Abstract
The stability of transgenes was investigated in highbush blueberry cultivars transformed with either gus A (and npt II for selection with kanamycin) in ‘Aurora’ or bar in ‘Legacy’. Transgenic ‘Aurora’ shoots were cultured on selection medium with 50 mg L−1 kanamycin and non-selection medium separately for 5 years. They showed no apparent morphological differences in comparison to non-transgenic shoots. Histochemical GUS staining revealed expression of gus A in all 19 transgenic events regardless of the culture media. Three-year old bar-transgenic ‘Legacy’ plants were grown in the greenhouse and also showed a normal phenotype compared to non-transgenic plants. Presence of the bar gene was detected by polymerase chain reaction in all young leaf samples derived from six transgenic events. Herbicide tolerance was observed in transgenic plants after application of 750 mg L−1 glufosinate ammonium through leaf painting or whole plant spraying. For both gus A and bar, there was no evidence that the transgenes were unstable in transgenic blueberry plants.
INTRODUCTION
Vaccinium fruits are health-promoting foods that contain relatively high antioxidant and anti-inflammatory capacities (CitationPrior et al., 1998; CitationEhlenfeldt and Prior, 2001; CitationConner et al., 2002a, Citation2002b; CitationZheng and Wang, 2003; CitationUSDA-ARS, 2007). Of the three major domesticated Vaccinium fruit crops (blueberry, cranberry, and lingonberry), highbush blueberry is by far the most important commercial crop (CitationGalletta and Ballington, 1996; CitationLyrene et al., 2003; CitationHancock et al., 2008).
Genetic transformation provides a powerful approach to supplement conventional methods for blueberry breeding by introducing individual genes of interest. Blueberry is susceptible to infection by Agrobacterium tumefaciens strains (CitationRowland, 1990; CitationCao et al., 1998), and A. tumefaciens-mediated transformation is the only reported approach that has yielded transgenic blueberry plants (CitationGraham et al., 1996; CitationSong and Sink, 2004, 2006; CitationSong et al., 2007). As a consequence of random insertion of transgenes or somaclonal mutations associated with tissue culture, it is a common phenomenon that primary transformants show variations in either phenotype or expression levels of transgenes. Accordingly, desirable transgenic events with stable transgene expression must be selected after plant transformation. Unintended instability of transgenes or endogenous gene(s) is a prominent concern for crop improvement via genetic engineering (CitationAhuja, 2009). Investigation of transgene(s) stability is, therefore, a necessary step before commercializing any transgenic crop.
Field trials were conducted to evaluate the performance of transgenic highbush blueberry cv. Legacy plants with herbicide resistance during 2005–2007 (CitationSong et al., 2008), but to date, no transgenic plants of any Vaccinium species have been commercialized. Investigation of transgene stability has not been reported. In this study, transgene stability in transgenic blueberry plants with the ß-glucuronidase (gusA) or the bialaphos resistance gene (bar) was investigated.
MATERIALS AND METHODS
Plant Materials
Northern highbush blueberry ‘Aurora’ was transformed with the neomycin phosphotransferase gene (nptII) and an intron interrupted gusA directed by the chimeric super promoter (Aocs)3AmasPmas in 2003 using A. tumefaciens-mediated transformation (CitationSong and Sink, 2004). Nineteen transgenic events were investigated. In vitro shoots for each transgenic event were subcultured at 8-week intervals for 5 years separately on kanamycin (Km) (50 mg L−1) selection and non-selection proliferation medium containing WPM salts (CitationLloyd and McCown, 1980) with MS vitamins (CitationMurashige and Skoog, 1962) + 4.0 mg L−1 zeatin, 2% sucrose, and solidified with 0.6% Bacto-agar, pH 5.2 (herein WPM4Z).
The southern highbush blueberry ‘Legacy’ was transformed with the chimeric bar gene driven by either the nos or the CaMV 35S promoter (CitationSong et al., 2007, 2008). In vitro shoots of six transgenic events, including three for each of the 35S-bar and the nos-bar, were cultured on WPM4Z + 0.5 mg L−1 glufosinate ammonium (GS) for 4 years.
