ABSTRACT
Multiple shoot cultures established from shoot tip cultures of an elite cultivar of banana ‘Giant Cavendish’ (Musa spp. AAA group) were irradiated with gamma rays (5, 10, and 30 Gy) and the resulting plant populations were field evaluated for their agronomical performance. A few variant plants were selected for their agronomic traits, including dwarf stature and early flowering and characterized through RAPD analysis. In the case of 30 Gy irradiated plants, although dwarf stature was noted, the yield was comparable to control plants. The GC rich primers exhibited DNA amplification in the variants with a total of 717 bands, of which 44% were polymorphic. These results suggest that the gamma irradiation can be employed for recovering agronomic variants in ‘Cavendish’ bananas.
Abbreviations BAP, 6-Benzylaminopurine; AH, Adenine hemisulphate; NAA, Naphthalene acetic acid; MS, CitationMurashige and Skoog (1962)
INTRODUCTION
Bananas and plantains are one of the most important crops in the world and are a staple food for millions in the tropical and subtropical countries. The crop is grown on 10 million ha with a production of 97.5 million metric tons (CitationSingh, 2002). India is the largest producer of bananas in the world, contributing 26% of the total production. Bacterial, fungal, and viral diseases and other pests threaten banana production. In this context, generation of resistant cultivars using conventional or biotechnological approaches assumes significance. Bananas and plantains are propagated vegetatively through suckers, as most of the edible bananas do not set seeds and the fruit develops parthenocarpically. Additionally most of the commercial clones are triploids and are male and female sterile (CitationNovak and Micke, 1990). Therefore, conventional breeding has become difficult and cumbersome. Owing to these constraints, mutation breeding and biotechnological methods have become useful tools for crop improvement.
The ability to culture and manipulate a large number of totipotent plant cells provides a greater opportunity for in vitro mutagenesis and selection (CitationSuprasanna et al., 2006). Mutation induction is empowered by in vitro techniques as many examples related to different plant species showed that the combination of in vitro culture and mutagenesis is relatively inexpensive, simple, and efficient (CitationJain, 2000). During a study on the effect of gamma irradiation on in vitro multiple shoot cultures in six banana cultivars belonging to different genomic groups, CitationKulkarni et al. (1997) isolated a few morphological variants. It has been suggested that the use of multiple shoot cultures for mutagenesis has several advantages, like higher multiplication of shoots resulting in the proper separation of chimeras, requirement of less space and time, treatment of large number of shoots, and in vitro selection of mutagenized population for biotic and abiotic stresses.
Genetic stability and maintenance of variant germplasm are important for any crop improvement program and, in this regard, molecular markers have become useful in the detection of genetic variation (CitationMartin et al., 1998). Among different molecular markers, random amplified polymorphic DNA (RAPD) requires only small amounts of DNA sample without involving radioactivity and is simpler as well as faster, making this an attractive method for the detection of somaclonal variations in banana (CitationSahijram et al., 2003). The RAPD method has successfully been employed in the detection of genetic variation among micropropagated banana plants (CitationMartin et al., 2006; CitationVenkatachalam et al., 2007). In case of ‘Cavendish’ group cultivars (AAA group), increased attention is given to the application of biotechnological approaches for crop improvement (CitationSmith et al., 2005). Our laboratory is engaged in utilizing radiation-induced mutagenesis of in vitro cultures of banana for improvement of some Indian banana cultivars (CitationBapat et al., 2007). In this communication, we report the field evaluation of the gamma-irradiated plant population and molecular characterization using RAPD of the selected variants in an elite cultivar of banana ‘Giant Cavendish’ (AAA).
