Abstract
The biochemical changes in fruit of an early ‘Lonet-Mesaed’ and a late ‘Helali’ date palm cultivar during development and ripening including the activities of various degradative enzymes were studied. During the 2009 and 2010 seasons, in both cultivars, fruit growth, based on fruit and flesh weight, followed a smooth sigmoidal curve. The fruit and flesh weight gradually increased during development reaching a maximum at the immature green (Bisir) stage, but slightly decreased thereafter during ripening. Moisture percentage was highest at early stages and then gradually decreased to lower levels during the Bisir and the mature firm full-colored (Rutab) stages in both cultivars, with a further sharp decrease at the raisin-like stage (Tamer) in ‘Helali’. The accumulation of both total and reducing sugars in fruit slightly increased during development with a vast increase during maturation and ripening of both cultivars. The concentration of total proteins was highest at early stages and then gradually decreased during development to lower concentrations during ripening. A steady decrease in the membrane stability index (MSI %), as measured by the leakage of ions, was observed upon the progression of fruit development, especially during the Bisir and the Rutab stages, indicates a gradual loss of the membrane's stability due to changes occurring in the biochemical and biophysical properties of cell membranes. During the 2009 season, both cultivars possessed polygalacturonase, cellulase xylanase, and α-amylase activities. Within the same fruit, the slightly softened apical half had activity of about 15 and 2 times for polygalacturonase and cellulase, respectively, higher than that of the firm basal half of the same fruit. Moreover, the activity of both xylanase and α-amylase was only detected in the apical tissues. The activities of these enzymes and fruit ripening were closely associated, suggesting their involvement in the ripening process of dates. The differences between the two cultivars in developmental and ripening patterns in conjunction with enzyme activities are discussed.
INTRODUCTION
Date palm is the most successful and extremely important subsistence crop in many of the hot arid regions of the world (CitationChao and Krueger, 2007; CitationAwad, 2007). The fruit of all date palm cultivars pass through five distinct stages of development and ripening. These stages are designated by Arabic terms and are used universally. Hababouk, Kimri, Bisir or Khalal, Rutab, and Tamer are used to represent, respectively, the cell division, cell elongation or the immature green, the mature firm full-colored, the soft brown, and the hard raisin-like stages of date palm development.
Generally, whole dates are harvested and marketed at three stages of their development—mainly Bisir or Khalal, Rutab, and Tamer—depending on cultivar characteristics, especially soluble tannins level, climatological conditions, and market demand (CitationGlasner et al., 1999; CitationAwad, 2007). Accordingly, in many districts in the Arabic Gulf region, some of the date palm cultivars reach the Bisir or the Rutab stage early in the season (late May to early June), such as ‘Lonet-Mesaed’, while others reach these stages late in the season (mid August to late September), such as ‘Helali’.
Both ‘Lonet-Mesaed’ and ‘Helali’ are important date palm cultivars being extensively cultivated in Saudi Arabia. At the Bisir stage, ‘Lonet-Mesaed’ dates are slightly astringent due to a low level of soluble tannins, and thus can be consumed at both the Bisir and Rutab stages, while ‘Helali’ dates are astringent at the Bisir stage as a result of high contents of soluble tannins, and removal of tannins is necessary for the fruit to be edible (CitationAwad, 2007). The fruit do not ripen at one time, even in the same bunch; consequently, several harvests are required during the harvesting season. In some regions in Saudi Arabia and in some other countries in the Gulf region, only 40–50% of the total ‘Helali’ dates might normally ripen on the tree and the remaining fruit fail to ripen, which causes a great economic loss (CitationAwad, 2007). Most of the research work has mainly concerned changes in physical and chemical parameters during development and ripening related to the marketing quality of dates (CitationRouhani and Bassiri, 1976; CitationSawaya et al., 1982a, Citation1982b, Citation1982c; CitationAhmed et al., 1995). However, limited consideration is being given to the biochemistry of dates and biochemical changes with development and ripening especially, the activities of the hydrolytic and degradative enzymes, and other physiological events. Also, most of the available studies have been performed on dates that were picked during the commercial harvest period (similar phonological age) and sorted into different physiological stages based on color (different physiological age) (CitationHasegawa et al., 1969; CitationHasegawa and Smolensky, 1971; CitationMustafa et al., 1986). It is generally accepted that the degradation of the polysaccharide components of the cell wall and reduction of cell to cell adhesion, as a result of middle lamella degradation, are the main factors causing softening of the fruit (CitationHasegawa et al., 1969; CitationAndrews and Li, 1995; CitationMohamed, 2004; CitationMercadoa et al., 2010).
Both polyuronides and matrix glycans are modified during ripening, but the cell wall change that most closely correlates with softening is the depolymerization of matrix glycans (CitationBrummell, 2006), xyloglucans being the major component of this cell wall fraction in dicotyledons (see CitationMercadoa et al., 2010). However, our understanding of these changes is hampered by limited knowledge of the structure of the cell wall in the fruit and the enzymes that modify the cell wall polysaccharides. This article reports the changes in one pectic enzyme (polygalacturonase) and three glycanases enzymes (cellulase, xylanase, and α-amylase) that accompany the biochemical and physiological events that take place in dates during development and ripening of an early ‘Lonet-Mesaed’ and a late ‘Helali’ cultivar.