All the cultures were maintained under a 16 h photoperiod of 30 μE m−2 s−1 at 25°C. All basal media were autoclaved at 121°C for 20 min at 105 kPa. Stock solutions of zeatin, Km, and GS were filter-sterilized (0.22 μm Millipore filters; Millipore Corporation, Bedford, MA, USA), and added to media cooled to 50–60°C after autoclaving.
Rooting and greenhouse care of transgenic plants were performed as described in a previous report (CitationSong and Sink, 2006; CitationLiu et al., 2010). Three-year-old plants, including one for each of the 19 transgenic events with gusA and three for each of the six events with bar, were grown in a non-heated greenhouse. Non-transformed plants of ‘Aurora’ and ‘Legacy’ were grown as controls.
Histochemical GUS Assay
The histochemical GUS assay was modified from CitationJefferson et al. (1987). All tissues were washed with 100 mM phosphate buffer and stained in X-Gluc (2 mM X-Gluc, 100 mM phosphate buffer, 100 μM EDTA and 100 mM Triton X-100) overnight at 37°C. After staining, the chlorophyll was removed in 70% ethanol washes.
Polymerase Chain Reaction (PCR)
Genomic DNA was isolated from young leaf tissues of greenhouse-grown plants transformed with bar. Three leaf samples from each plant were randomly collected from separate branches. PCR was carried out on 100–150 ng DNA per reaction. The primers corresponding to a 563 bp bar fragment were 5′- tct aga atg agc cca gaa cga cgc c-3′ (forward primer) and 5′- gga tcc tca gat ctc ggt gac gg-3′ (reverse primer). The reaction conditions were: 94°C for 2 min, 35 cycles of 45 sec at 94°C, 60 sec at 58°C, and 90 sec at 72°C, with a final 10 min extension at 72°C. PCR products were electrophoresed on 1% agarose gel and visualized under UV light after staining with ethidium bromide.
Herbicide Resistance Assays
Following previous experiments on 1-year old plants (CitationSong et al., 2008), three 3-year-old plants for each of the six transgenic events with the bar gene, including three events with nos-bar and three with 35S-bar, were evaluated for herbicide resistance. Non-transgenic ‘Legacy’ plants were used as a control. Prior to the herbicide spraying experiment, six expanding leaves from three randomly selected branches of each plant were painted with 750 mg L−1 of GS (Bayer CropScience, Research Triangle Park, NC, USA). Injury to the leaves was photographed weekly. Whole plant sprays with GS at 750 mg L−1 were carried out using a track sprayer. Leaves from two randomly selected branches of each plant were scored for herbicide tolerance. Herbicide injury to each leaf was scored from 0–10 according to the percentage of dead tissue: 0—no injury; 10—90 to 100% of the leaf brown.
RESULTS AND DISCUSSION
Phenotype Stability in Transgenic Plants with the Reporter Genes
Phenotypically, no apparent variation was observed between 3-year-old transgenic and non-transgenic plants. In the greenhouse, transgenic plants of both ‘Aurora’ and ‘Legacy’ showed a normal phenotype; they flowered and set fruits normally (). In field trials, no obvious variations in appearance were found between all 96 transgenic plants from four independent transgenic events and the 24 non-transgenic plants (CitationSong et al., 2008); investigations on flowering and fruiting was not carried out during field trials, as flowers were removed to prevent the flow of transgenic pollen. These trials are now terminated.
FIGURE 1 Three-year-old transgenic and non-transgenic blueberry plants cv. Legacy in the greenhouse (color figure available online).

