Abstract
The underutilized fruit of Cordia dichotoma, which is popularly known as Indian cherry, was studied at its sequential stages of growth and ripening. The biochemical analysis of this fruit revealed that it is a climacteric type and is a rich source of sugars, proteins, and phenolic compounds, indicating its potential as a nutritious food. The change in the specific activities of enzymes, such as amylase, invertase, catalase, peroxidase, pectinmethylesterase, cellulase, and polygalacturonase, was correlated with the compositional changes of the fruit during growth and ripening.
INTRODUCTION
Due to an ever increasing population and escalating urbanization, highly productive agricultural land is being used for urban development, which in turn places pressure on the remaining agricultural land. The difficulties have become more acute due to the over-dependency of humans on fewer plant species. Over 50% of the daily global requirement of proteins and calories is met by just three crops—maize, wheat, and rice (CitationFAO, 1996). Hence, diversifying production and/or consumption of a broader range of plant species, including those currently identified as ‘underutilized’, can therefore contribute significantly to improve human health, nutrition, income generation, and maintain ecological sustainability (CitationICUC, 2006).
India is endowed with a wide variety of food plants, which contribute to the diet of the people. Diversity of agro-climatic conditions and people's preference for taste offer great chances to identify the most promising fruits for commercial cultivation. Nutritional contributions and nutritive value of the most common foods have been extensively studied and compiled (CitationICUC, 2006), but the lack of information regarding the nutritional value of some less commonly consumed foods limits the use of these underutilized fruits.
Indian cherry (C. dichotoma) is a small- to moderate-sized deciduous tree, locally known as Lehsora or Gunda. The fruit of lehsora is underutilized. Its immature fruits are pickled and/or used as a vegetable (CitationDuhan et al., 1992). The globose or ovoid and drupaceous fruit when ripe turns yellow or pinkish-yellow (). The seed kernel of Indian cherry can be used as a potential cattle feed (CitationSingh, 1982). Moreover, the fruit extract of lehsora is known to have various medicinal uses (CitationWarrier et al., 1994).
However, a review of the literature reveals that although the fruit of C. dichotoma is known to possess high nutritional and medicinal value, so far due consideration has not been given to understand the compositional changes that occur during the sequential stages of growth and ripening of Indian cherry fruit. Hence, the present study.
MATERIALS AND METHODS
The fruits of C. dichotoma were collected at their successive developmental stages from the University Botanical Garden, Sardar Patel University, Gujarat, India. After recording the measurements of fresh weight, length, and diameter (), they were subjected to biochemical analyses using the methods as described earlier by CitationPatel and Rao (2009). The statistical analysis of the data obtained was analyzed as per the method of CitationBliss (1967).
TABLE 1 Measurements of Fresh Weight, Diameter, Length, pH, and Total Acidity of C. dichotoma Fruit at Successive Stages of Growth and Ripening
RESULTS
The pH of the pulp of the Indian cherry at its young stage was found to be 6.1, but it remained more or less unchanged in all the subsequent developmental stages (). In contrast, the total acidity of the fruit increased by two-fold with 0.13% citric acid equivalent at the young stage to 0.26% citric acid equivalent in the pre-ripened stage. However, with the onset of ripening, sharp reduction in the total acidity (0.16% citric acid equivalent) was observed ().
As the fruit of C. dichotoma continues its growth and proceeds towards the ripening stage, a visual change occurs in its color from green to pink. A quantitative analysis of pigments revealed that the amount of chlorophyll ‘a’ and chlorophyll ‘b’ increases by three- and two-fold, respectively, from the young stage to the mature stage, but thereafter it decreases. Also, the amount of total chlorophylls at the mature stage was recorded to be as high as 1.41 mg/100 gm, but subsequently it showed a declining trend (). In contrast, the quantity of total carotenoids in the fruit measured 0.09 mg/100 gm at the young stage, but increased significantly by two-fold measuring 0.21 mg/100 gm at the ripened stage. Similarly, anthocyanins also accumulated to the highest levels of 1.05 mg/100 gm at the ripened stage ().
