ABSTRACT
High temperatures induce physiological changes within the mango fruit. A study was conducted to determine the influence of the rate of transpiration and respiration on spongy tissue formation in ‘Alphonso’ mango. Spongy tissue is a physiological disorder in ‘Alphonso’ mango. Both artificially and naturally induced spongy tissue showed low rates of transpiration and high rates of respiration as evidenced by stable oxygen isotope analysis and portable photosynthetic system readings. This condition builds up high temperatures within the fruits, leading to tissue breakdown. The results of this study indicated that the rate of transpiration and respiration has an influence on fruit temperature, which in turn has an influence on normal fruit ripening processes.
INTRODUCTION
India grows many of the finest mangoes in the world. Although more than 1000 cultivars and selections exist, only few cultivars are grown on commercial scale. ‘Alphonso’ mango is the most important cultivar of Mangifera indica L., belonging to Anacardiaceae family. It is considered the “king of mango cultivars” and is highly suited to the coastal climate of the west coast of India. ‘Alphonso’ mangoes are oval in shape and are about 4 to 6 inches long. The skin is inedible and upon ripening it turns golden yellow in color. The pulp is golden yellow in color and is widely considered to be the tastiest fruit in India. This mango has a warm sweet taste and is sometimes pleasantly tart. This fruit has a rich aromatic flavor and is an excellent source of vitamins A and C.
Because of its excellent fruit qualities, ‘Alphonso’ is one of the important commercial mango cultivars in India, but it suffers from spongy tissue disorder that can be detected only when the fruit is cut open. It accounts for nearly 60% of the mango export trade from India (CitationRavindra and Shivashankar, 2004). Prolonged exposure to sunlight or high temperatures can affect the physiology of the fruit. While the effect of temperature on specific plant processes is reasonably characterized around the optimum range, the question of how high temperatures affect fruit physiology remains unanswered. Two types of high-temperature stress are generally considered: chronic exposures (days and weeks) to temperatures that exceed the optimum and acute exposures (minutes to hours) to relatively high temperatures. The nature of injury resulting from these types of thermal stress may be vastly different and should be considered in proposing a mechanism of injury (CitationLeitsch, 1916). Cytoplasmic changes observable upon acute exposures to high temperature include coagulation of the protoplasm, cytolysis, nuclear changes, and altered mitosis (CitationBelehradek, 1935). Inhibition of protoplasmic streaming, increased protoplasmic viscosity, and loss of membrane semipermeability suggest that the various manifestations occur in succession, protoplasmic streaming being the most sensitive (CitationAlexandrov, 1967). Thermodenaturation of proteins has long been suggested as the principle cause of high-temperature injury and thermal disruption of membrane integrity (CitationBjorkman et al., 1980).
Several factors such as time and season of harvest, effect of mulching, location, and biological factors are known to influence spongy tissue formation. The exact reason for the increase in temperature within fruit that leads to spongy tissue formation has not been reported. The rate of transpiration and respiration during fruit ripening regulates temperature within fruits. During fruit respiration, water vapor molecules containing the lighter isotope of oxygen (16O) diffuse relatively faster than do molecules with the heavy isotope of oxygen. Further, the isotope enrichment occurs during evaporation also because the vapor pressures of 18O water are lower compared to that of the 16O water. This favors faster evaporation of H2 16O (water), leading to 18O enrichment. Transpiration, an evaporative process, would result in the enrichment of H2 18O in leaf water. This oxygen isotopic signature subsequently finds its path into the leaf biomass. Thus, 18O in leaf biomass has emerged as a surrogate for transpiration rate (CitationMadhava, 2000; CitationMadhava et al., 1999). Therefore, studying the rates of transpiration and respiration and 18O enrichment during fruit ripening is important. The main objective of this study is to document the rate of transpiration and respiration in ‘Alphonso’ mango fruit and leaf tissues to understand their influence on spongy tissue formation. This will also pave way to understanding other similar physiological disorders caused by heat.
MATERIALS AND METHODS
Collection of the Fruits
Mature green fruits of uniform size were hand-picked from the tree without causing any damage to the fruit. The fruits were washed with water and were air-dried at room temperature. These fruits were used to artificially induce spongy tissue.