Morphological variation in transgenic plants may be caused by differential transgene expression or position effects of transgenes as well as somaclonal variation associated with tissue culture. The expression levels of the reporter genes, such as nptII, gusA, or bar, are generally not responsible for any morphological alteration in transgenic blueberry plants. However, random insertion of transgenes and somaclonal variation may result in altered morphology of transgenic plants. In this study, none of the transgenic blueberry plants from 25 separate events showed apparent morphological differences compared to non-transgenic plants. This indicates that transformation and expression of reporter genes has little impact on the morphology of transgenic blueberry.
Stability of gusA
The generation of somatic mutations unrelated to the inserted transgene is one of the major concerns associated with plant transformation; however, none were observed in any of the previous studies using A. tumefaciens-mediated transformation of leaf explants. Over 90 transgenic events were obtained via organogenesis from transformation of three northern and one southern highbush blueberry cultivars (CitationSong and Sink, 2004, 2006). No chimeric transformants were detected; all the shoots from the different transgenic events proliferated on WPM4Z containing 20 mg L−1 Km (a level that inhibits non-transgenic shoots from proliferating). Additionally, all sampled shoots from each event were GUS-positive, although the blue staining varied among and within different tissues, such as among the leaves of the same shoot (CitationSong and Sink, 2004).
Since the selection pressure included in the culture medium might prevent identification of chimeric transformants, further investigation was carried out by comparing GUS expression in transgenic shoots cultured on selection versus non-selection medium for 5 years. No chimeric transformants were observed in any of the 19 transgenic events tested. For each transgenic event, no individual shoot or shoot clusters were fully GUS-negative without showing any blue staining; in addition, GUS staining in the shoots cultured on Km-selection medium had a similar pattern to those cultured on non-selection medium (). These results indicate that A. tumefaciens-mediated transformation of leaf explants is a reliable approach for production of putative transgenic plants of blueberry.
FIGURE 2 Histochemical GUS assay in transgenic blueberry cv. Aurora: (A) Shoots cultured on Km-selection medium (upper wells) and non-selection medium (lower wells); (B) 6-month-old plants; (C) root parts of one transgenic and one non-transgenic as in B but at higher magnification; (D) shoot tips; (E) mature leaves; (F) immature flowers; (G) mature flowers; and (H) immature fruits. NT: Non-transformant. A1, A2, A6, and A9: Independent transgenic events. White arrows show the areas with blue staining (color figure available online).
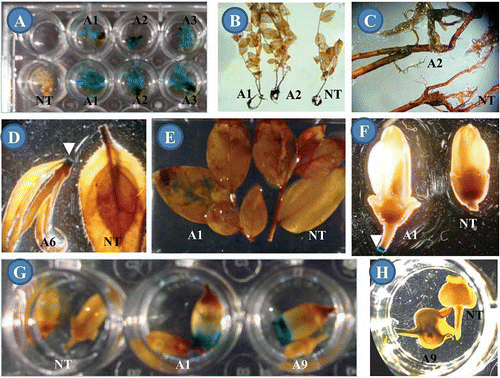
While it was an effective approach for screening transformed cells at in vitro culture stages, the histochemical GUS assay was not a reliable method for detection of the gusA expression in in vivo cultured transformed tissues. It is likely that endogenous compounds in blueberry plants inhibited GUS detection either by direct interference in the enzymatic reaction between ß-glucuronidase and the substrate, or by masking the blue color once it was produced.
Histochemical GUS assays on 6-month-old plants from putative transgenic events produced blue staining in young root tissues and newly wounded areas of shoot tips, but this blue color was absent in any non-transgenic tissues (–D). All 3-year-old, greenhouse-grown plants transformed with gusA were GUS positive with visible blue staining observed in transgenic stems, leaves, flowers, and fruits; and blue staining at cut edges of shoot tips, leaves, and peduncle was often more intense (–G). Faint blue color was present in immature fruits (). In intact mature transgenic leaves, blue staining was occasionally observed at random and unpredictable locations (). Similarly, the random and unpredictable area of histochemical GUS staining was also reported in transgenic leaf tissues of another Vaccinium species-cranberry (V. macrocarpon Ait.), where the results of GUS assays were found to be very unreliable in interpreting the gusA expression due to the endogenous inhibitors of GUS detection (CitationSerres et al., 1997). Despite the difficulty in using GUS assays to evaluate the gusA expression in blueberry, GUS staining detected in tissues from all 19 transgenic events still indicated that transgenes were stably carried in in vivo plants.