MATERIALS AND METHODS
Plant Materials and the Establishment of Multiple Shoot Cultures
The sword suckers of the banana cultivar ‘Giant Cavendish’ (AAA) were collected from the farmer's fields and established in the departmental greenhouse. The shoot tips from these plants were used to establish the cultures as reported previously (CitationGanapathi et al., 1995). Shoot tip cultures were then maintained on liquid MS medium supplemented with 2 mg L−1 BAP and 30 mg L−1 AH, 3% W/V sucrose on a filter paper support. After 2 to 3 weeks the established shoots were transferred to semi-solid shoot multiplication medium supplemented with 2 mg L−1 BAP, 30 mg L−1 AH, 3% W/V sucrose, and 0.2% phytagel. Multiple shoots that formed on this medium were subcultured once in 3 to 4 weeks. The pH of the medium was adjusted to 5.8 and all the cultures were maintained under fluorescent light (1000 lux) for 10 h/day, at a temperature of 25 ± 2°C and 55–60% relative humidity.
Irradiation of Multiple Shoots
Multiple shoot cultures (six culture tubes per treatment, each with four to six shoots) were irradiated at 0-, 5-, 10-, and 30-Gy doses with gamma rays @ 20 Gy/min. These were immediately subcultured onto shoot multiplication medium (M1V0) and subcultured up to M1V3.
Rooting of Shoots, Acclimatization, and Field Planting
Individual shoots from M1V3 were separated from the multiple shoot cultures and transferred to MS medium supplemented with 1 mg L−1 NAA and 0.1% W/V activated charcoal for obtaining the rooted plantlets (M1V4). After 30–40 days, these were transferred to a greenhouse for hardening. The plantlets were removed from the culture vessel and washed thoroughly in tap water and transferred to polythene bags containing 1:1 mixture of good horticultural soil and farmyard manure. These were hardened in the greenhouse for 60–75 days and field planted (along with control mother plants) in a random block design at the experimental farm at the Nuclear Power Corporation of India Limited (NPCIL), Kaiga, Karnataka state, India.
Isolation of DNA and Polymerase Chain Reaction Using RAPD Primers
Total genomic DNA was isolated from young leaves of individual control and gamma-irradiated plants using a modified cetyltrimethylammoniumbromide (CTAB) method (CitationStewart and Via, 1993). The quantity and quality of DNA samples were estimated by comparing band intensities on agarose gel. Twenty primers from Integrated DNA Technology (Series-A) of Biogene (Coralville, Iowa), were used for the RAPD analysis. The sequences of these primers are given in . Fifty microliters of PCR mix contained the primers (100 ng); Taq DNA polymerase (1.0 unit); 200 μM each of dNTP and 1X PCR buffer; and 100 ng of genomic DNA as a template. The PCR conditions were 94°C initial denaturation for 5 min followed by 35 cycles of amplification with each cycle consisting of following steps: 94°C for 1 min, 37°C for 2 min, and 72°C for 2 min with a final extension for 10 min. The amplified products were analyzed using agarose gel (1.5%) electrophoresis. The gels were visualized and photographed over a UV transilluminator after staining with ethidium bromide according to CitationSambrook et al. (1989).
TABLE 1. Primers selected for the RAPD analysis
Data Analysis
Data were scored as discrete variables, using 1 to indicate presence and 0 to indicate absence of a band. Data were used for similarity-based analysis using the program NTSYS-PC (version 2. 02). The SIMQUAL program was used to calculate Jacard's coefficient (F′). Similarity coefficients were used to construct a UPGMA (unweighted pair group method with arithmetic average) dendrogram (CitationRohlf, 1990).
