Abstract
Four concentrations of salicylic acid (0, 1, 2, and 4 mM) were applied postharvest to ‘Bidaneh Ghermez’ grapes (Vitis vinifera); to investigate their potential impact on the fruit quality and storage life. They were stored for up to 45 days at 0°C, followed by 2 days at 20°C. The results indicated that postharvest treatment of grapes with salicylic acid could potentially improve berry and cluster appearance while significantly reducing fruit quality deterioration. Fruits treated with salicylic acid had greener and softer stems, and at the same time decay incidence was more efficiently controlled.
INTRODUCTION
Table grapes are non-climacteric fruits with a low rate of physiological activity. Postharvest quality of table grapes is affected by stem and rachis browning, softening, water loss, berry shatter, and decay, which is caused mainly by Botrytis cinerea (CitationChen et al., 2006). While the predominant method of controlling fungi in grapes is SO2 fumigation, this treatment during storage has been proven to be harmful (CitationArtés-Hernández et al., 2004), therefore, such applications of SO2 have been restricted in many countries. Hence, there is a tendency to find natural alternatives for postharvest treatment of fresh production.
Salicylic acid (SA) is a simple phenolic compound that plays a substantial role in many processes of plant growth and development (CitationRaskin, 1992a, Citation1992b). Postharvest application of SA at non-toxic concentrations has been shown to be affective at (a) retarding ripening and softening of banana (CitationSrivastava and Dwivedi, 2000) and kiwi (CitationZhang et al., 2003); (b) reducing chilling injury of loquat (CitationCai et al., 2006), tomato (CitationDing et al., 2007), peach (CitationWang et al., 2006), and sweet peppers (CitationFung et al., 2004); and (c) improving quality characteristics of strawberry fruits (CitationBabalar et al., 2007; CitationShafiee et al., 2010). SA's role as an essential signal molecule has been well documented in the defense reactions and particularly induction of local and systemic resistance response against pathogen attack (CitationHayat et al., 2010). Recently, it has been discovered that exogenous application of SA could enhance resistance to pathogens and control postharvest decay in sweet cherry (CitationXu and Tian, 2008), strawberry (CitationBabalar et al., 2007; CitationShafiee et al., 2010) and peach fruits (CitationWang et al., 2006). These results showed that SA could be introduced as a potent alternative to chemicals.
‘Bidaneh Germez’ grape is a seedless and red-skinned cultivar. It is one of the most popularly consumed grapes in Iran. The main purpose of the present study was to determine postharvest treatment of SA in maintaining table grapes’ quality during the storage period.
MATERIALS AND METHODS
Plant Material and Treatments
‘Bidaneh Ghermez’ grapes (a seedless red-skinned cultivar) were harvested at their commercial maturity (total soluble solid [TSS] > 22 °Brix) during the 2008–2009 growing season in Hamedan, Iran. Fruits were immediately transported to the laboratory of the Department of Horticultural Science, and were sorted based on their berry size. Then, those without any physical injuries or apparent decay were selected and randomly divided, to be used in research's different treatments. Later, clusters were selected to obtain 48 samples and were divided into 4 groups per replicate. The grapes were then treated by dipping them into a solution of distilled water (as control), 1, 2, and 4 mM SA containing 0.05% Tween-20 for 5 min. After treatment, all clusters were left to dry at room temperature before being stored for 45 days at 0 ± 0.5°C temperature plus 2 days at 20°C, as a shelf-life (SL) indicator. In each replicate, one cluster was allotted for water loss, decay incidence, berry shatter, rachis browning, and visual berry appearance monitoring, while the others remained for the rest of the experiment. Finally, the measurements were made on the 15th, 30th, and 45th day of the storage period, plus at the end of the SL.
Determination of Fruit Quality Parameters
Soluble solids content (SSC), titratable acidity (TA), and firmness of ten berries were determined as follows: SSC was calculated using an Atago refractometer (N1, Atago Co., Tokyo, Japan) at a temperature of 20°C. TA of diluted juice (1:10 with distilled water) was ascertained by titration with 0.1 N sodium hydroxide to a pH of 8.2. TA was calculated as percentage of tartaric acid.
Data on flesh firmness was established using a hand-held penetrometer equipped with a plunger (6 mm in diameter) (FDK; Wagner Instruments, Greenwich, CT, USA) on peeled cheeks of berries.