Stability of bar
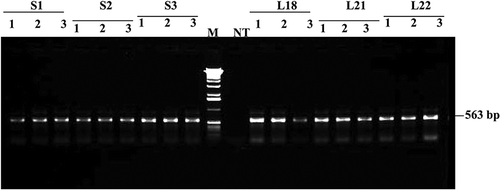
For the transgenic events with the bar gene, in vitro cultured shoots of all six events had consistent levels of resistance to 0.5 mg L−1 of GS during subculturing. Leaf painting with 750 mg L−1 GS on 3-year-old plants confirmed herbicide resistance in all bar-containing transgenic plants (). Consistent with a previous report on 1-year-old plants, variations in herbicide resistance were observed among the different transgenic events when the GS was applied through whole plant spraying; and the 35S-bar yielded higher levels of herbicide resistance than the nos-bar (data not shown) (CitationSong et al., 2008). The presence of a 563-bp fragment of the bar gene was detected by PCR in all samples from six bar-containing transgenic events; in contrast, the 563-bp band was not found in non-transgenic samples (). These results suggest that the bar gene was stable in all the selected transgenic events.
FIGURE 3 Detection of bar gene-based herbicide resistance in blueberry cv. Legacy, 1 week following leaf painting assay with 750 mg L−1 glufosinate ammonium. S1–S3: Independent transgenic events with 35S-bar. NT: Non-transformant (color figure available online).
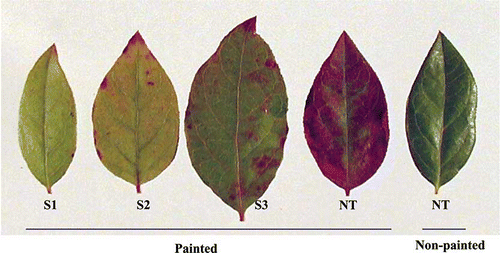
FIGURE 4 PCR amplification of a 563-bp fragment of the bar gene in randomly sampled leaf tissues of 3-year-old blueberry cv. Legacy. Lanes 1–3: Three randomly selected samples for each plant. S1–S3: Trangenic events with the 35S-bar. M: 1 Kb λ DNA marker. NT: Non-transformant. L18, L21, and L22: Transgenic events with the nos-bar.
CONCLUSIONS
Overall, based on qualitative analyses of gusA or bar, there was no evidence of transgene instability in long-term cultured, in vitro and in vivo tissues of 25 independent transgenic events with blueberries.
LITERATURE CITED
- Ahuja , M.R. 2009 . Transgene stability and dispersal in forest trees . Trees , 23 : 1125 – 1135 .
- Cao , X. , Liu , Q. , Rowland , L.J. and Hammerschlag , F.A. 1998 . GUS expression in blueberry (Vaccinium spp.): Factors influencing Agrobacterium-mediated gene transfer efficiency . Plant Cell Rep. , 18 : 266 – 270 .
- Conner , A.M. , Luby , J.J. and Tong , C.B.S. 2002a . Variability in antioxidant activity in blueberry and correlations among different antioxidant assays . J. Amer. Soc. Hort. Sci. , 127 : 238 – 244 .
- Conner , A.M. , Luby , J.J. , Tong , C.B.S. , Finn , C.E. and Hancock , J.F. 2002b . Genotypic and environmental variation in antioxidant activity, total phenolics and anthocyanin content among blueberry cultivars . J. Amer. Soc. Hort. Sci. , 127 : 89 – 97 .
- Ehlenfeldt , M.K. and Prior , R.L. 2001 . Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry . J. Agri. Food Chem. , 49 : 2222 – 2227 .