MATERIALS AND METHODS
Plant Materials and Experimental Procedure
During both the 2009 and 2010 growing seasons, six 23-year-old uniform ‘Lonet-Mesaed’ and ‘Helali’ date palm trees growing in a sandy loamy soil, drip irrigated, and receiving normal cultural practices were used. The trees were located at the experimental orchard of the Faculty of Meteorology, Environment, and Arid land Agriculture at Hada Al-Sham, Saudi Arabia. The experiment was designed as a completely randomized design with three replicates (two trees for each replicate). All trees were pollinated from one male tree during the flowering period (March 1–5 in the 2009 season and February 13–16 in the 2010 season for ‘Lonet-Mesaed’ and March 7–10 in the 2009 season and February 21–24 in the 2010 season for ‘Helali’). Following fruit set, the crop load was adjusted to eight bunches per palm. In both seasons, three fruit samples of 20 fruits each were collected at 6, 8, 10, 12, 14, and 16 weeks from pollination and at 6, 8, 10, 12, 14, 17, 23, and 27 weeks from pollination for ‘Lonet-Mesaed’ and ‘Helali’, respectively. The fruit samples were immediately frozen and kept at −20°C until biochemical determinations.
Fruit Growth, Total Sugars, Reducing and Non-Reducing Sugars, and Total Proteins
In both growing seasons, fruit and flesh weight were recorded independently in each of the 20 fruits per replicate at each sampling date. A homogeneous sample was prepared from these 20 fruits per replicate for measuring total and reducing sugars and total crude proteins. Total crude proteins were measured by the micro-Kjeldahl procedure. The fruit samples were oven dried for 48 hr at 65°C in paper bags. Samples were ground and wet digested as described by CitationChapman and Pratt (1961). Percentage of total proteins was calculated as N% × 6.25. Total sugars were determined by phenol–sulfuric acid reaction (CitationDubois et al., 1956) and reducing sugars were determined by the dinitrosalicylic acid method (CitationMiller, 1959). Glucose served as the calibration standards for total and reducing sugars.
Leakage of Ions
In both growing seasons, leakage of ions from fruit disks was measured according to CitationSairam et al. (1997) with some modifications and was expressed as membrane stability index percentage (MSI %). Five grams of fruit disks (including skin and flesh) per replicate/treatment was taken from fruits (previously washed with distilled water and lightly cleaned with tissue papers) and placed in 30 ml of deionized water at ambient temperature for 4 hr in a shaker. Conductivity before killing (C1) was measured with an electrical conductivity digital meter (Orion 150A+, Thermo Electron Corporation, USA). The same disks were kept in a boiling water bath (100°C) for 30 min to release all electrolytes, cooled to 22 ± 2°C with running water, and conductivity after killing was recorded (C2). MSI was expressed in percentage using the formula: [1–(C1/C2)] × 100.
Enzyme Measurements
Crude extract
During the 2010 growing season, ten grams of fruit tissue (including skin and flesh) per replicate/treatment was taken from the middle of at least five fruits (previously washed with distilled water and lightly cleaned with tissue papers). In order to study the distribution of enzyme activity within fruit, additional samples from ‘Lonet Mesaed’ at the beginning of ripening were separately taken from the apical end and the basal (stem) end of fruit. The fruit tissues were homogenized in 10 ml of cold 0.02 M Tris-HCl buffer (pH 7.2) containing 1% (w/v) soluble polyvinyl pyrrolidone (PVP-40). The homogenate was centrifuged at 15,000 × g for 15 min. The supernatant was designated as crude extract and dialyzed against cold 0.02 M Tris-HCl buffer (pH 7.2) over night. Aliquots were taken for assay of enzymes.
Enzyme Assays
Polygalacturonase (EC 3.2.1.15), cellulase (EC 3.2.1.21), xylanase (EC 3.2.1.8), and α-amylase (EC 3.2.1.1) activities were assayed by determining the liberated reducing end products using galacturonic acid, glucose, xylose, and maltose as standards, respectively (CitationMiller, 1959). The reaction mixture (0.5 ml) contained 1% substrate, 0.05 M sodium acetate buffer (pH 5.5), and a suitable amount of crude extract. Assays were carried out at 37°C for 1 hr, then 0.5 ml dinitrosalicylic acid reagent was added to each tube and heated in a boiling water bath for 10 min. After cooling to room temperature, the absorbance was measured at 560 nm. Substrates used were polygalacturonic acid, CM-cellulose, xylane, and starch for polygalacturonase, cellulase, xylanase, and α-amylase, respectively. One unit of enzyme activity was defined as the amount of enzyme that liberated 1 μmol of reducing sugar per hour under standard assay conditions.
Statistical Analysis
The obtained data were statistically analyzed as a completely randomized design with three replicates by analysis of variance (ANOVA) using the statistical package software SAS (SAS Institute Inc., 2000, Cary, NC., USA). Comparisons between means were made by F-test and the least significant differences (LSD) at P = 5%.