Sample Preparation
Matured healthy pulp tissue was collected from green fruits immediately after harvest. Spongy and healthy tissues were collected from the control fruits ripened at room temperature to analyze the 18O and 16O ratio. Spongy and healthy tissues were collected from the same fruit. Utmost care was taken during sampling to avoid contamination. Matured healthy, spongy and healthy tissue from spongy tissue affected fruits and leaf samples were packed separately in polyethylene bags, sealed, frozen in liquid nitrogen, and stored at −80°C. Before oxygen isotopic analysis the samples were oven dried at 60°C for 48h and milled to a fine powder.
Determination of 18O Enrichment and Respiration Rate
The oxygen isotopic composition of leaf and pulp is generally determined by quantitative pyrolysis of the sample at high temperature in the complete absence of oxygen. The dried leaf, spongy tissue, healthy tissue from spongy tissue affected fruit and pulp from matured unriped fruits were loaded into silver capsules and placed in a sample carousel in the autosampler. The autosampler dropped the sample at precisely designated times sequentially into the pyrolysis column, which contained a graphite tube filled with glassy carbon catalyst. The graphite tube was placed in a ceramic column heated at 1400°C. The pyrolysis of organic matter resulted in the production of carbon monoxide (CO) and nitrogen (N2) gas. CO2 is generally <5%. These gases were swept by a helium (He) carrier gas (purity > 99.996%) into a GC column and then into the ion source of isotope ratio mass spectrometry (IRMS).
Since the mass-to-charge ratio (m/z) of CO and N2 is the same, it was essential to separate these two gases before introducing them into the ion source for isotopic ratio determination. These gases were passed through a GC-column containing 5 Å molecular sieve heated to 90°C. The CO travels slower than N2 gas through the molecular sieve; hence, the two gas species can be quantitatively separated. The m/z ratio for 28 and 30 masses corresponding to C16O and C18O, respectively, was determined by the IRMS. An appropriate standard (Craig-corrected against v SMOW) was also introduced in the run to determine the accuracy of mass detection and standard deviation of the run. The 18O enrichment over and the source water was computed as follows:
The δ 18O was determined by CO2 equilibrium technique using the gas bench coupled to the IRMS.
Artificial Induction of Internal Breakdown in ‘Alphonso’ Fruits
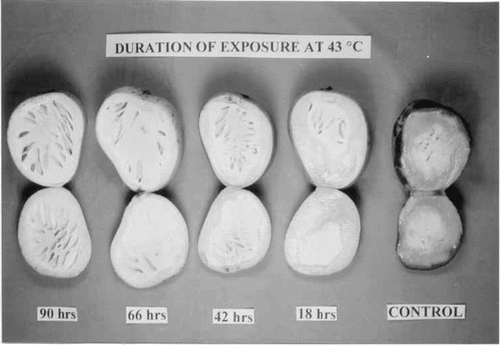
In order to further analyze the influence of heat on rates of transpiration and respiration during fruit ripening, the freshly harvested fruits were incubated at 43°C in an incubator for a period of 18 to 114 h. Five fruits were kept at room temperature (25°C) and were used as controls. The extent of damage at different durations of exposure of the fruit tissues was determined visually.
Although essentially a biochemical process, photosynthesis is often regarded as a diffusive process. The rate of diffusion of CO2 is largely controlled by two factors, the stomatal conductance (gs) and the CO2 concentration gradient between the carboxylation site and the ambient air. Since the ambient CO2 concentration is fairly constant (Ca), the intercellular concentration (Ci) largely determines the CO2 gradient for diffusion at any given gs. Therefore, both gs and Ci regulate assimilation rates in plants.
The stomatal factors, besides regulating the diffusion of substrate CO2 into the leaf, also determine the rates of transpiration and respiration. Gas exchange traits were measured using Portable Photosynthetic System LI-6200 (LI-COR 6200, Lincoln, Neb.).