TABLE 2 Changes in the Biochemical Composition of C. dichotoma Fruit at Successive Stages of Growth and Ripening
Sugars are said to play an essential role in imparting attractive flavor, appearance, and texture to the fruits. The quantitative analysis of non-reducing sugars in the fruit exhibited an increase in quantity from 28.2 mg/gm at its young stage to 31.2 mg/gm at the ripened stage, which represents a 25% rise. In contrast, the amount of reducing sugars was found to increase by more than four-fold, being at 12.5 mg/gm at the young stage and 53.8 mg/gm at the ripened stage (). Starch, which is considered to be the major storage polysaccharide found in the fruits, was at 15.9 mg/gm at the young stage, but decreased by more than five-fold to 2.8 mg/gm at the ripened stage ().
Proteins are known to occur in low concentrations within the fruits and they are also known to be involved in the metabolism during the growth and ripening of the fruit. The amount of protein in the fruit of Indian cherry is higher at its young stage at 19.4 mg/gm than during its subsequent stages of development and ripening. The protein content of the fruit exhibited inconsistency in its amount with 13.2 mg/gm at mature stage, increasing to 19.0 mg/gm at the pre-ripened stage, and eventually declining to 14.7 mg/gm during ripening (). The quantity of amino acids in the fruit decreased from 2.3 mg/gm at the young stage to 0.94 mg/gm at the mature stage, but a significant increment in their quantity occurs at the pre-ripened stage (2.95 mg/gm). The phenolic compounds, which are said to be important in determining the flavor and color of fruits, measured 0.97 mg/gm in the young fruit. An increase in their quantity to 1.68 mg/gm at maturity was found, but with the onset of ripening then decreased significantly ().
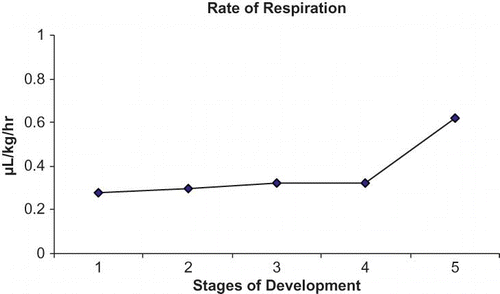
The present study, further revealed that the levels of ethylene evolution in the fruit of Indian cherry was 0.23 μL/kg/hr during the young stage, but with the onset of ripening, the rate of ethylene evolution increased by more than three-fold reaching 0.78 μL/kg/hr (). Although the rate of respiration remained more or less consistent from the young stage (0.28 μL/kg/hr) to the pre-ripened stage (0.32 μL/kg/hr), towards ripening it increased by two-fold to 0.62 μL/kg/hr ().
FIGURE 2 Changes in the levels of ethylene in C. dichotoma fruit during successive stages of growth and ripening.
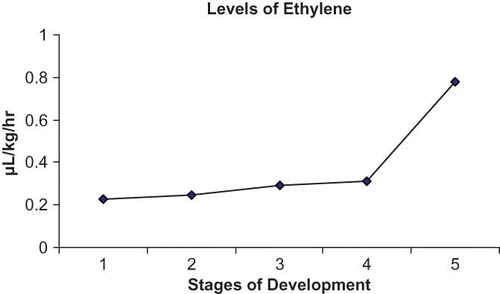
FIGURE 3 Changes in the rate of respiration in C. dichotoma fruit during successive stages of growth and ripening.