RESULTS AND DISCUSSION
Multiple shoot cultures () established from shoot tip cultures of an elite cultivar of banana ‘Giant Cavendish’ (Musa spp. AAA group) were irradiated with gamma rays (5, 10, and 30 Gy) and the resulting plant populations () were field evaluated for their agronomical performance. A total of 200 plants: control, unirradiated group of 60 plants; and irradiated population of 140 plants of 5, 10, and 30 Gy were field evaluated and the data were recorded for different agronomic characters (). Morphological differences were noticed for pseudostem height and foliage development after 5 months of planting in the field. Significant differences were noted for plant height as most of the plants derived from the 30-Gy irradiation treatment were dwarf in stature compared to control (). The plants derived from the 5- and 10-Gy treatments were comparable to control plants. In case of 30-Gy irradiated plants, although dwarf stature was observed, the yield was comparable to control plants. The number of fruits per bunch was more (141–145/bunch) in the 30-Gy irradiation-derived plants as compared to the control (118–121/bunch). The number of suckers was more in 5-Gy irradiated plants (8/plant) compared to the 30-Gy treatment (5/plant). Two plants, one from tissue culture–derived control and one from 10-Gy irradiated group, flowered very early (175 days; data not shown) and these are being further evaluated to see their stability.
FIGURE 1. A. Multiple shoot cultures established on MS medium supplemented with BAP (2 mg/L) + adenine hemisulfate (30 mg/L) used for gamma irradiation. B. Two-month-old hardened plants in the green house, inset: closeup of the leaves showing the characteristic red pigments of Cavendish banana. C. Irradiated population growing in the field. D. Ten-gray irradiated plant bearing a fruit bunch.

FIGURE 2. RAPD analysis based on amplification with primers A-6 (top) and A-19 (bottom). Nos. 1–20 are control and selected gamma radiation–induced variants in banana var. Giant Cavendish. 1, 5 Gy; 2, 5 Gy; 3, 30 Gy; 4, 30 Gy; 5, 30 Gy; 6, 30 Gy; 7 & 8, tissue culture–derived control plant; 9 & 10, 30 Gy; 11, 5 Gy; 12, 5 Gy; 13 to 15, 10 Gy; 16 & 17; 5 Gy; 18, tissue culture–derived control, early-flowering plant; 19 & 20, sucker-derived control plant.
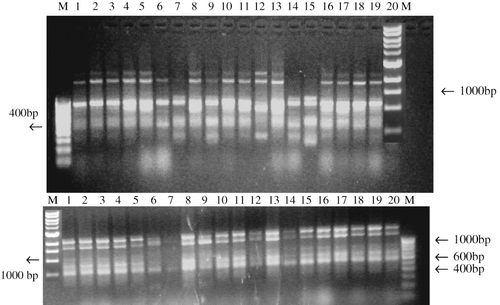
TABLE 2. Field performance of gamma irradiated plant population of banana var. ‘Giant Cavendish’. Each line consisted of 20 plants and data indicate means
The variants selected from the control and irradiation-derived population were used for RAPD analysis (Fig. 5). The salient features of these selected variant plants are given in . RAPD analysis showed that all the primers exhibited DNA amplification in the variants (). Each primer generated a unique set of amplification products and some revealed more polymorphism than others. The primers used were GC rich with G+C content equal to 60–70%. A total of 717 band positions were produced, of which 318 were polymorphic, producing 44% polymorphism. The average number of bands per primer was three, with a minimum of one and a maximum of five. Primer A8 yielded only monomorphic bands, whereas primers A7, A11, A16, A14, and A20 produced 100% polymorphism. Primer A8 detected minor polymorphism in regenerants (). Primer A5 showed a specific band (1.2 kb) associated with 5- and 10-Gy irradiated plants. The least similarity index (SI) was 0.48 in the case of GC14 (10 Gy) and the similarity index (SI) was in the range of 0.48 to 0.93 for the sucker-derived control plants compared to the others. On average, the similarity index was 0.78 for tissue culture–derived control plants and 0.58% to 80% for the irradiated plants.
TABLE 3. Distinctive features of the selected variants. Each line consisted of 20 plants and data indicate means
FIGURE 3. Dendrogram showing genetic relationship between the control and gamma radiation–induced variants in banana var. Giant Cavendish. GC1, 5 Gy; GC2, 5 Gy; GC3, 30 Gy; GC4, 30 Gy; GC5, 30 Gy; GC6, 30 Gy; GC7 and 8, tissue culture–derived control plant; GC9 and 10, 30 Gy; GC11, 5 Gy; GC12, 5 Gy; GC13 to GC15, 10 Gy; GC16 and 17, 5 Gy; GC18, tissue culture–derived control, early-flowering plant; GC19, sucker-derived control plant.