RESULTS
Fruit Growth, Total Sugars, Reducing and Non-Reducing Sugars, and Total Proteins
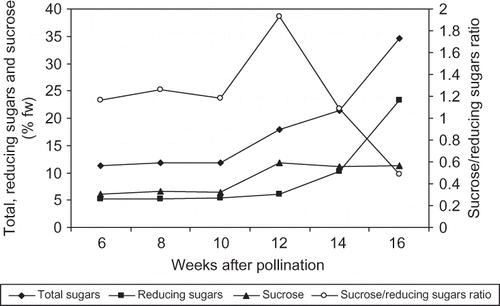
Because of similarity between the results of the two growing seasons (no significant interactions between seasons), data are presented as the means of both seasons. For ‘Lonet Mesaed’, the fruit and flesh weight gradually increased during development until week 14 from pollination at the Bisir stage, but slightly decreased thereafter at the Rutab stage at week 16 from pollination in a sigmoidal pattern (). Moisture percentage was highest at the early stage (80.2%) with a slight decrease from week 6 to week 10 and then it gradually decreased to reach lower levels during the Bisir (65.4%) and the Rutab (42.9%) stages at week 14 and 16 from pollination, respectively (). For ‘Helali’, the fruit and flesh weight gradually increased during development until week 17 at the Bisir stage, slightly decreased at the Rutab stage at week 23, but sharply decreased during the Tamer stage at week 27 from pollination in a sigmoidal pattern (). Moisture percentage was highest at the early stage (82.3%) with slight changes from week 6 to week 14 and then gradually decreased at the Bisir stage (76.6%) at week 17 with a sharp decrease during the Rutab (46.1%) and the Tamer (21.3%) stages at weeks 23 and 27 from pollination, respectively (). In ‘Lonet Mesaed’ fruit, both of the total and reducing sugars slightly increased from week 6 to week 10, and then rapidly increased from week 12 to week 16 from pollination to reach a high concentration of 34.7 and 23.3% fw, for total and reducing sugars, respectively (). Meanwhile, sucrose concentration slightly fluctuated from week 6 to week 10, then sharply increased at week 12 from pollination with a slight decrease thereafter during maturation and ripening reaching a level of 11.4% fw. The sucrose/reducing sugars ratio was 1.2 at the early stage and reached a maximum (1.9) at week 12 before the Bisir stage and then sharply decreased to 1.1 and 0.5 at the Bisir and the Rutab stages, respectively.
FIGURE 1 Changes in fruit and flesh weight and moisture percentage of ‘Lonet-Mesaed’ date palm during development and ripening. Data are the means of the 2009 and 2010 seasons. LSD at 5% for time effect is 0.061, 0.045, and 0.773 for fruit and flesh weight and moisture percentage, respectively.
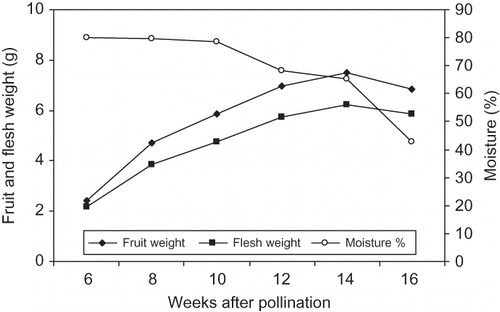
FIGURE 2 Changes in fruit and flesh weight and moisture percentage of ‘Helali’ date palm during development and ripening. Data are the means of the 2009 and 2010 seasons. LSD at 5% for time effect is 0.047, 0.046, and 1.01 for fruit and flesh weight and moisture percentage, respectively.
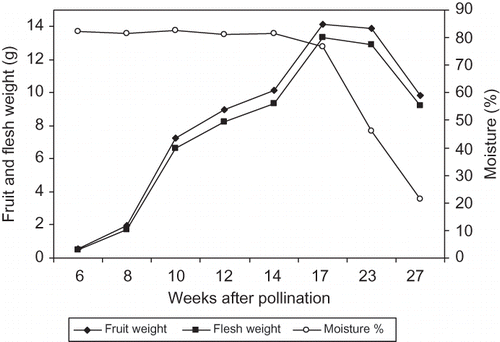
FIGURE 3 Changes in total and reducing sugars and sucrose concentration and sucrose/reducing sugars ratio of ‘Lonet-Mesaed’ date palm fruit during development and ripening. Data are the means of the 2009 and 2010 seasons. LSD at 5% for time effect is 0.126, 0.056, 0.137, and 0.019 for total and reducing sugars and sucrose concentration and sucrose/reducing sugars ratio, respectively.