Various metabolic changes that occur in the fruit during its growth and ripening are partly or wholly due to the activity of the enzyme systems present in it. During the course of the present study, the specific activity of the amylase enzyme increased from the young stage (0.007 mg maltose released/min/mg protein) to the mature stage (0.07 mg maltose released/min/mg protein), but subsequently it declines to 0.003 and 0.002 mg maltose released/min/mg protein during the pre-ripened and ripened stages, respectively (). In contrast, a consistent and gradual increase in the specific activity of enzyme invertase was found with 0.008 mg glucose released/min/mg protein at the young stage to 0.019 mg glucose released/min/mg protein at the premature stage. It, however, showed inconsistency in its activity with its decrease to 0.002 mg maltose released/min/mg protein in the mature and pre-ripened stages and a minor increment (0.006 mg maltose released/min/mg protein) with the onset of ripening ().
TABLE 3 Changes in the Specific Activity of Enzymes in C. dichotoma Fruit During Successive Stages of Growth and Ripening
One mechanism by which plants defend against the deleterious effects of free radicals is by the help of catalase, peroxidase, and superoxide dismutase, which prevent the formation of potent free radicals. During the course of the present study, no significant changes are noticed in the specific activity of catalase, while the specific activity peroxidase increases from 0.001 units/min/mg protein at the young fruit stage to 0.057 units/min/mg protein at the premature stage, but, thereafter, the specific activity remarkably decreases to the tune of 0.0008 units/min/mg protein ().
The enzymes that are involved in fruit cell wall metabolism during ripening, include pectinmethylesterase (PME), polygalacturanase (PG), cellulase, and β-galactosidase. Throughout the course of the currrent study, the specific activity of PME was found to be 0.004 mg A620/min/mg protein at the young stage, but it increased significantly during ripening to 0.006 mg glucose released/min/mg protein (). However, the specific activity of cellulase exhibited inconsistent results (0.048 mg A620/min/mg protein at the young stage, 0.072 mg glucose released/min/mg protein at premature stage, 0.038 mg glucose released/min/mg protein at mature stage, 0.089 mg glucose released/min/mg protein at pre-ripened stage, and 0.027 mg glucose released/min/mg protein during ripening). In comparison with the specific activity of other enzymes, the specific activity of PG was higher with 1.385 mg glucose released/min/mg protein at the young stage and also it subsequently increased further at the pre-ripened stage to 3.024 mg glucose released/min/mg protein ().
DISCUSSION
During the successive stages of growth and development of the Indian cherry fruit, the pH of the fruit pulp remained more or less stable. The total acidity, which measured high at the premature stage, decreased with the onset of ripening. This decrease in the acidity of the fruit changes the flavor of the fruit pulp from sour to sweet. Moreover, ripening is accompanied by changes in acidity, flavor, texture, color, and aroma of the fruit (CitationWhite, 2002).
The decrease in the quantity of chlorophylls and concurrent increase in the carotenoids and anthocyanins in the fruit of C. dichotoma may be due to the unmasking of previously present pigments by degradation of chlorophyll and dismantling of the photosynthetic apparatus and synthesis of different types of anthocyanins and their accumulation in vacuoles, and accumulation of carotenoids, such as β-carotene, xanthophylls, and lycopene (CitationTucker and Grierson, 1987; CitationLizada, 1993).
The results of the present study regarding the starch accumulation during the early stages supports the opinion of CitationMattoo et al. (1975) that starch is the main carbohydrate present in the fruits and that it decreases to lower levels with the advancement of maturity. This accumulated starch is hydrolyzed into sugars, which is a characteristic event during fruit ripening. The results of the present study also support the view of CitationHulme (1970) who noted that the sugar levels within the fruit tend to increase progressively at all successive stages of growth and ripening. He suggested that the increase in sugars may be mainly due to the hydrolysis of starch, which generally accumulates during the early stage of growth and with the onset of ripening results in increasing sugar content. In addition, activation or de novo synthesis of hydrolytic enzymes like amylase was suggested to have an active role in starch degradation (CitationYoung et al., 1975). Moreover, the increase in the reducing sugar in the fruit of C. dichotoma may be due to more rapid and partial breakdown of non-reducing sugars and other polysaccharides and their subsequent inversion to reducing sugars in the course of fruit ripening (CitationSagar and Khurdiya, 1996).