Water Loss and Berry Drop
To measure water loss, fruits were weighted before, during, and after the storage periods. Percentages of weight loss were collated to be used as data from this stage of investigation. Berry drop for each box was quantified by subtracting freed berries’ weight from total grapes’ weight, and reported as percentage of shatter.
Fruit Decay
Decay incidence was assessed according to a subjective scale of: no decay, up to 5 decayed, up to 10 decayed, up to 20 decayed, and eventually over 20 decayed berries per bunch were quantified as 1, 2, 3, 4, and 5, respectively (CitationLurie et al., 2006).
Rachis Browning and Berry Appearance
Rachis condition was scored based on a 5-point scale in which 1 meant healthy, 2 implied slightly, 3 stood for moderate, and 4 indicated severe damage (Crisisto et al., 2002). Visual appearance of berries was rated according to CitationXu et al. (2007), shown in the following scale: 1 = excellent condition, 2 = good, 3 = slightly dull, 4 = less than 50% brownish and soft, and 5 = more than 50% brownish and soft.
Total Phenolic Content
The Folin-Ciocalteau method (CitationSlinkard and Singleton, 1977)for colorimetric measurement of total phenols was utilized. Phenolic compounds were extracted from skins of 20 berries of three clusters using MeOH/HCl (99:1 v/v). First, the skins were powdered in liquid nitrogen using mortar and pestle, and then stored at a temperature of −24°C until further investigation. Later in the lab, 0.5 g of powdered skins were weighed and extracted with 3 ml of solvent. This homogenate was filtered through a Whatman no. 1 paper filter. Afterwards, 300 μl of Filterate was thoroughly mixed with 1.5 ml of Folin-Ciocalteau reagent. After 5 min incubation at room temperature, 1.2 ml of 7% sodium carbonate was added. Solutions were shaken at room temperature and held in darkness for about one and a half hours. The absorbance was measured at 765 nm with a UV/vis spectrophotometer (Carry 100, Varian Analytical Instruments, Walnut Creek, CA, USA). Finally, values were quantified using the standard curve generated with different concentrations of gallic acid, and reported as mg gallic acid equivalents per g of fresh weight (FW) ± SD.
Total Anthocyanin Content
Total anthocyanin content (TAC) of powdered grape skins was quantified using a pH differential method (CitationDiamanti, 2008). The absorbance was measured at 520 and 700 nm with an UV/vis spectrophotometer (Carry 100, Varian Analytical Instruments, Walnut Creek, CA, USA). Then, the TAC was calculated and expressed as mg malvidin 3-glucoside equivalent per gram of FW (mg/g).
Statistical Analysis
All data were subjected to analysis of variance (ANOVA). Means were compared by a Duncan's multiple range test at a significance level of 0.05. All of the analyses were performed with SAS software (version 9.1, 2002–2003, SAS Institute Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
TSS and TA
TSS for all treatments increased at the beginning of the storage, but later slightly decreased. The initial increases in soluble solids over the storage period could be due to weight loss and, therefore, fruit juice concentration (CitationMoreno et al., 2008), while later reductions, particularly in SL, might be related to an increase in respiratory activities.
TA increased slightly in the first 30 days and then reduced considerably until the 45th day. Afterwards, there was a negligible reduction at the SL period again. However, the differences were not significant among treatments (P > 0.05).
In this study, and in comparison with the control, SA treatments did not change the total soluble solids or titratable acidity, which is in agreement with results reported by CitationSayyari et al. (2009) and CitationDing et al. (2007).
Firmness and Water Loss
Flesh firmness decreased during the storage period as expected, while SA treatment only at highest concentration significantly delayed the process. After 2 days of shelf-life, clusters treated with the highest dose (4 mM) significantly retained their firmness ().
FIGURE 1 Effects of SA treatment on fruit firmness (a) and decay index (b) of Bidaneh Ghermez grapes stored at 0°C for 45 days, followed by 2 days shelf-life. The bars represent standard errors (n = 3) of the means followed by different letters showing significance according to Duncan Multiple Range Test at P < 0.05 (color figure available online).