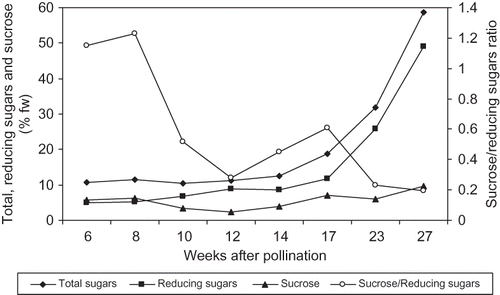
In ‘Helali’ fruit, both of the total and reducing sugars concentration slightly fluctuated until week 12, and then sharply increased from week 12 to week 27 from pollination reaching a high concentration of 58.6 and 49.0% fw for total and reducing sugars, respectively (). Meanwhile, sucrose concentration increased from week 6 to week 10, then sharply decreased at week 10 with a slight fluctuation until week 17 and then sharply increased to reach a concentration of 9.6% fw at week 27 from pollination. The sucrose/reducing sugars ratio was 1.2 at the early stage and reached a maximum (1.2) at week 8 from pollination and then sharply decreased with fluctuation to reach 0.61, 0.23, and 0.20 at the Bisir, the Rutab, and the Tamer stage, respectively. In both cultivars, the concentration of total proteins was highest early in the season (2.8 and 3.4% for ‘Lonet Mesaed’ and ‘Helali’, respectively), and then gradually decreased during development to reach a lower concentration during ripening (1.1 and 1.5% for ‘Lonet Mesaed’ and ‘Helali’, respectively) ( and ).
FIGURE 4 Changes in total and reducing sugars and sucrose concentration and sucrose/reducing sugars ratio of ‘Helali’ date palm fruit during development and ripening. Data are the means of the 2009 and 2010 seasons. LSD at 5% for time effect is 0.066, 0.170, 0.165, and 0.026 for total and reducing sugars and sucrose concentration and sucrose/reducing sugars ratio, respectively.
Membrane Stability Index
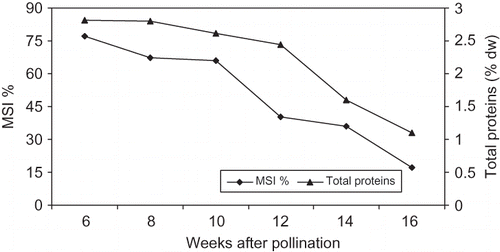
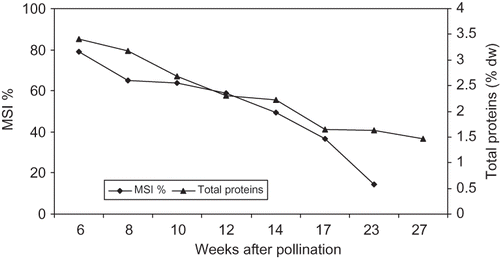
In both seasons, the membrane stability index of ‘Lonet Mesaed’ fruit, as measured by the leakage of ions, was highest (77.0%) early in the season, gradually decreased from week 6 to week 10, and then sharply decreased from week 12 to week 16 from pollination reaching a low level (17.3%) at the Rutab stage. While in ‘Helali’ fruit, the membrane stability index was highest (79.0%) early in the season, gradually decreased during development until week 17, and then sharply decreased at week 23 (the Rutab stage) to reach a low level (14.6%) at week 27 from pollination (the Tamer stage) ( and ).
Enzyme Activities
During the 2010 season, the activity of polygalacturonase in ‘Lonet Mesaed’ fruit was relatively high at week 6 (5.10 units/h/g fw) and increased to reach a maximum level at the Bisir stage at week 14 (10.35 units/h/g fw), followed by a sharp decrease during the Rutab stage at week 16 from pollination (3.0 units/h/g fw, respectively) (). The activity of cellulase was detected at a relatively low level at early stages, decreased from week 6 to week 8 reaching a zero level at week 10 and 12 and then sharply increased to maximum level (1.0 units/h/g fw) at week 14 (the Bisir stage) followed by a slight decrease at week 16 from pollination (the Rutab stage). Xylanase activity gradually increased from week 6 to reach a maximum level at week 14 (4.3 units/h/g fw) followed by a slight decrease at week 16 from pollination. The activity of α-amylase increased from week 6 to week 8, sharply decreased at week 10 and 12, and then sharply increased to a maximum level at week 14 (the Bisir stage) (1.4 units/h/g fw) with a slight decrease at week 16 from pollination (). Softening of dates commences at the apical end and progresses toward the basal (stem) end until the whole fruit is ripe. Within the same fruit, the apical tissues showed higher activity for all the measured enzymes (3.2, 4.0, 1.5, and 0.9 units for polygalacturonase, xylanase, α-amylase, and cellulase, respectively) than the basal (stem end) tissues (0.2, 2.3, 0.0, and 0.0 units/h/g fw for polygalacturonase, xylanase, α-amylase, and cellulase, respectively) ().
FIGURE 7 Changes in polygalacturonase, cellulase, xylanase, and α-amylase activities of ‘Lonet-Mesaed’ date palm fruit during development and ripening during the 2010 season. LSD at 5% for time effect is 0.147, 0.052, 0.115, and 0.058 for polygalacturonase, cellulase, xylanase, and α-amylase activities, respectively.
