettini, A. 1972. Olivicoltura. Ramo Editoriale degli Agricoltori.
Zucconi, F., 2003. Declino del suolo e stanchezza del terreno. Pitagora (ed.)
LITERATURE CITED
- Bingham , I. and Bengough , A. 2003 . Morphological plasticity of wheat and barley roots in response to spatial variation in soil strength . Plant and Soil , 250 : 273 – 282 .
- Bonanomi , G. , Giorgi , V. , Del Sorbo , G. , Neri , D. and Scala , F. 2006 . Olive mill residues affect saprophytic growth and disease incidence of foliar and soilborne plant fungal pathogens . Agr. Ecosystems and Environ. , 115 : 194 – 200 .
- Calvet , C. , Pagès , L. and Estaun , V. 1985 . Composting olive marc . Acta Hort. , 172 : 255
- Cegarra , J. , Paredes , C. , Roig , A. , Bernal , M. and Garcia , D. 1996 . Use of olive mill wastewater compost for crop production . Intl. Biodeterioration & Biodegradation , 1 : 193 – 203 .
- Deak , K. and Malamy , J. 2005 . Osmotic regulation of root system architecture . The Plant J. , 43 : 17 – 28 .
- Doussan , C. , Pagès , L. and Pierret , A. 2003 . Soil exploration and resource acquisition by plant roots: An architectural point of view . Agronomie , 23 : 419 – 431 .
- Forde , B. and Lorenzo , H. 2001 . The nutritional control of root development . Plant and Soil , 232 : 51 – 68 .
- Fransen , B. , Blijjenberg , J. and De Kroon , H. 1999 . Root morphological and physiological plasticity of perennial grass species and the exploitation of spatial and temporal heterogeneous nutrient patches . Plant and Soil , 211 : 179 – 189 .
- Fransen , B. , Kroon , H. d. and Berendse , F. 1998 . Root morphological plasticity and nutrient acquisition of perennial grass species from habitats of different nutrient availability . Oecologia , 115 : 351 – 358 .
- Hodge , A. 2004 . The plastic plant: Root responses to heterogeneous supplies of nutrients . New Phytologist , 162 ( 1 ) : 9 – 24 .
- Hodge , A. 2006 . Plastic plant and patchy soils . J. Expt. Bot. , 57 : 401 – 411 .
- Leyser , O. and Fitter , A. 1998 . Roots are branching out in patches . Trends in Plant Sci. , 3 ( 6 ) : 203 – 204 .
- Lopez-Bucìo , J. , Cruz-Ramirez , A. and Herrera-Estrella , L. 2003 . The role of nutrient availability in regulating root architecture . Current Opinion in Plant Biol. , 6 : 280 – 287 .
- Maina , G. , Brown , J. and Gersani , M. 2002 . Intra-plant versus inter-plant root competition in beans: Avoidance, resource matching or tragedy of the commons . Plant Ecol. , 160 : 235 – 247 .
- Malamy , J. 2005 . Intrinsic and environmental response pathways that regulate root system architecture . Plant, Cell and Environ. , 28 : 67 – 77 .
- Marques , I. 2001 . Anaerobic digestion treatment of olive mill wastewater for effluent re-use in irrigation . Desalination , 137 : 233 – 239 .
- Martin , J. , Sampedro , I. , Garcia-Romera , J. and Ocampo , J. 2002 . Arbuscular mycorrhizal colonization and growth of soybean (Glicine max) and lettuce (Lactuca sativa) and phytotoxic effects of olive mill residues . Soil Biol. Biochem. , 34 : 1769 – 1775 .
- Morettini, A. 1972. Olivicoltura. Ramo Editoriale degli Agricoltori.
- Neri , D. , Sugiyama , N. and Inujima , A. 2005 . Effects of organic residues on strawberry root growth . Intl. J. Fruit Sci. , 5 ( 1 ) : 127 – 139 .
- Paredes , C. , Cegarra , J. , Roing , A. , Sanchez-Monedero , M. and Bernal , M. 1999 . Characterization of olive mill wastewater (alpechin) and its sludge for agricultural purposes . Bioresource Technol. , 67 : 111 – 115 .
- Rana , G. , Rinaldi , M. and Introna , M. 2003 . Volatilisation of substances after spreading olive oil wastewater on the soil in a Mediterranean environment . Agr. Ecosystems and Environ. , 96 : 49 – 58 .
- Tagliavini , M. and Marangoni , B. 1992 . Growth of peach as affected by decomposition of own root residues in soil . Plant and Soil , 145 : 253 – 260 .
- Tomati , U. , Galli , E. , Fiorelli , F. and Pasetti , L. 1996 . Fertilizers from composting olive-mill wastewaters . Intl. Biodeterioration & Biodegradation. , 38 ( 3/4 ) : 155 – 162 .
- Zucconi , F. , Pera , A. , Forte , M. and De Bertoldi , M. 1981 . Evaluating toxicity of immature compost . BioCycle , 22 ( 2 ) : 54 – 57 .
- Zucconi, F., 2003. Declino del suolo e stanchezza del terreno. Pitagora (ed.)