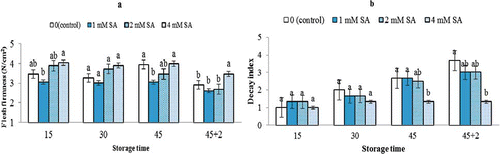
Retention of flesh firmness as a result of SA treatment has been proven in several crops. Fruit application of SA has been shown to be effective in retarding softening in the peach (CitationWang et al., 2006), kiwifruit (CitationZhang et al., 2003), and banana (CitationSrivastava and Dwivedi, 2000). It has been previously documented (CitationAsghari and Soleimani Aghdam, 2010) that during ripening, SA decreases ethylene production and inhibits major enzymes, such as polygalacturonase (PG), lipoxygenase (LOX), cellulose, and ectinemethylesterase (PME), leading to decreased fruit softening, that eventually affects fruit firmness. Moreover, CitationLeshem et al. (1986) reported that softening of a fruit can be related to the production of free radicals as a result of senescence and its effect on the cell wall. The explanation for maintaining fruit firmness could be related to inhibitory effects of SA on LOX activity, leading to reduction in the production of free radicals (CitationZhang et al., 2003). In this experiment, SA was shown to have no effect on water loss (data not shown). The rate of water loss noticeably increased with the storage time, as expected.
Decay
In the present study, it was shown that SA treatment at 4 mM was effective in preventing decay incidence of grapes in both cold storage and during the SL period. After 2 days at 20°C, the control clusters had a score of 3.7, whereas in 4 mM, SA treatment decay was 1.3. Generally speaking, SA treatment at 4 mM has significantly suppressed any development of decays, reaching a minimum score of 1.33 on day 45 + 2 ().
These were similar to the findings obtained by CitationWang et al. (2006) on peach, CitationXu and Tian (2008) on sweet cherry, and CitationBabalar et al. (2007) on strawberry. Previous research results have indicated that SA treatment is probably effective in inducing a defense system (CitationChan and Tian, 2006)by enhancing activities of antioxidant enzymes (CitationXu and Tian, 2008), resulting in improved resistance against fungal attack in treated fruits. It also has been reported that SA pretreatment could activate defensive enzymes in young pear fruit (CitationCao et al., 2006). These studies indicate a key role of SA in activating fruit defense responses against any fungal contamination.
Shattering
Berry shatter index after 45 days of cold storage was similar to the control regardless of treatment. It was significantly controlled during the SL period among grapes treated with 4 mM SA.
CitationDeng et al. (2006) indicated that ethylene accompanied by falling auxin levels stimulates berry drop. Likewise, the inhibitory effects of SA on both ethylene production and action in harvested fruit has been proven (CitationSrivastava and Dwivedi, 2000; CitationZhang et al., 2003). Accordingly, SA by interfering with biosynthesis and/or action of ethylene could restrict berry shatter. In addition, some evidence has shown that the low incidence of shattering observed in SA-treated berries is related to the antifungal effects of SA (CitationXu et al., 2007, CitationZoffoli et al., 2009). It has to be noted that ‘Bidaneh Ghermez’ grape is susceptible to shattering.
Rachis Browning
During the 45 days of cold storage and the following 2 days of SL, there was a significant difference in rachis browning between SA-treated fruits and the control. Rachis browning in fruits treated with 4 mM SA was not observed during the 47 days of the study (). Moreover, control fruits had an unacceptable appearance compared to 4 mM SA-treated ones at the end of the 2 day SL period. No change in visual appearance of treated fruits in 4 mM SA was observed after 45 days of cold storage and the following SL ().
FIGURE 2 Effects of SA treatment on rachis browning (a) and berry appearance (b) of Bidaneh Ghermez grapes stored at 0°C for 45 days, followed by 2 days shelf-life. The bars represent standard errors (n = 3) of the means followed by different letters showing significance according to Duncan Multiple Range Test at P < 0.05 (color figure available online).
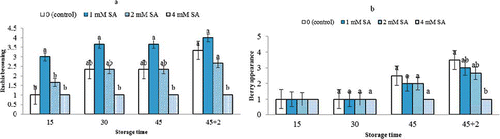
According to the current results, it appears that at low concentrations (1 mM) SA could stimulate related enzymes, unlike previous studies on the Chinese water chestnut, in which CitationPeng and Jiang (2006) reported that application of SA increased PPO and POD activities significantly only at high concentrations in vitro. One of the major concerns with regard to quality loss and marketability of table grapes is rachis and berry browning. It is well known that tissue browning can be related to phenolic-related enzymes, polyphenol oxidase (PPO), peroxidase (POD), and phenylalanine ammonia lyase (PAL) activities. CitationPeng and Jiang (2006) demonstrated that SA treatment inhibits the activity of the above mentioned enzymes and consequently stops browning in fresh-cut Chinese water chestnut. CitationCai et al. (2006) reported that acetylsalicylic acid (a derivative of salicylic acid) reduced PAL and G-POD activities, and also postharvest tissue browning of loquat fruit stored at 0°C. In our study, SA-treated fruits in 4 mM had a better visual appearance than the control after both cold storage and the SL period that followed.