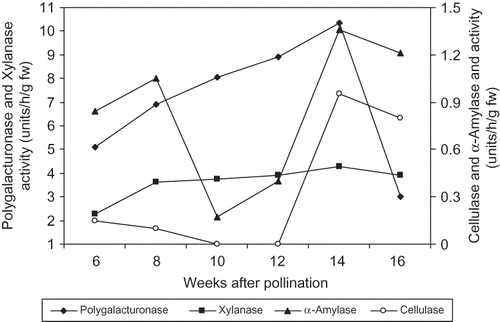
TABLE 1 Enzyme Activities in Apical and Basal (Stem) End Tissues of ‘Lonet-Mesaed’ Date Palm Fruit During the 2010 Season
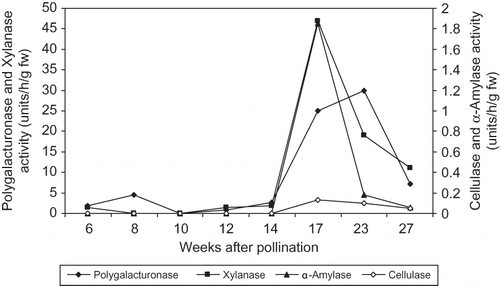
In ‘Helali’ fruit, the activity of polygalacturonase increased from week 6 (1.8 units/h/g fw) to week 8 (4.6 units/h/g fw) followed by a rapid decrease at week 10, but sharply increased to a maximum level at week 17 (the Bisir stage) (25.0 units/h/g fw) and week 23 (the Rutab stage) (30.0 units/h/g fw) followed by a sharp decrease at week 27 (the Tamer stage) (7.2 units/h/g fw) from pollination (). Cellulase activity was not detected until week 17 (0.13 units/h/g fw) followed by a gradual decrease at week 23 and 27 during the Rutab and the Tamer stage, respectively. Xylanase activity was 1.5 units/h/g fw at week 6, not detected from weeks 8 to 10, and then increased from week 12 to reach a maximum level (47 units/h/g fw) at week 17 followed by a sharp decrease to a lower level at week 23 (19 units/h/g fw) and at week 27 (11 units/h/g fw) from pollination. The activity of α-amylase was not detected until week 17 from pollination at the Bisir stage (1.9 units/h/g fw), and then sharply decreased to lower levels of 0.18 and 0.06 units/h/g fw at week 23 (the Rutab stage) and week 27 (the Tamer stage) from pollination, respectively ().
FIGURE 8 Changes in polygalacturonase, cellulase, xylanase, and α-amylase activities of ‘Helali’ date palm fruit during development and ripening during the 2010 season. LSD at 5% for time effect is 0.327, 0.017, 1.25, and 0.042 for polygalacturonase, cellulase, xylanase, and α-amylase activities, respectively.
DISCUSSION
In order to relate the changes in the physical and biochemical properties to fruit growth, the fruit and flesh weight were measured during development and ripening. Based on fruit and flesh weight, fruit growth followed a smooth sigmoidal type of curve for both ‘Lonet Mesaed’ and ‘Helali’ cultivars ( and ). These results confirm those of CitationRouhani and Bassiri (1976) and CitationShabana et al. (1981) for other date palm cultivars. In both cultivars, the fruit reached the maximum weight at the Bisir stage (at 14 and 17 weeks from pollination for ‘Lonet Mesaed’ and ‘Helali’, respectively) with a growth rate of 0.076 and 0.118 g fresh weight per day for ‘Lonet Mesaed’ and ‘Helali’, respectively. The accumulation of both total and reducing sugars in fruit slightly increased during development with a vast increase during maturation and ripening ( and ) mainly due to gain in sugars and loss of moisture ().
In ‘Lonet Mesaed’, the concentration of sucrose was higher than reducing sugars throughout the developmental stages until the Rutab stage at which reducing sugars greatly increased to form the dominant sugar. While in ‘Helali’ the concentration of sucrose was higher than reducing sugars only during early stages until week 8 from pollination after which the concentration of reducing sugars greatly increased to form the dominant sugar. This pattern of sugar accumulation is similar to that observed with other soft date cultivars in other studies (CitationAshmawi et al., 1956). In both cultivars, the vast increase in reducing sugars occurred at the Bisir and Rutab stages with a further increase also at the Tamer stage in ‘Helali’, which is possibly due to the rising activity of the soluble invertase enzyme as a result of loss of the integrity of the cell membrane system during maturation and ripening allowing invertase to come into contact with its substrate (CitationCoggins and Knapp, 1969; CitationMustafa et al., 1986). Date ripening was associated with an increase in total fruit soluble solids, which appears linked in increase to cell wall hydrolyzing enzyme during ripening as reported in other climacteric fruits, such as banana (CitationPathak and Sanwal, 1998) and mango (CitationSingh et al., 2007). Indeed, a steady decrease in the membrane stability index (MSI %), as measured by the leakage of ions, was observed upon the progression of fruit development, especially during the Bisir and Rutab stages in both cultivars ( and ).