The protein content was observed to exhibit inconsistency during the growth and ripening of the fruit. The results of the present study are in agreement with the findings of CitationGomez-Lim (1997) in several fruits. CitationTressel et al. (1975) also reported an increase in the amounts of some proteins and enzymes, while CitationMathooko (2000) suggested that the dramatic increase in protein reflects the enzyme required for the process of ripening. The reduction in the quantity of total free amino acids in the fruit of C. dichotomamay be due to their incorporation into proteins required for the synthesis of various ripening enzymes, and, subsequently, their utilization may also decline causing an increase in their quantity at the later stages (CitationFrankel et al., 1968).
The declining trend of phenols from high levels during early growth to lower levels when the fruit attains maturity and thereafter may be due to phenolic compounds being easily oxidized to quinines (CitationVicente et al., 2007). Furthermore, CitationDiley (1972) is of the opinion that the biosynthetic mechanisms of certain phenolic compounds are suspected of being involved in some types of stress responses that occur mainly during ripening.
Moreover, the Indian cherry fruit also exhibited an increase in autocatalytic endogenous ethylene production and an increase in respiration during the ripened stage. This kind of increase in ethylene and respiration is thought to be necessary to provide ATP and substrates for various anabolic processes associated with fruit ripening (CitationBlanke, 1991).
Fruit ripening is regarded as an oxidative phenomenon (CitationBrenan and Frenkel, 1977). This oxidation causes an imbalance in the production of reactive oxygen species (ROS) that leads to negative cellular alterations. During the onset of ripening, the ROS, such as singlet oxygen, hydrogen peroxide, superoxide, or hydroxyl radical (CitationAsada, 1999), are removed by the activities of oxygen-detoxifying enzymes, such as catalase, peroxidase, and superoxide dismutase (CitationBowler et al., 1992). Whereas catalase plays an important role in removing the toxic hydrogen peroxide within the cell (CitationDeDuve, 1983), it also has a role in the removal of electrons that lead to the production of O2- free-radical (CitationAbassi et al., 1998). Moreover, peroxidase enzyme is said to act during ripening or senescence (CitationGrover and Sinha, 1985) as peroxidases have free radical scavenger properties (CitationBurris, 1960). The results of the present study reveal that the enzyme peroxidase has a more major role than that of catalase in detoxifying the cells.
The major textural changes resulting during the softening of fruit are due to the enzyme-mediated alterations in the structure and composition of the cell wall and partial or complete solubilization of cell wall polysaccharides, such as pectins and cellulose (CitationTucker and Grierson, 1987). In the fruit of Indian cherry, the enzyme PG exhibits high specific activity followed by cellulase and PME. Similar results of increased pectic solubilization, loss of tissue firmness, and a rapid rise in the PG activity is said to accompany normal ripening in many fruits (CitationBrady, 1987; CitationTucker, 1993; CitationFisher and Bennett, 1991).
Since pectic polymers begin to acquire solubility only after PG has become active, it is believed that the enzyme PG is involved in the breakdown of the insoluble complex polysaccharides by reducing the length of the chains cross-linked by calcium (CitationWong, 1995). PG hydrolyzes the α-1, 4-glycosidic bonds between the galacturonic acid residues in galacturonans, while PME catalyzes the hydrolysis of pectin methyl ester groups, resulting in desterification. As PME is specific for galacturonide esters, its action is to remove methoxyl groups from methylated pectin (CitationPrasanna et al., 2008). This results in the formation of an acyl enzyme intermediate with the release of methanol, followed by hydrolysis to generate the enzyme and a carboxylic acid. Thus, the process of de-esterification appears to proceed linearly along the chain resulting in blocks of free carboxyl groups (CitationRexova-Benkova and Markovic, 1976).
