Abstract
Fruit from the annual replicated yield assessments for the USDA-ARS strawberry (Fragaria x ananassa Duchesne ex Rozier) breeding program at Beltsville, MD in 2010 were evaluated for postharvest decay development after storage at 5°C. A statistically significant correlation between percentage decay of fruit in the field and percentage decay of fruit from post-harvest evaluation was observed when data were analyzed on a genotypic mean basis (r = 0.37) or a field plot basis (r = 0.25) across all harvests. Analysis of the same data on a plot by harvest combination basis resulted in a statistically significant correlation for only one harvest date. While significant, the level of correlation on a genotypic mean basis is not strong enough to dismiss the need for post-harvest evaluation. The percentage postharvest decay increased over harvests, while the percentage decay at harvest, in the field, did not. Weather data from 2010 indicated that field conditions just a few days before harvest can affect percentage decay at harvest differently than percentage decay in postharvest storage; rain events were correlated with increased percentage decay in the field but not postharvest decay, while dry air was correlated with decreased percentage decay postharvest but not decay in the field. These findings suggest that, in some environments, conditions after flowering can have a more significant role in the fruit decay than previously has been reported.
Keywords:
INTRODUCTION
Strawberry (Fragaria × ananassa Duchesne ex Rozier) is a highly perishable horticultural fruit crop with a short storage life (CitationMitcham, 2002). The major postharvest disease of strawberry is “Botrytis,” “gray mold,” “ash mold,” or “Botrytis fruit rot,” primarily caused by Botrytis cinerea Pers.:Fr. (CitationCeponis et al., 1987). Development of decay in the field is favored by cool wet conditions. Several cultural practices can be used to reduce percentage decay in the field (CitationSutton, 1998). These, combined with fungicide application, especially during flowering (CitationMertely et al., 2002), reduce both pre-harvest and postharvest botrytis infection (CitationMertely et al., 2009). However, when environmental conditions are favorable for Botrytis development, in other words, a year with many rain events, fungicide applications are difficult. An important cultural practice for disease control is the use of resistant cultivars. Although, no strawberry cultivar is completely resistant to Botrytis, some cultivars and selections show lower levels of the disease than others, so that development of cultivars with decreased susceptibility is possible.
Field evaluation methods harvest fruit into two containers that are weighed separately and a percent decayed value is obtained for transformation and statistical analyses. This approach is relatively straightforward and has been successful in determining differences among genotypes (CitationMaas, 1978). Interestingly, previous studies have shown that fruit decay in the field, measured at harvest, and postharvest decay were not correlated when data were analyzed across harvests (CitationBarritt, 1980; CitationDaubney and Pepin, 1977). In studies analyzing data from several harvest dates, a few of the harvest dates show statistically significant but weak correlation between percentage decay at harvest and percentage postharvest decay, while the majority of harvest dates showed no correlation, and a few showed negative correlation. It was determined that selection methods need to include field and postharvest evaluation data (CitationBarritt, 1980; CitationMaas, 1978).
MATERIALS AND METHODS
Production System and Field Planting
The annual replicated yield and fruit size assessments for the USDA-ARS strawberry breeding program at Beltsville, Maryland, for 2010 were used to determine if a correlation between fruit decay at harvest and fruit decay after postharvest storage could be detected using advanced statistical analyses, and if any detected correlation was strong enough to eliminate the need for postharvest evaluation. The production system was the annual hill system described by CitationBlack et al. (2002) with raised beds, black plastic mulch, and below surface trickle irrigation. The plants were established in August 2009. Each genotype was represented once in each of three blocks, and plots of each genotype were randomly assigned positions in each block. A plot of each genotype consisted of six plants in two rows of three, staggered across from each other in the planting bed. A total of 58 genotypes were evaluated, including ten cultivars: AC® Wendy, Allstar, Chandler, Cle des Champs, Darselect, Earliglow, Eros, Northeaster, Ovation, and Record. Breeding selections from four programs were evaluated. Forty selections were from the USDA-ARS Beltsville program. Five selections (MNUS674, MNUS691, MNUS796, MNUS818, and MNUS950) were evaluated from a joint program between the University of Minnesota and the Beltsville USDA-ARS program. Two selections (LL0220-10 and LL0311-43) were contributed from a collaborative program between Lareault Nursery (Lavaltrie, Quebec, Canada) and Agriculture and Agri-Food Canada, and one selection (EM995) was contributed from East Malling Research (East Malling, Kent, UK). Daily maximum, minimum, and average temperature; maximum and minimum relative humidity; and total rainfall were recorded during the fruiting period using a weather station located ∼300 m from the field.