RESULTS AND DISCUSSION
Spongy tissue is a physiological ripening disorder in ‘Alphonso’ mango. The spongy tissue develops as yellowish white corky patches with or without air pockets in the breakdown tissues. Spongy tissue is caused by various factors and hence it is difficult to attribute an exact and specific reason for its cause. Recently, it has become a major problem in ‘Alphonso’ mango cultivation and has impacted its export. Several horticultural, biochemical, and molecular aspects related to spongy tissue occurrence have been reported, but there are limited reports on the influence of rates of fruit transpiration and respiration on fruit temperature. This study will further enhance our understanding about spongy tissue formation.
Measurement of Stable Isotopes of Oxygen
A study was conducted to determine the effect of respiration on the occurrence of spongy tissue. Plants grown at low relative humidity were more enriched in 18O than those grown at high relative humidity. Within each humidity environment, increasing ABA concentration increased oxygen isotope enrichment of leaf cellulose (Δ18Oc) and whole leaf tissue (Δ18Oi; CitationBarbour and Farquhar, 2000). CitationCraig and Gordan (1965) originally developed a model of isotopic fractionation for the process occurring during evaporation of water. They discovered that during evaporation water molecules with the lighter isotope of oxygen (H2 16O) tend to diffuse and evaporate faster than the heavier isoform (H2 18O). This, they showed, resulted in an enrichment of H2 18O at the evaporating surface of the water body.
Oxygen isotope study of different tissues of mango revealed that there was a reduction in 18O in healthy fully ripened tissue compared to others, which is mainly due to the formation of H2 16O during respiration. Higher 16O is being found in the tissues where respiration and transpiration rate is higher. The data on 18O of unripe, spongy tissue, and healthy tissue (33.968, 33.085, and 32.761) indicated a high respiration and in mature fruit followed by spongy and healthy tissues (). This also indicated that 16O fixation was less in spongy tissue.
TABLE 1. 18O analysis depicting the rate of respiration in leaf, healthy, spongy, and unripened tissues of ‘Alphonso’ mango
This clearly showed that there was a high rate of respiration and a lower rate of transpiration in spongy tissue compared to others, which leads to a buildup of high temperatures within the fruit, causing tissue breakdown. To further confirm the rates of respiration and transpiration in the fruits affected with spongy tissue, the fruits were incubated at 43°C for different durations. Earlier studies have shown that heat treatment increase the spongy tissue occurrence (CitationLad et al., 1992).
Artificial Induction of Internal Breakdown in Fruit by Increasing Temperature During Ripening
A study was undertaken to know the effects of respiration and transpiration rate on ‘Alphonso’ mango fruits during different exposure periods at 43°C. The data showed that the increase in duration of exposure from 18 to 114 h at 43°C reduced the climacteric respiration from 15.65 to 5.96 mmol CO2/kg/h (). Beyond 18 h of exposure there was no increase in climacteric respiration. Statistically there was no significant difference from control to 66 h of temperature treatment, but there was a significant difference in the rate of respiration at 90 and 114 h of exposure of fruits to 43°C. The extent of spongy tissue formation was related to increased damage to respiratory machinery. Prolonged duration of exposure from 18 to 114 h at 43°C increased the spongy tissue occurrence from 27% to 90% (). Control fruits incubated at room temperature (25°C) showed a respiration rate of 16.82 mmol CO2/kg/h and the extent of damage to tissue was also less (10%) when compared to the fruits treated at 43°C. With respect to the rate of transpiration there was a significant difference statistically ().
TABLE 2. Climacteric respiration rate measured at room temperature after different durations of exposure of fruits at 43°C and percentage spongy tissue occurrence (respiration rate in mmol CO2/kg/h)
TABLE 3. Climacteric transpiration rate measured at room temperature after different durations of exposure of fruits at 43°C (transpiration rate in mmol H2O/kg/h)
This study indicates that the tissue affected with spongy tissue has a low transpiration and a high respiration rates when compared to healthy ripened tissue. The rates ultimately increase the temperature within these tissues and affect normal fruit ripening. High external temperatures affect the normal respiration rate of the fruit, thereby leading to tissue breakdown. Since ‘Alphonso’ has a low transpiration rate, it builds up 18O, which in turn increases reactive oxygen species that also affect normal ripening. Effective control of this factor will help in reducing the incidence of spongy tissue occurrence. Molecular analysis of spongy tissue in an earlier study also revealed similar results (CitationVasanthaiah et al., 2006). Several spongy and healthy tissue specific genes were isolated and characterized. Higher expression of catalase, ubiquitin, coproporphrinogen III oxidase, and keratin genes indicated that the tissue was experiencing oxidase stress. They concluded that oxidative stress is one of the probable causes for spongy tissue formation in mango (CitationVasanthaiah et al., 2006).