- Galletta , G.J. and Ballington , J.R. 1996 . “ Blueberries, cranberries and lingonberries ” . In Vine and Small Fruit Crops , Edited by: Janick , J. and Moore , J.N. Vol. II , 1 – 107 . New York : John Wiley and Sons . Fruit breeding
- Graham , J. , Greig , K. and McNicol , R.J. 1996 . Transformation of blueberry without antibiotic selection . Ann. Appl. Biol. , 128 : 557 – 564 .
- Hancock , J.F. , Lyrene , P. , Finn , C.E. , Vorsa , N. and Lobos , G.A. 2008 . “ Blueberries and cranberries ” . In Temperate Fruit Crop Breeding , Edited by: Hancock , J.F. 115 – 149 . New York : Springer .
- Jefferson , R.A. , Kavanagh , T.A. and Bevan , M.W. 1987 . GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants . EMBO J. , 6 : 3901 – 3907 .
- Liu , C. , Callow , P. , Rowland , L.J. , Hancock , J.F. and Song , G.-Q. 2010 . Adventitious shoot regeneration from leaf explants of southern highbush blueberry cultivars . Plant Cell Tiss. Org. Cult. , doi: 10.1007/s11240-010-9766-9
- Lloyd , E. and McCown , B. 1980 . Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture . Proc. Intl. Plant Prop. Soc. , 30 : 421 – 427 .
- Lyrene , P.M. , Vorsa , N. and Ballington , J.R. 2003 . Polyploidy and sexual polyploidization in the genus Vaccinium . Euphytica , 133 : 27 – 36 .
- Murashige , T. and Skoog , F. 1962 . A revised medium for rapid growth and bioassay with tobacco tissue cultures . Physiol. Plant , 15 : 473 – 497 .
- Prior , R.L. , Cao , G. , Martin , A. , Sofic , E. , McEwen , J. , ’Brien , C. O , Lischner , N. , Ehlenfeldt , M. , Kalt , W. , Krewer , G. and Mainand , C.M. 1998 . Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species . J. Agr. Food Chem. , 46 : 2686 – 2693 .
- Rowland , L.J. 1990 . Susceptibility of blueberry to infection by Agrobacterium tumefaciens . HortScience , 25 : 1659
- Serres , R. , McCown , B. and Zeldin , E. 1997 . Detectable β-glucuronidase activity in transgenic cranberry is affected by endogenous inhibitors and plant development . Plant Cell Rep. , 16 : 641 – 647 .
- Song , G.-Q. and Sink , K.C. 2004 . Agrobacterium tumefaciens-mediated transformation of blueberry (Vaccinium corymbosum L.) . Plant Cell Rep. , 23 : 475 – 484 .
- Song , G.-Q. and Sink , K.C. 2006 . Agrobacterium-mediated transformation of highbush blueberry (Vaccinium corymbosum L.) cultivars , 2nd , Edited by: Wang , K. and Agrobacterium Protocols . 37 – 44 . Humana , , Totowa, NJ : Methods in Molecular Biology 344 .
- Song , G.-Q. , Sink , K.C. , Callow , P.W. , Baugham , R. and Hancock , J.F. 2008 . Evaluation of different promoters for production of herbicide-resistant blueberry plants . J. Amer. Soc. Hort. Sci. , 133 : 605 – 611 .
- Song , G.-Q. , Roggers , R.A. , Sink , K.C. , Particka , M. and Zandstra , B. 2007 . Production of herbicide-resistant highbush blueberry ‘Legacy’ by Agrobacterium-mediated transformation of the Bar gene . Acta Hort. , 738 : 397 – 407 .
- USDA-ARS. 2007. Oxygen radical absorbance capacity of selected foods—2007. http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/ORAC/ORAC07.pdf (http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/ORAC/ORAC07.pdf) (Accessed: 26 March 2009 ).
- Zheng , W. and Wang , S.Y. 2003 . Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries . J. Agr. Food Chem. , 51 : 502 – 509 .