Fruit Harvest
Mature fruits, full color to 75% color, were harvested twice a week, usually Monday and Thursday, from 10 May to 7 June. From each plot, fruits were harvested into two containers: one for fruit that appeared decayed and one for fruit that showed no visible signs of decay. The weight of each container was recorded separately. Decayed fruits were then discarded in the field. From the remaining container for each plot, individual fruits were selected to represent the type of fruits that are packed commercially for local sale. These fruits were mature but not over ripe (light bright red color) and did not show outward signs of damage or infection. The selected fruits were placed in one-quart plastic vented “clamshell” containers (Indiana Berry and Plant Co., Plymouth, IN, USA). The containers were transported to a walk-in refrigerator pre-set at 5°C a week before the first harvest.
Postharvest Storage and Evaluation
Individual clamshells were stacked on wire racks and covered (unsealed) with gas permeable polyethylene films to reduce water loss during storage. Fruit were stored in the dark at 5°C ± 0.5°C with air circulation provided by an overhead fan. After several days of storage, the fruit were evaluated for quality retention and decay development. During each evaluation, the fruit in each container were counted, and the total number of fruits showing visual signs of decay was recorded. The decay percentage was calculated based on the number of fruit decayed divided by the total number of fruit for each clamshell container. The number of days of storage was recorded for each harvest.
Data Analysis
To stabilize within-genotype variability, the observed p decay values were transformed by square root, followed by arcsine, to obtain approximately normally-distributed data. Means were back-transformed to the p decay scale for presentation of the results. An analysis of variance (ANOVA) was conducted for percent decay in the field at harvest and for percent decay after postharvest storage to determine estimates of means for: (1) each harvest across all genotypes and field plots; (2) each genotype across field plots and harvests; and (3) each genotype by field plot combination across harvests. To maximize replication and, hence, maximize statistical power, a replicate was defined to be a plot by harvest (clamshell of fruit) per genotype instead of a plot planted per genotype (summed over harvests), one per block. An ANOVA also was conducted to obtain genotypic estimates of yield; in this case, a replicate was defined as a field plot. Yield was multiplied by the proportion of fruit that did not decay in either the field or in postharvest storage and the resulting term was called “marketable yield,” though this term is sometimes used by others to mean the yield multiplied by the proportion of fruit that did not decay at harvest only. The ANOVAs were conducted using the PROC MIXED statement of SAS (version 9.2, 2008; SAS Institute Inc., Cary, NC, USA) and the PDMIX800.SAS means comparisons macro to obtain letters by the genotypic means (CitationSaxton, 1998).
Correlations between percentage of decayed fruit in the field at harvest and percentage of decayed fruit after postharvest storage were calculated using: (1) genotypic means across field plots and harvests; (2) genotype by field plot combination across harvests; and (3) field plot by harvest combination. Correlations also were calculated for the change between harvests in percentage decay in the field and percentage postharvest decay and weather conditions between harvests: maximum, minimum, and average temperature; maximum and minimum relative humidity; and total rainfall. Correlation coefficients (r) and the p-value for H0: |r| = 0 vs. H0: |r| > 0 were calculated using SAS PROC CORR (version 9.2, 2008; SAS Institute Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
Percentage Decay Means by Genotype
The percentage of fruit decayed at harvest was significantly different for the 58 genotypes and ranged from 5% to 26%, averaging 13% (). Use of the letters assigned by the means comparison procedure resulted in the selection of 30 genotypes (any genotype with a “t” beside the percentage field decay estimate) with as high as 13% field decay, resulting in a selection rate of almost 52% for improving percentage field decay. Eight genotypes (those with an “a” beside the percentage field decay estimate) would be considered unsuitable for release and used only as parents for improving other traits.
Percentage of fruit decayed postharvest also was significantly different for the 58 genotypes and ranged from 30% to 88%, averaging 54%, much higher than the percentage of fruit decayed in the field (). Means comparison resulted in selection of 21 genotypes (any genotype with a “w” beside the percentage postharvest decay estimate) with as high as 52% decay, resulting in a 36% selection rate for improving postharvest decay. Ten genotypes (those with an “a” beside the percentage postharvest decay estimate) would be considered unsuitable for release and used only as parents for improving other traits.