Thus, a steady decrease in MSI % upon the progression of fruit development and ripening indicates a gradual loss of the membrane's stability due to changes occurring in the biochemical and biophysical properties of cell membranes. The relatively high concentration of sucrose in both cultivars, especially in ‘Lonet Mesaed’, might be due to a high rate of influx of this sugar into the fruit during ripening rather than any changes in the form or activity of invertase enzyme as suggested by CitationMustafa et al. (1986) for some Sudanese date cultivars. A substantial accumulation of sucrose during maturation and ripening has been also observed for other soft date cultivars (CitationHussein et al., 1976; CitationMustafa et al., 1986). On the basis of the texture and moisture level of the ripe fruit as well as the relative abundance of reducing sugars to sucrose (), both cultivars are classified as soft-date type (CitationSawaya et al., 1982b, Citation1982c; CitationMustafa et al., 1986). In both cultivars, the concentration of total crude protein was highest at early stages and then gradually decreased to reach a lower concentration during ripening ( and ). It has been shown that during plant tissues senescence, proteins, phospholipids, and pigments may be degraded by free radicals as the free radical scavenging system declines (CitationProchazkova et al., 2001).
It is believed that fruit ripening occurs largely as a result of the enzyme-mediated hydrolysis of cell wall components, especially polysaccharides. Our results showed that fruit of the both cultivars possess polygalacturonase, cellulase, xylanase, and α-amylase activities ( and ). In ‘Helali’, polygalacturonase activity was relatively low and fluctuated at the early stages but sharply increased to a maximum level during the Bisir and Rutab stages. However, in ‘Lonet-Mesaed’, the activity of polygalacturonase was relatively high during early stages of fruit growth and reached a maximum level at the Bisir stage. In ‘Helali’, cellulase activity was not detected until week 17 from pollination at the Bisir stage followed by a gradual decrease at the Rutab and the Tamer stages ( and ). Whereas, in ‘Lonet-Mesaed’, cellulase activity was detected at a relatively low level at early stages, decreased from week 6 to week 8, and was absent from week 10 to week 12 from pollination, and then sharply increased to a maximum level at the Bisir stage followed by a slight decrease at the Rutab stage ( and ). These results partly confirm those of earlier work reported for other date cultivars by CitationHasegawa et al. (1969), CitationHasegawa and Smolensky (1971), and CitationMustafa et al. (1986) in which the greatest increase in the activity of both polygalacturonase and cellulase occurred during the Bisir and Rutab stages. Accordingly, it is reasonable to assume that both enzymes are involved in the ripening process of dates. In ‘Deglet Noor’ dates, both polygalacturonase and cellulase activities were not detected until the early and late red stage (the Bisir stage) of development (CitationHasegawa et al., 1969; CitationHasegawa and Smolensky, 1971). However, in our study, such a case was only true for cellulase activity in ‘Helali’ fruit. In three Sudanese date cultivars, CitationMustafa et al. (1986) found that the activities of both polygalacturonase and cellulase gradually rose in a fairly uniform manner throughout the maturation and ripening processes.
Xylanase activity increased to a maximum level in ‘Helali’ and ‘Lonet-Mesaed’ fruits at the Bisir stage and then decreased thereafter at the Rutab and Tamer stages. Also, the activity of α-amylase increased to a maximum level at the Bisir stage and then decreased at the Rutab stages in both cultivars with further decreases at the Tamer stage in ‘Helali’ fruit. Both xylanase and α-amylase, like cellulase, are glycanases enzymes that hydrolysis cell wall polysaccharides. Since the greatest increase in the activity of both xylanase and α-amylase occurred during the maturation and ripening stages, it is also reasonable to assume that both enzymes are involved in the ripening process of dates.
CitationMustafa et al. (1986) found no significant accumulation of starch, the substrate of α-amylase, in three Sudanese date cultivars picked at commercial harvest period and sorted to different developmental classes upon color. They reported that the concentration of starch declined with fruit ripening from 2.15, 0.19, and 1.02% in ‘Jawa’, ‘Bentamoda’, and ‘Mishrig Wad Laggai’ cultivars, respectively, at the green stage to nil at the 100% soft stage. This is different than other climacteric fruits, such as banana and mango, where starch constitutes about 20–25% of the fresh weight, which is almost entirely converted into soluble sugars during ripening with about 2–5%, being lost via respiration (CitationChang and Hwang, 1990; CitationSingh et al., 2007). Comparing the enzymes activities in the two cultivars, the ‘Lonet-Messaed’ fruit showed lower activities of both polygalacturonase and xylanase during maturation and ripening (10.35 and 3.0 units/h/g/fw for the Bisir and Rutab stage, respectively) than ‘Helali’ (25.0 and 30.0 units/h/g/fw for the Bisir and Rutab stage, respectively). However, at earlier developmental stages, ‘Lonet-Messaed’ fruit retained considerably higher levels of both enzymes than ‘Helali’. Also, cellulase activity was higher in ‘Lonet-Messaed’ fruit (0.95 and 0.80 units/h/g/fw for the Bisir and Rutab stage, respectively) than ‘Helali’ (0.13 and 0.10 units/h/g/fw for the Bisir and Rutab stage, respectively) and was considerably detected only in ‘Lonet-Messaed’ fruit at earlier developmental stages ( and ).