Total Phenolic and Total Anthocyanin Content
With the extension of cold storage, in all treatments, total phenolic content (TPC) as well as total anthocyanin content (TAC) declined, and then during the SL period, TPC sharply increased while TAC raised slightly (). TPC in SA-treated fruit showed higher values compared to the control, but its effect was not significant.
FIGURE 3 Effects of SA total phenolic content (a) and total anthocyanin (b) of Bidaneh Ghermez grapes stored at 0°C for 45 days, followed by 2 days shelf-life. The bars represent standard errors (n = 3) of the means (color figure available online).
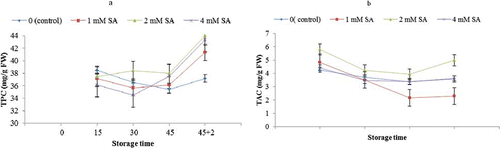
Berry skin compounds and color are important parameters in the quality determination of grapes. In the present study, postharvest application of SA had no significant effect on TPC and TAC during cold storage and the subsequent SL period. CitationChen et al. (2006), working on grape, reported that SA treatment enhanced accumulation of phenolics while it did not affect two of the hydroxybenzoic acid derivatives (protocatechuic and gentisic acid). The results in this study appear to be associated with the crucial role of the related enzymes in phenolic production. However, there is not enough evidence on the effect of postharvest SA treatment on phytochemical properties of grapes with only one report (CitationChen et al., 2006).
In conclusion, this experiment showed the effectiveness of postharvest treatment of SA on ‘Bidaneh Ghermez’ grape fruit quality. It can be concluded from this study that 4 mM postharvest application of SA was mostly effective in maintaining a high level of firmness and in delaying rachis browning after SL. Furthermore, among all four treatments investigated, 4 mM SA was beneficial with regard to visual appearance of berries and could be applied to reduce decay incidence during postharvest storage. However, SA application had no effect on the accumulation of total phenolics and total anthocyanin. In summary, SA can be used as an appropriate alternative to chemicals in postharvest technology of table grapes to assure food safety. The SA grapes could attract health conscious customers and also increase the marketability of fresh fruits.
LITERATURE CITED
- Asghari , M. and Soleimani Aghdam , M. 2010 . Impact of salicylic acid on post-harvest physiology of horticultural crops . Trends Food Sci. Technol , 2 : 502 – 509 .
- Artés-Hernández , F. , Aguayo , E. and Artés , F. 2004 . Alternative atmosphere treatments for keeping quality of ‘Autumn Seedless’ table grapes during long-term cold storage . Postharvest Biol. Technol , 31 : 59 – 67 .
- Babalar , M. , Asghari , M. , Talaei , A. and Khosroshahi , A. 2007 . Effect of pre- and post-harvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit . Food Chem , 105 : 449 – 453 .
- Cai , C. , Li , X. and Chen , K.S. 2006 . Acetylsalicylic acid alleviates chilling injury of post-harvest loquat (Eriobotrya japonica Lindl.) . fruit. Eur. Food Res. Technol , 223 : 533 – 539 .
- Cao , J. , Zeng , K. and Jiang , W. 2006 . Enhancement of postharvest disease resistance in Ya Li pear (Pyrus bretschneideri) fruit by salicylic acid sprays on the trees during fruit growth. Eur . J. Plant Pathol , 114 : 363 – 378 .
- Chan , Z. and Tian , S. 2006 . Induction of H202-metabolizing enzymes and total protein synthesis by antagonist and salicylic acid in harvested sweet cherry fruit . Postharvest Biol. Technol , 39 : 314 – 320 .
- Chen , J.Y. , Wen , P.F. , Kong , W.F. , Pan , Q.H. , Zhan , J.C. , Li , J.M. , Wan , S.B. and Huang , W.D. 2006 . Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries . Postharvest Biol. Technol , 40 : 64 – 72 .
- Crisosto , C.H. , Garner , D. and Crisosto , G. 2002 . Carbon dioxide-enriched atmospheres during cold storage limit losses from Botrytis but accelerate rachis browning of Redglobe table grapes . Postharvest Biol. Technol , 26 : 181 – 189 .
- Deng , Y. , Wu , Y. and Li , Y. 2006 . Physiological responses and quality attributes of ‘Kyoho’ grapes to controlled atmosphere storage . Food Sci. Technol , 39 : 584 – 590 .