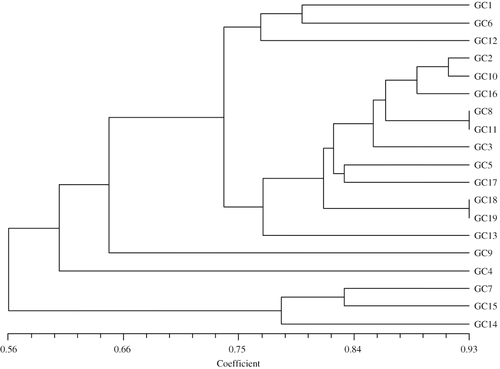
The cluster analysis resulted in three main cluster groups based on secondary branching: in group A, irradiated plants of 5 and 30 Gy were included; group B consisted of irradiated plants, tissue-cultured control plants, and sucker-derived control plants (). Among these, the tissue-cultured control plant (GC18) and sucker-derived control plant (GC19) were in close proximity. Group C included a tissue-cultured plant control (GC7) and irradiated plants (GC14 and 15); irradiated plants (GC9 and 4) were not associated to any group.
Improvement of banana using mutation induction and somaclonal variation has been the major interest for banana researchers (CitationHwang and Ko, 2004; CitationSahijram et al., 2003). In this study, gamma irradiation was employed to induce genetic variability in a commercial ‘Cavendish’ cultivar of banana. The irradiated population showed significant morphological variation with respect to some traits including plant stature. At a higher dose of 30 Gy, irradiated plants exhibited dwarfism. CitationHernandez et al. (2007) reported the morphoagronomic and molecular characteristics of three triploid banana clones. The dwarf variants were characterized by the shorter pseudostem and leaves, as these plants had the same number of leaves and identical bunch weight. In addition, the dwarfs have the advantage of possessing lodging resistance, which is a benefit to the farmers. In the present study, some dwarf variants from the 30-Gy treatments were observed that were significantly shorter compared to control plants with a comparable yield. Somaclonal variation for dwarfism, growth cycle, and disease resistance has been suggested as useful in banana improvement (CitationIsraeli et al., 1996; CitationMartin et al., 2006; CitationSahijram et al., 2003). CitationRamage et al. (2004) found that the dwarf somaclones were relatively stable and did not generally revert to a normal phenotype. Another common undesirable somaclonal variant is the mosaic type heterogeneity, and such an incident of variation was reported in ‘Cavendish’ banana (CitationReuveni and Israeli, 1990). Combining gamma irradiation with in vitro culture has also been effective for the induction of mutations in banana (CitationSmith et al., 2005). For example, CitationSmith et al. (2006) employed gamma irradiation of a dwarf ‘Cavendish’ cultivar for improvement in agronomic characteristics.
DNA based markers have been successfully used in the fingerprinting of plants, especially for the identification of cultivars and characterization of variants (CitationMartin et al., 1998). RAPD markers have been used for cultivar identification and genetic similarity studies due to their simplicity and speed. CitationKulkarni et al. (1999) used RAPD profiles to distinguish 29 banana genotypes and gamma (10 and 30 Gy)-irradiated banana plants. The irradiated plants clearly diverged from the unirradiated control plants. Our results revealed that among the irradiation-derived variants of banana cultivar ‘Giant Cavendish’, there were different clusters, indicating that genomic alterations were seen in all the plant populations irrespective of the origin of variation (tissue culture or mutation derived). With respect to sucker-derived controls, tissue culture–derived control plants exhibited an average of 78% SI and irradiated plants ranged from 0.58 to 0.80 SI. It is possible that the tissue culture–derived controls (nonirradiated) accumulated genetic variation (somaclonal) and gamma-irradiated variants differed more from sucker-derived control plants. The irradiated plants, which were included in group B, were mostly tall, with the exception of GC10. In rice, somaclones generated from mature seed-derived callus cultures differed significantly from the original material based on RAPD characterization, indicating that genomic alterations occurred in all families (CitationGodwin et al., 1997).