It appears that PME preferentially attacks the methyl ester bonds of a galacturonate unit next to a non-esterified galacturonate unit (CitationPilnik and Voragen, 1970). Therefore, they deesterify the esterified pectic substances, making them vulnerable for PG action. Thus, the action of PME may be a prerequisite for the action of PG during ripening.
From the foregoing account, it may be concluded that the fruit of the Indian cherry is a rich source of total sugars, starch, proteins, and phenolic compounds, which indicated its potential as a nutritious food. Moreover, the present study provides the baseline information as a meaningful indicator for commercial exploitation of this underutilized fruit. Hence, utilization of these underutilized fruits would help in diversifying production, which can, therefore, contribute significantly to human health, nutrition, income generation, and maintenance of ecological sustainability.
LITERATURE CITED
- Abassi , N.A. , Kushad , M.M. and Endress , A.G. 1998 . Active oxygen-scavenging enzymes activities in developing apple flowers and fruits . Sci. Hort. , 74 ( 3 ) : 183 – 194 .
- Asada , K. 1999 . The water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons . Annu. Rev. Plant Physiol. Plant Mol. Biol. , 50 : 601 – 639 .
- Blanke , M.M. 1991 . Respiration of apple and avocado fruits . Postharvest News & Info. , 2 : 429 – 436 .
- Bliss , C.I. 1967 . “ Statistical methods for research in the natural sciences ” . In Statistics in biology , New York : McGraw Hill Book Company .
- Bowler , C. , VanMontagu , M. and Inze , D. 1992 . Superoxide dismutase and stress tolerance . Ann. Rev. Plant Physiol. , 43 : 83 – 116 .
- Brady , C.J. 1987 . Fruit ripening . Ann. Rev. Plant Physiol. , 38 : 155 – 178 .
- Brenan , T.I. and Frenkel , C. 1977 . Involvement of hydrogen peroxide in the regulation of senescence in pear . Plant Physiol. , 598 : 411 – 416 .
- Burris , R.H. 1960 . “ Hydroperoxidase (peroxidase and catalases) ” . In Encyclopedia of plant physiology , Edited by: Ruhland , W. 365 – 400 . Berlin : Springer-Verlag .
- DeDuve , C. 1983 . Microbodies in the living cells . Sci. Amer. , 248 : 42 – 52 .
- Diley , D.R. 1972 . Hypobaric storage—A new concept for preservation of perishables . 102nd Ann. Rept. Mich. State Hort. Soc. , 132 : 146
- Duhan , A. , Chauhan , B.M. and Punia , D. 1992 . Nutritional value of some non-conventional plant foods of India . Plant Foods for Human Nutr. , 42 ( 3 ) : 193 – 200 .
- FAO . 1996 . Report on the state of the world's plant genetic resources for food and agriculture 511 Rome, , Italy
- Fisher , R.L. and Bennett , A.B. 1991 . Role of cell wall hydrolases in fruit ripening . Ann. Rev. Plant Physiol. Plant Mol. Biol. , 42 : 675 – 703 .
- Frankel , C. , Klein , I. and Dilley , D.R. 1968 . Protein synthesis enzymes in pome fruits . Plant Physiol. , 43 : 11 – 46 .
- Gomez-Lim , M.A. 1997 . “ Post-harvest physiology ” . In The mango , Edited by: Litz , R.E. 425 – 446 . New York, , USA : Botany, production, and uses. CAB Intl .
- Grover , A. and Sinha , S.K. 1985 . Senescence of detached leaves in pigeon pea and chick pea: Regulation by developing pods . Physiol. Plant. , 65 : 503 – 507 .
- Hulme , A.C. 1970 . The biochemistry of fruits and their products , London : Academic Press .
- ICUC . 2006 . “ Strategic framework for underutilized plant species research and development with special reference to Asia, the Pacific and Sub-Saharan Africa ” . In Intl. Centre for Underutilized Crops , 33 Colombo : Sri Lanka & Global Facilitation Unit for Underutilized Species, Rome, Italy .