- Diamanti , J. 2008 . “ Comparison of analytical methodologies for the evaluation of nutraceutical parameters in strawberries fruits. Marche Polytechnic University ” . In A short term scientific mission (STSM) within the COST 863 Euroberry Research Acona, , Italy
- Ding , Z.S. , Tian , S.P. , Zheng , X.L. , Zhou , Z.W. and Xu , Y. 2007 . Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress . Physiol. Plant , 130 : 112 – 121 .
- Fung , R. , Wang , C. , Smith , D. , Gross , K. and Tian , M. 2004 . MeSA and MeJA increase steady-state transcript levels of alternative oxidase and resistance against chilling injury in sweet peppers (Capsicum annuum L.) . Plant Sci , 166 : 711 – 719 .
- Hayat , Q. , Hayat , S. , Irfan , M. and Ahmad , A. 2010 . Effect of exogenous salicylic acid under changing environment: A review . Environ. Expt. Bot , 68 : 14 – 25 .
- Leshem , Y.Y. , Halevy , A.H. and Frenkel , C. 1986 . Processes and control of plant senescence , Amsterdam : Elsevier .
- Lurie , S. , Pesis , E. , Gadiyeva , O. , Feygenberg , O. , Ben-Arie , R. , Kaplunov , T. , Zutahy , Y. and Lichter , A. 2006 . Modified ethanol atmosphere to control decay of table grapes during storage . Postharvest Biol. Technol , 42 : 222 – 227 .
- Moreno , J.J. , Cerpa-Caldero , F. , Cohen , S.D. , Fang , Y. , Qian , M. and Kennedy , J.A. 2008 . Effect of postharvest dehydration on the composition of Pinot Noir grapes (Vitis vinifera L.) and wine . Food Chem , 109 ( 4 ) : 755 – 762 .
- Peng , L. and Jiang , Y. 2006 . Exogenous salicylic acid inhibits browning of fresh-cut Chinese water chestnut . Food Chem , 94 : 535 – 540 .
- Raskin , I. 1992a . Salicylate, A New Plant Hormone . Plant Physiol , 99 : 799 – 803 .
- Raskin , I. 1992b . Role of salicylic acid in plants . Annu. Rev. Plant Physiol. Plant Mol. Biol , 43 : 439 – 463 .
- Sayyari , M. , Babalar , M. , Kalantari , S. , Serrano , M. and Valero , D. 2009 . Effect of salicylic acid treatment on reducing chilling injury in stored pomegranates . Postharvest Biol. Technol , 53 : 152 – 154 .
- Shafiee , M. , Taghavi , T.S. and Babalar , M. 2010 . Addition of salicylic acid to nutrient solution combined with post-harvest treatments (hot water, salicylic acid, and calcium dipping) improved post-harvest fruit quality of strawberry. Sci . Hort , 124 ( 1 ) : 40 – 45 .
- Slinkard , K. and Singleton , V.L. 1977 . Total phenol analyses: automation and comparison with manual methods . Am. J. Enol. Vitic , 28 : 49 – 55 .
- Srivastava , M.K. and Dwivedi , U.N. 2000 . Delayed ripening of banana fruit by salicylic acid . Plant Sci , 158 ( 1–2 ) : 87 – 96 .
- Wang , L. , Chen , S. , Kong , W. , Li , S. and Archbold , D.D. 2006 . Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage . Postharvest Biol. Technol , 41 : 244 – 251 .
- Xu , X. and Tian , S. 2008 . Salicylic acid alleviated pathogen-induced oxidative stress in harvested sweet cherry fruit . Postharvest Biol. Technol , 49 : 379 – 385 .
- Xu , W.T. , Huang , K.L. , Guo , F. , Qu , W. , Yang , J.L. , Liang , Z.H. and Luo , Y.B. 2007 . Postharvest grapefruit seed extract and chitosan treatments of table grapes to control Botrytis cinerea . Postharvest Biol. Technol , 46 : 86 – 94 .
- Zhang , Y. , Chen , K.S. , Zhang , S.L. and Ferguson , I. 2003 . The role of salicylic acid in post-harvest ripening of kiwifruit . Postharvest Biol. Technol , 28 : 67 – 74 .
- Zoffoli , J.P. , Latorre , B.A. and Naranjo , P. 2009 . Postharvest applications of growth regulators and their effect on postharvest quality of table grapes during cold storage . Postharvest Biol. Technol , 51 : 183 – 192 .