The results presented in this article suggest that gamma irradiation can be usefully employed in banana for the induction of desirable mutations (dwarfism) and that the RAPD technique can be adopted to detect variation among the plant population at the molecular level. The advent of genetic transformation techniques has generated hope of obtaining useful plants; however, until the effectiveness of foreign gene expression and field evaluation is conclusively validated, mutation breeding offers as an alternative and simple feasible approach for banana improvement.
The authors thank Shri G. Nageshwara Rao, Executive Director (Operations), NPCIL, Mumbai; Shri Amol Revankar, Engineer-in-Charge (civil, township), KGS, Kaiga; and Dr. P. M. Ravi, Environmental Survey Laboratory, KGS, Health Physics Division, BARC, for their help and cooperation in conducting the field studies.
LITERATURE CITED
- Bapat , V.A. , Ganapathi , T.R. , Kulkarni , V.M. , SunilKumar , G.B. , Srinivas , L. and Suprasanna , P. 2007 . “ Cellular and molecular approaches for the improvement of banana ” . In Banana: Technological advancements , Edited by: Singh , H.P. and Uma , S. 160 – 176 . Trichy, , India : Association for the Improvement in Production and Utilization of Banana, Natl. Research Center for Banana .
- Ganapathi , T.R. , Mohan , J.S.S. , Suprasanna , P. , Bapat , V.A. and Rao , P.S. 1995 . A low cost strategy for in vitro propagation of banana . Curr. Sci. , 68 : 646 – 649 .
- Godwin , I.D. , Sangduen , N. , Kunanuvatchaidach , R. , Piperidis , G. and Adkins , S.W. 1997 . RAPD polymorphisms among variant and phenotypically normal rice (Oryza sativa var. indica) somaclonal progenies . Plant Cell Rep. , 16 : 320 – 324 .
- Hernandez , R. , Rodriguez , R. , Ramirez , T. , Canal , M.J. , Guillen , D. , Noceda , C. , Escalona , M. , Corujo , M. and Ventura , J. 2007 . Genetic and morphoagronomic characterization of plantain variants of Musa AAB clone ‘CEMSA ¾’ . J. Food Agr. Environ. , 5 ( 1 ) : 220 – 223 .
- Hwang , S.C. and Ko , W.H. 2004 . Cavendish banana cultivars resistant to Fusarium wilt acquired through somaclonal variation in Taiwan . Plant Dis. , 88 : 580 – 588 .
- Israeli , Y. , Ben-Bassat , D. and Reuveni , O. 1996 . Selection of stable banana clones which do not produce dwarf somaclonal variants during in vitro culture . Sci. Hort. , 67 : 197 – 205 .
- Jain , S.M. 2000 . Tissue culture induced variation in crop improvement . Euphytica , 118 : 153 – 166 .
- Kulkarni , V.M. , Ganapathi , T.R. , Suprasanna , P. , Bapat , V.A. and Rao , P.S. 1997 . Effect of gamma irradiation on in vitro multiple shoot cultures of banana (Musa sp.) . J. Nuclear Agr. Biol. , 26 : 232 – 240 .
- Kulkarni , V.M. , Ranade , S.A. , Ganapathi , T.R. , Suprasanna , P. , Bapat , V.A. , Ussuf , K.K. and Rao , P.S. 1999 . RAPD-profile variation amongst cultivated, wild and irradiation derived variants of banana. Asia Pacific . J. Mol. Biol. Biotechnol. , 7 ( 2 ) : 159 – 166 .