Percentage decay in the field and percentage decay after storage were significantly but weakly correlated on a genotype basis (r was 0.367; p > r = 0.0043), averaged across the three plots in the field and across harvests. The correlation between field and postharvest decay also was significant but weak when based on plot means across harvests (r was 0.249 (p > r = 0.0008). When correlation was examined on a plot × harvest combination, so that the percentage decay in the field was compared at each harvest with the percentage postharvest decay, a significant but weak correlation was observed only for the 1 June harvest date (r was 0.184; p > r = 0.0174). The highest level of correlation between percentage decay in the field and percentage decay postharvest was obtained by comparing genotype means for these two traits averaged across both field plots and harvests. But the level of correlation (r was 0.367; p > r = 0.0043) was not high enough to predict postharvest decay from decay at harvest, and selection for field decay would not give any indication of a genotype's postharvest decay percentage. Of the eight selections that would be discarded based on percentage decay in the field at harvest, two also would have been discarded for high postharvest decay, but two would have been selected as having some of the lowest postharvest decay percentages ().
TABLE 1 Percentage decay of fruit from 58 strawberry genotypes evaluated in 2010 in a field on the North Farm of the USDA-ARS Beltsville Agricultural Research CenterFootnote z
When the percentages of decay from harvest and from postharvest were combined, the total percentage of decayed berries ranged from 35% to 91%, averaging 60% (). When the total berry yields were multiplied by the total percentage of berries that did not decay in the field or after harvest to obtain an estimate for “marketable yield,” six of the seven genotypes with the highest marketable yield also had the highest total yields. The exception, B1806, had a moderate yield but among the lowest field decay and also among the lowest postharvest decay and could be considered as possible release along with the other six genotypes, although Eros already was released as a cultivar. ‘Eros’ had one of the highest percentages of field decay and was identified as a genotype for discard based on that trait. However, a breeder needs to consider many traits in determining whether or not a selection should be released as a cultivar, used as a parent, or discarded; many genotypes are outstanding for at least one important trait, but very few are among the best for all important traits. A breeder also needs to consider that commercial growers may be willing to apply fungicides to a cultivar with reduced disease resistance if that cultivar performs well in all other important ways.
Percentage Decay Means by Harvest
The percentage of decayed fruit at harvest in the field was significantly different among the nine harvest dates and ranged from 9% for the first harvest on 10 May, to 55% for the 24 May harvest in the middle of the season (). Percentage of decayed fruit in the field seemed to be highest after a rainfall event. For example, the highest level of decay was on the 24 May harvest, which was the day after the heaviest rainfall event of the season (23 mm). The correlation between total rainfall and percentage decay in the field was significant (r was 0.803 (p > r = 0.0092), and the correlation between total rainfall and change in percentage rot in the field from harvest to harvest was even stronger (r was 0.863 (p > r = 0.0057). It is generally accepted that rain and high humidity during flowering are conducive to disease development, and that wet surfaces are a key environmental factor for the development of botrytis fruit decay in the field (CitationSutton, 1998). This study shows that rainfall also can be important after flowering, and that a rain event even a few days prior to harvest can be correlated with an increase in the visible signs of decay in the field. Neither percentage decay in the field nor change in percentage decay in the field was correlated with either average temperature or minimum humidity between harvests. Therefore, although cool temperatures and high humidity in the field during flowering have been associated with field decay, those conditions a few days prior to harvest did not seem to increase visible symptoms of decay at harvest.
FIGURE 1 Daily total rainfall (mm) and minimum relative humidity (%) during the strawberry fruiting season at the USDA-ARS Beltsville Agricultural Research Center, Beltsville, MD, in 2010 (George Meyers, BARC-RSS, personal communication, December 2010), plus percentage decay of fruit from 58 strawberry genotypes evaluated at each of nine harvest dates from 10 May through 7 June 2010. Percentage decay in the field was calculated at each harvest for each of three plots of each genotype from the weight of fruits harvested separately into a container of decayed fruit and a container of fruit showing no signs of decay. Fruits from these harvests were placed into containers and stored at 5°C for 9 to 12 days. After storage, the percentage decayed fruit was calculated from the number of decayed fruits and the total number of fruits.