CONCLUSIONS
Several factors have been reported to influence spongy tissue occurrence. Low transpiration and high respiration rates in ‘Alphonso’ mango fruit influence spongy tissue formation. This condition causes high temperatures within the fruit, leading to tissue breakdown. This study indicates that critical rates of transpiration and respiration are important for fruit ripening, alteration of which will affect the normal ripening process. Therefore, the recommended strategy is to use the available molecular approach to address this unsolved problem and to obtain ‘Alphonso’ mango free of spongy tissue.
The author thanks the Division of Biotechnology and Division of Plant Physiology and Biochemistry, Indian Institute of Horticultural Research, Hessarghatta, Bangalore, India, and the Department of Crop Physiology, University of Agricultural Sciences, GKVK Campus, Bangalore, India, for their help in carrying out this study.
LITERATURE CITED
- Alexandrov , V.Y. 1967 . “ A study of the changes in resistance of plant cells to the action of various agents in the light of cytological considerations ” . In The cell and environmental temperature , Edited by: Troshin , A.S. 142 – 151 . New York, N.Y : Pergamon Press .
- Barbour , M.M. and Farquhar , G.D. 2000 . Relative humidity and ABA induced variation in carbon and oxygen isotope ratio of cotton leaves . Plant Cell Environ. , 23 : 473 – 483 .
- Belehradek , J. 1935 . Temperature and living matter , Borntraeger, Berlin : Protoplasm monographie .
- Bjorkman , O. , Badger , M. and Armond , P. 1980 . “ Response and adaption of photosynthesis to high temperature ” . In Adaptation of plants to water and high temperature stress , Edited by: Truner , N.C. and Kramer , P.J. 233 – 249 . New York, N.Y : Wiley-Interscience .
- Craig , H. and Gordan , L.I. 1965 . Deuterium and oxygen—18 variations in the ocean and marine atmosphere. Stable isotopes in oceanographic studies and paleotemperaturesStable isotopes in oceanographic studies and paleotemperatures. Spoleto , 1 – 22 . Pisa, , Italy : Consiglio Nazionale delle Ricerche, Laboratorio di Geologia Nucleare .
- Lad , B.L. , Gunjate , R.T. and Salvi , J. 1992 . Causes and control measures of spongy tissue disorder in Alphonso mango fruit: An integrated approach . Maharashtra J. Hort. , 6 ( 2 ) : 25 – 32 .
- Leitsch , I. 1916 . Some experiments on the influence of temperature on the rate of growth of Pisum sativum . Ann. Bot. , 30 : 25 – 46 .
- Madhava , B.H. 2000 . Oxygen (Δ18O) and carbon (Δ13C) isotope composition in plants—An approach to quantify transpiration and mesophyll factors associated with water use efficiency . Univ. of Agr. Sci. Bangalore, PhD Diss. ,
- Madhava , B.H. , Sheshshayee , M.S. , Devendra , A.V. , Prasad , T.G. and Kumar , U.M. 1999 . Oxygen (18O) isotopic enrichment in the leaves as a potential surrogate for transpiration and stomatal conductance . Curr. Sci. , 76 ( 11 ) : 1427 – 1428 .
- Ravindra , V. and Shivashankar , S. 2004 . Spongy tissue in Alphonso mango-significance of in situ seed germination events . Curr. Sci. , 87 ( 8 ) : 1045 – 1049 .
- Vasanthaiah , H.K.N. , Ravishankar , K.V. , Shivashankara , K.S. , Anand , L. , Narayanaswamy , P. , Mukunda , G. K. and Prasad , T.G. 2006 . Cloning and characterization of differentially expressed genes of internal breakdown in mango fruit (Mangifera indica L.) cv . Alphonso. J. Plant Physiol. , 163 ( 6 ) : 671 – 679 .