α-Amylase activity was rather similar in both cultivars at the Bisir stage but was higher at the Rutab stage in ‘Lonet-Messaed’ fruit than ‘Helali’. However, α-amylase activity was also considerably detected only in ‘Lonet-Messaed’ fruit at earlier stages in contrast to‘Helali’. These results might explain the earlier and faster ripening of ‘Lonet-Messaed’ fruit than ‘Helali’ ( and ). Cultivars variations in polygalacturonase and cellulase activities have been previously observed by CitationMustafa et al. (1986). Otherwise, as climacteric fruit, the rate and/or the pattern of ethylene production might be involved in such variation among date cultivars. Unfortunately, ethylene production during development and ripening was not measured in the current study. CitationAbdel-latif (1988) found that with three date cultivars—‘Zahdi’, ‘Derey’, and ‘Sultani’—ethylene production rate was very high during early stages of fruit development and decreased with maturity, but rose again to a high level as the fruit entered the ripening phase. She also found that the rise in ethylene production preceded the rise in respiration rate. However, in other cultivars, CitationAbbas and Ibrahim (1996) reported that ethylene emission was not detected in ‘Hillawi’ dates until the 91st day from pollination when ethylene production began with a rapid increase in emission, reaching a peak value within 15 days and then declining rapidly. These results might explain the observed relatively higher activity of some of the measured enzymes in ‘Lonet-Messaed’ fruit at early developmental stages than ‘Helali’ ( and ).
Our results showed that the activity of the four studied enzymes and fruit ripening are closely associated. This was also demonstrated by the measured activity of these enzymes at the apical and the basal (stem end) tissues in a single fruit of ‘Lonet-Mesaed’ (). The slightly softened apical half had activity of about 15 and 2 times for polygalacturonase and cellulase, respectively, higher than that of the firm basal half. Moreover, the activity of both xylanase and α-amylase was only detected in the apical tissues. These results confirm those of earlier work reported for polygalacturonase in ‘Deglet Noor’ date cultivar by CitationHasegawa et al. (1969). Also, internal variation in hydrolytic enzymes activities as polygalacturonase and/or cellulase within a single fruit during ripening has been reported in avocado (CitationPesis et al., 1978), tomato (CitationHall, 1987), and sweet cherry (CitationAndrews and Li, 1995).
In conclusion, it seems likely that the four measured hydrolytic enzymes in our study act in conjunction with each other to cause the ripening of dates.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Mohamed Ebraheem and Mr. Nageeb El-Masoudi at the Faculty of Meteorology, Environment, and Arid land Agriculture, King AbdulAziz University, Saudi Arabia, for their indispensable technical support.
LITERATURE CITED
- Abbas , M.F. and Ibrahim , M.A. 1996 . The role of ethylene in the regulation of fruit ripening in the hillawi date palm (Phoenix dactylifera L.) . J. Sci. Food Agr. , 72 : 306 – 308 .
- Abdel-latif , S.A. 1988 . The physiology of ripening of date palm fruit (Phoenix dactylgera L.) , Baghdad, , Iraq : M.Sc. Thesis, Baghdad University . in Arabic with English summary
- Ahmed , I.A. , Ahmed , A.K. and Robinson , R.K. 1995 . Chemical composition of date varieties as influenced by the stage of ripening . Food Chem. , 54 : 305 – 309 .
- Andrews , P.K. and Li , S. 1995 . Cell wall hydrolytic enzyme activity during development of nonclimacteric sweet cherry (Prunus avium L.) fruit . J. Hort. Sci. , 70 : 561 – 567 .
- Ashmawi , H. , Aref , H. and Hussein , A. 1956 . Compositional changes in Zagloul dates throughout the different stages of maturity . J. Sci. Food Agr. , 7 : 625 – 628 .
- Awad , M.A. 2007 . Increasing the rate of ripening of date palm fruit (Phoenix dactylifera L.) c.v. ‘Helali’ by preharvest and postharvest treatments . Postharvest Biol. Technol. , 43 : 121 – 127 .
- Brummell , D.A. 2006 . Cell wall disassembly in ripening fruit . Funct. Plant Biol. , 33 : 103 – 119 .
- Chang , W.H. and Hwang , Y.J. 1990 . Effect of ethylene treatment in ripening, polyphenol oxidase activity and water soluble tannin content of Taiwan northern banana at different maturity stages and the stability of the banana polyphenoloxidase . Acta Hort. , 275 : 603 – 610 .
- Chao , C.T. and Krueger , R.R. 2007 . The date palm (Phoenix dactylifera L.): Overview of biology, uses, and cultivation . HortScience , 42 : 1046 – 1311 .
- Chapman , H.D. and Pratt , F.P. 1961 . Methods of analysis for soils, plants, and water , Riverside, CA : University of California, Citrus Exp. Stat .
- Coggins , C.W. and Knapp , J.C.F. 1969 . Growth, development and softening of the Deglet Noor date fruits . Date Growers’ Ins. Rept. , 46 : 11 – 14 .