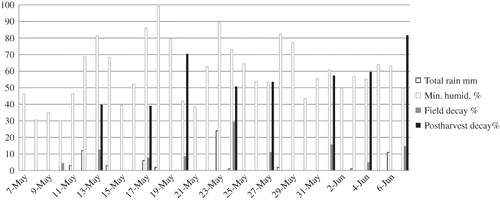
The percentage of decayed fruit after postharvest storage also was significantly different among the nine harvest dates, and appeared to
TABLE 2 Total yield and percentage of yield lost due to decay in the field or after storage at 5°C of fruit from 58 strawberry genotypes evaluated in 2010 in a field on the North Farm of the USDA-ARS Beltsville Agricultural Research CenterFootnote z
increase generally over time, ranging from 40%, for the first harvest for which postharvest data were recorded, to 82% for the last harvest (). Percentage of postharvest decay was much higher than the percentage decay at harvest. Postharvest decay per se did not seem to be directly affected by rain events a few days before harvest, as there was no correlation between the two. However, postharvest decay did seem to increase with consistently humid air in the field (r was 0.720 (p > r = 0.0440), and seemed to decrease if one of the days between harvests had low relative humidity. The change in percentage postharvest decay from harvest to harvest was even more strongly affected by the minimum humidity level between harvests (r was 0.861 (p > r = 0.0128). Association between high humidity during flowering and postharvest decay is well accepted (CitationSutton, 1998). This study shows that humidity also can be important well after flowering and that consistently high humidity between harvests is needed for higher levels of postharvest decay, while a day of low humidity may possibly reduce postharvest decay.
The majority of fruit showing postharvest decay are infected through the flower (CitationPowelson, 1960), but this study showed that, under some environmental conditions, a day of low humidity in the field, just a few days before harvest, long after flowering, can strongly reduce percentage postharvest decay. This might be due to effects of low humidity on latent infection that originated at flowering, or it could be due to an increased role of infection post-flowering, either in the field or after harvest. If a day of low humidity in the field can negatively affect latent infection that originated at flowering, then it should reduce percentage decay both in the field and postharvest. Since reduced humidity was not correlated with percentage field decay, it is possible that infection after flowering, either in the field or after harvest played a larger role in postharvest decay in this study than previously has been found.
This study also showed that a rain event long after flowering, just prior to harvest, was correlated with an increase in percentage of decayed fruit at harvest, but not after storage. If nearly all infection were through the flower, then it could be assumed that a rain event just prior to harvest simply increased the percentage decay in the field by inducing sporulation, and making it more obvious which berries were infected. The harvest date of 24 May could be viewed as an example. It follows a heavy rain event of 23 mm, and, in fact, an additional 1 mm of rain was recorded for the morning of 24 May. The percentage of field decay, 29%, increased sharply from the previous harvest on 20 May, 8%, but the percentage postharvest decay, 51%, dropped significantly in comparison with the 20 May harvest, 70%. However, if rainfall events simply revealed latent infection, then they also should be associated consistently with decreased postharvest decay, as occurred on this single harvest date. Contrary to this explanation, rain events just prior to harvest were not correlated with percentage postharvest decay. This also supports a larger role of post-flowering infection in the field in postharvest percentage decay in this study.
However, similar analyses of data from 2007 and 2008 did not show a significant correlation between percentage postharvest decay and any of the environmental measurements recorded, including minimum relative humidity. The 2007 season had mild temperatures and was very dry with the most severe drought in 25 years and lower overall humidity. Disease expression in the field was low (data not shown), and the percentage postharvest decay across the seven harvests was lower than for 2010. Spring of 2008 was cool and very wet early in the season then turned warm and dry, resulting in a compact harvest season. Decay in the field was high, resulting in fewer berries available for postharvest evaluation, and the postharvest decay percentage was similar among the five harvests (data not shown). Compared with 2007 and 2008, the 2010 harvest season was earlier and more extended, having nine harvest dates. It is possible that the correlation between postharvest percentage decay and a day of low humidity just prior to harvest would only be detectable under certain circumstances, such as an extended season with multiple harvests to evaluate, or when humidity levels bordered some critical threshold for the fungus.