- Lizada , C. 1993 . “ Mango, p ” . In Biochemistry of fruit ripening , Edited by: Seymour , G.B. , Taylor , J.E. and Tucker , G.A. 255 – 271 . London : Chapman and Hall .
- Mathooko , F.M. 2000 . Manual of third country group training programme in applied food analysis: Postharvest Physiol , Kenya : JKUAT .
- Mattoo , A.K. , Murata , T. , Pantastico , E.B. , Chachin , K. , Ogata , K. and Phan , C.T. 1975 . “ Chemical changes during ripening and senescence ” . In Postharvest physiology, handling and utilization of tropical and subtropical fruits and vegetables , Edited by: Pantastico . 103 – 127 . AVI Publishing, Westport .
- Patel , P.R. and Rao , T.V.R. 2009 . Physiological changes in relation to growth and ripening of khirni [Manilkara hexandra (Roxb.) Dubard] fruit . Fruits , 64 ( 3 ) : 139 – 146 .
- Pilnik , W. and Voragen , A.G.J. 1970 . “ Pectic substances and other uronides ” . In The biochemistry of fruits and their products , Edited by: Hulme , A.C. 53 – 87 . New York : Academic Press .
- Prasanna , V. , Prabha , T. N. and Tharanathan , R.N. 2008 . Fruit ripening phenomena—An overview . Crit. Rev. Food Sci. Nutr. , 47 : 1 – 19 .
- Rexova-Benkova , L. and Markovic , O. 1976 . Pectic enzymes . Adv. Carbohydrate Chem. Biochem. , 33 : 323 – 385 .
- Sagar , V.R. and Khurdiya , D.S. 1996 . Effect of ripening stages on quality of dehydrated ripe mango slices. J. Food Sci . Technol. , 33 ( 6 ) : 527 – 529 .
- Singh , R.V. 1982 . Fodder trees of India , New Delhi, , India : Oxford & IBH Co .
- Tressel , R. , Holzer , M. and Apertz , M. 1975 . “ Biosynthesis of volatiles ” . In Intl. Symp. on Aroma Research. Zeist, the Netherlands Central Inst. for Nutr. Food Res., TNO Edited by: Maarse , H. and Groenen , P.J. 41 – 62 .
- Tucker , G.A. 1993 . “ Introduction ” . In Biochemistry of fruit ripening , Edited by: Seymour , G.B. , Taylor , J.E. and Tucker , G.A. 1 – 51 . London : Chapman and Hall .
- Tucker , G.A. and Grierson , D. 1987 . “ Fruit ripening ” . In The biochemistry of plants , Edited by: Davies , D. Vol. 12 , 265 – 319 . New York : Academic Press Inc .
- Tucker , G.A. , Robertson , N.G. and Grierson , D. 1982 . Purification and changes in activities of tomato pectinesterase isoenzymes . J. Sci. Food Agr. , 3 : 396 – 400 .
- Vicente , A.R. , Saladie , M. , Rose , J.C.K. and Labavitch , J.M. 2007 . The linkage between cell wall metabolism and fruit softening, looking to the future . J. Sci. Food Agr. , 87 : 1435 – 1448 .
- Warrier , P.K. , Nambiar , V.P.K. and Ramankutty , C. 1994 . Indian medicinal plants: A compendium of 500 species , Vol. 2 , 416 India, Hyderabad : Orient Longman .
- White , J.P. 2002 . Recent advances in fruit development and ripening: An overview . J. Expt. Bot. , 53 : 1995 – 2000 .
- Wong , D.W.S. 1995 . “ Pectic enzymes ” . In Food enzymes. Structure and mechanisms , 212 – 236 . New York : Chapman and Hall .
- Young , R.E. , Salsinen , S. and Facteuset , S. 1975 . “ Enzyme regulation associated with ripening in banana fruit ” . In Regulation de la maturation des fruits , Edited by: Olrich , K. 271 – 280 . Paris : Colloq. Intl. CNRS .