- Dubois , M. , Gilles , K.A , Hamilton , J.K. , Rebers , P.A. and Smith , F. 1956 . Colorimetric method for determination of sugars and related substances . Anal. Chem. , 28 : 350 – 356 .
- Glasner , B. , Botes , A. , Zaid , A. and Emmens , J. 1999 . “ Date harvesting, packing house management, and marketing aspects, p ” . Edited by: Zaid , A. and Arias , E.J. 177 – 198 . Date palm cultivation. FAO Plant Production and Protection Paper, No. 156. Publishing Management Service, Information Division, FAO, Rome, Italy .
- Hall , C.B. 1987 . Firmness of tomato fruit tissues according to cultivar and ripeness . J. Amer. Soc. Hort. Sci. , 112 : 663 – 665 .
- Hasegawa , S. and Smolensky , D.C. 1971 . Cellulase in dates and its role in fruit softening . J. Food Sci. , 36 : 966 – 967 .
- Hasegawa , S. , Maier , V.P. , Kaszycki , H.P. and Crawford , J.K. 1969 . Polygalacturonase content of dates and its relation to maturity and softness . J. Food Sci. , 34 : 527 – 529 .
- Hussein , F. , Moustafa , S. and El-Zeid , A. 1976 . Compositional changes during growth and ripening of Barhee and Sukkari dates grown in Saudi Arabia . Indian J. Hort. , 3 : 45 – 53 .
- Mercadoa , J.A. , Trainotti , L. , Jiménez-Bermúdezc , L. , Santiago-Doménecha , N. , Poséa , S. , Donollia , R. , Barcelóc , M. , Casadorob , G. , Pliego-Alfaroa , F. and Quesadaa , M.A. 2010 . Evaluation of the role of the endo-B-(1,4)-glucanase gene FaEG3 in strawberry fruit softening . Postharvest Biol. Technol. , 55 : 8 – 14 .
- Miller , G.L. 1959 . Use of dinitrosalicylic acid reagent for the determination of reducing sugar . Anal. Chem. , 31 : 426 – 429 .
- Mohamed , S.A. 2004 . Carbohydrate metabolism during development of apricot fruit. Partial purification and characterization of pectin methylesterase . Bul. NRC. Egypt , 29 : 379 – 398 .
- Mustafa , A.B. , Harper , D.B. and Johnston , D.E. 1986 . Biochemical changes during ripening of some Sudanese date varieties . J. Sci. Food Agr. , 37 : 43 – 53 .
- Pathak , N. and Sanwal , G.G. 1998 . Multiple forms of polygalacturonase from banana fruits . Phytochemistry , 48 : 249 – 255 .
- Pesis , E. , Fuchs , Y. and Zaubermann , G. 1978 . Cellulase activity and fruit softening in avocado . Plant Physiol. , 61 : 416 – 419 .
- Prochazkova , D. , Sairam , R.K. , Srivastava , G.C. and Singh , D.V. 2001 . Oxidative stress and antioxidant activity as the basis of senescence in maize leaves . Plant Sci. , 161 : 765 – 771 .
- Rouhani , I. and Bassiri , A. 1976 . Changes in the physical and chemical characteristics of Shahani dates during development and maturity . J. Hort. Sci. , 51 : 489 – 494 .
- Sairam , R.K. , Deshmukh , P.S. and Shukla , D.S. 1997 . Tolerance to drought and temperature stress in relation to increased antioxidant enzyme activity in wheat . J. Agron. Crop Sci. , 178 : 171 – 177 .
- Sawaya , W.N. , Khatchadourian , H.A. , Khalil , J. K. , Safi , W.M. and Al-Shalhat , A. 1982a . Growth and compositional changes during the various developmental stages of some Saudi Arabian date cultivars . J. Food Sci. , 47 : 1489 – 1497 .
- Sawaya , W.N. , Safi , W.M. , Khalil , J.K. and Mashadi , A.S. Physical measurements, proximate analysis, and nutrient elements content of twenty-five date cultivars grown in Saudi Arabia at the khalal (mature color) and tamer (ripe) stages . Proceeding of the First International Symposium on the Date Palm . Al-Hassa, Saudi Arabia. pp. 454 – 467 . King Faisal University .
- Sawaya , W.N. , Khalil , J.K. , Khatchadourian , H.A. , Safi , W.M. and Mashadi , A.S. Sugars, tannins and some vitamins contents of twenty-five date cultivars grown in Saudi Arabia at the khalal (mature color) and tamer (ripe) stages . Proceeding of the First International Symposium on the Date Palm . Al-Hassa, Saudi Arabia. pp. 468 – 478 . King Faisal University .
- Shabana , H.R. , Benjamen , N.D. and Mohammed , S. 1981 . Pattern of growth and development in date palm fruit . Date Palm J. , 1 : 31 – 42 .
- Singh , R. , Singh , P. , Pathak , N. , Singh , V.K. and Dwivedi , U.N. 2007 . Modulation of mango ripening by chemicals: Physiological and biochemical aspects . Plant Growth Regulat. , 53 : 137 – 145 .