- Martin , K.P. , Pachathundikandi , S.K. , Zhang , C.L. , Slater , A. and Madassery , J. 2006 . RAPD analysis of a variant of banana (Musa sp.) cv. Grande Naine and its propagation via shoot tip culture. In Vitro Cell . Dev. Biol. Plant. , 42 ( 2 ) : 188 – 192 .
- Martin , N.J.G. , Grillo , G.S. and Dominguez , A.M. 1998 . The use of randomly amplified polymorphic DNA (RAPD) for the study of genetic diversity and somaclonal variation in Musa . Intl. Soc. Hort. Sci. , 490 : 445 – 454 .
- Murashige , T. and Skoog , F. 1962 . A revised medium for rapid growth and bioassays with tobacco tissue cultures . Physiol. Plant. , 15 : 473 – 497 .
- Novak , F.J. and Micke , A. 1990 . “ Advancement of in vitro mutation breeding technology for bananas and plantain ” . In In vitro mutation breedings of banana and plantain 1 , 56 – 65 . Vienna, , Austria : Report of the first research co-ordination meeting of the FAO/IAEA CRP on mutation breeding of bananas and plantain. International Atomic Energy Agency .
- Ramage , C.M. , Borda , A.M. , Hamill , S.D. and Smith , M.K. 2004 . A simplified PCR test for early detection of dwarf off-types in micropropagated Cavendish banana (Musa spp. AAA) . Sci. Hort. , 103 : 145 – 151 .
- Reuveni , O. and Israeli , Y. 1990 . Measures to reduce somaclonal variation in in vitro propagated bananas . Acta Hort. , 275 : 307 – 313 .
- Rohlf , F.J. 1990 . NTSYS.Pc. Numerical taxonomy and multivariate analysis system, version 2.02 , New York, N.Y : Applied Biostatistics .
- Sahijram , L. , Soneji , J.R. and Bollamma , K.T. 2003 . Analyzing somaclonal variation in micropropagataed bananas (Musa spp.) . In Vitro Cell. Dev. Biol. Plant , 39 : 351 – 356 .
- Sambrook , J. , Fritsch , E.F. and Maniatis , T. 1989 . Molecular cloning: A laboratory manual , Cold Spring Harbor, N.Y : Cold Spring Harbor Laboratory Press .
- Singh , H.P. 2002 . Indian bananas: Issues and strategies , 1 – 2 . Bangalore, , India : Global conference on bananas and plantain . Association for the Improvement in Production and Utilization of Banana, Trichy, India.
- Smith , M.K. , Hamill , S.D. , Becker , D.K. and Dale , J.L. 2005 . “ Musa spp. banana and plantain ” . In Biotechnology of fruit and nut crops , Edited by: Litz , R.E. 366 – 391 . Wallingford, Oxfordshire, , U.K : CABI Publishing .
- Smith , M.K. , Hamill , S.D. , Longdon , P.W. , Giles , J.E. , Doogan , V.J. and Pegg , K.G. 2006 . Towards the development of a Cavendish banana resistant to race 4 of fusarium wilt: Gamma irradiation of micropropagated dwarf parfitt (Musa spp., AAA group Cavendish sub group) . Austral. J. Exp. Agr. , 46 : 107 – 113 .
- Stewart , C.N. Jr. and Via , L.E. 1993 . A rapid CTAB DNA isolation technique for RAPD finger print and other PCR applications . Biotechniques , 14 : 748 – 750 .
- Suprasanna , P. , Meenakshi , S. and Bapat , V.A. 2006 . “ Integrated approaches of mutagenesis and in vitro selection for crop improvement ” . In Plant tissue culture, molecular markers and their role in crop productivity , Edited by: Kumar , A. and Shekhawat , N.S. New Delhi : IK International Publ . , p. 73–92
- Venkatachalam, L., S.R. Venkataramareddy, and B. Neelwarne. 2007. Molecular analysis of genetic stability in long-term micropropagated shoots of banana using RAPD and ISSR markersElectronic Journal of Biotechnology 10:1. Available online http://www.ejbiotechnology.info/content/vol10/issue1/full/12/