Significant but very weak, or, practically speaking, lack of correlation between percentage decay of fruit at harvest and percentage decay of fruit after storage was observed in this study and also has been reported by others (CitationBarritt, 1980; CitationDaubney and Pepin, 1977). Since correlation of two traits is dependent on a certain level of variability for both traits, this can sometimes occur if either of the traits is at a static level. Biological explanations for the lack of correlation may lie in the effect that rainfall and humidity have on the fungus at different stages of the life cycle or in the differing levels and stages of infection of fruit in the field, including those caused by differing host responses to infection. A possible increased role for post-flowering infection in this study (2010) also may help explain the poor though significant correlation between percentage decay of fruit at harvest and postharvest. The low level of correlation may have been due to a significant level of direct infection of the receptacle, in some genotypes but not others, either while still in the field or after harvest. When infection through the flower and infection after flowering are both significant pathways leading to decay, then host resistance to decay in those environments will require significant defense of the receptacle as well as the flower, and the associated breeding effort should focus on both pathways until the genes controlling defense of one or both of the pathways are fixed in the population. The simplest approach is to consider resistance as measured postharvest in addition to that measured in the field, even though it requires additional time, personnel, and storage facilities.
ACKNOWLEDGMENTS
This project was funded by USDA-ARS Projects 1275-21220-189-00 and 1265-42000-004-00. The authors wish to thank the University of Minnesota, Agriculture and Agri-Food Canada, East Malling Research, and Lareault Nursery for donations of breeding selections; John Enns, Phil Edmonds, and the BARC Research Support Services for establishing and maintaining the fields; Ellen Turner, John Enns, and Yang Yang for evaluating fruit; George Meyers, RSS, for weather records; Stephen Rehner, Wayne Jurick, Bill Turechek, and John Maas for helpful discussions; and Wayne Jurick, John Maas, and the anonymous reviewers for their helpful comments.
Notes
This article not subject to US copyright law.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
LITERATURE CITED
- Barritt , B.H. 1980 . Resistance of strawberry clones to . Botrytis fruit rot. J. Amer. Soc. Hort. Sci. , 105 : 160 – 164 .
- Black , B.L. , Enns , J.M. and Hokanson , S.C. 2002 . A comparison of temperate-climate strawberry production systems using eastern genotypes . HortTechnology , 12 : 670 – 675 .
- Ceponis , M.J. , Cappellini , R.A. and Lightner , G.W. 1987 . Disorders in sweet cherry and strawberry shipments to the New York market, 1972–1984 . Plant Dis. , 71 : 472 – 475 .
- Daubney , H.A. and Pepin , H.S. 1977 . Evaluation of strawberry clones for fruit rot resistance . J. Amer. Soc. Hort. Sci. , 102 : 431 – 435 .
- Maas , J.L. 1978 . Screening for resistance to fruit rot in strawberries and red raspberries: A review . HortScience , 13 : 423 – 426 .
- Mertely , J.C. , MacKenzie , S.J. and Legard , D.E. 2002 . Timing of fungicide applications for Botrytis cinerea based on development stage of strawberry flowers and fruit . Plant Dis. , 86 : 1019 – 1024 .
- Mertely , J.C. , Seijo , T.E. , MacKenzie , S.J. , Moyer , C. and Peres , N.A. 2009 . Effect of timing of preharvest fungicide applications on postharvest Botrytis fruit rot of annual strawberries in Florida . Plant Health Progr. doi:10.1094/PHP-2009-0921-01-RS. ,
- Mitcham, E. 2002. Strawberry. In: K.C. Gross, C.Y. Wang, and M. Saltveit (eds.). The commercial storage of fruits, vegetables, and florist and nursery crops. USDA Agric. Hdbk No. 66. 8 Nov. 2002. < www.ba.ars.usda.gov/hb66/index (http://www.ba.ars.usda.gov/hb66/index)
- Powelson , R.L. 1960 . Initiation of strawberry fruit rot caused by . Botrytis cinerea. Phytopathology , 50 : 491 – 494 .
- Saxton , A.M. 1998 . “ A macro for converting mean separation output to letter groupings in Proc Mixed ” . In Proc. 23rd SAS Users Group Intl , 1243 – 1246 . Cary , ND, p : SAS Institute . (original reference); Revised in 2000, PDMIX800©
- Sutton , J.C. 1998 . “ Botrytis fruit rot (gray mold) and blossom blight, p ” . In Compendium of strawberry diseases , 2nd , Edited by: Maas , J.L. 28 – 31 . St. Paul , MN : American Phytopathological Society .