Abstract
A draft sequence of the diploid strawberry was released to the public early in 2011. This milestone was achieved by an international team of experts exploiting the newest advances in sequencing technology and bioinformatics. While an excellent resource, the accomplishment is simply a starting point for creating links between the genes within and the traits they control. The central thrust of many research programs is to now connect genes and traits using a variety of genetic, physiological, and developmental tests. Ultimately, the derivation of the parts list for a strawberry should expand the use of this organism as a system for studying questions important to the industry, as well as expanding our understanding of the basic science of plant physiology and development. This review is a supplement to the recent release of the genome sequence, focusing on the rationale for sequencing a strawberry genome, how it will be put to work, and some of the attributes of strawberry that make it an attractive system to answer questions in plant biology.
INTRODUCTION
A Long Sequence of Events to a Short Sequence
In January of 2008, our grasp of the molecular underpinnings of strawberry traits changed dramatically. Molecular-genetic research in strawberry had been gaining momentum for a decade, owing to development of agile transformation systems (CitationHaymes and Davis, 1998; CitationMezzetti and Costantini, 2006; CitationNehra et al., 1990; CitationOosumi et al., 2006; CitationSlovin et al., 2009), generation of increasingly complex genetic maps (CitationDavis and Yu, 1997; CitationSargent et al., 2004, 2006; CitationSpigler et al., 2010), and growing genomics-level resources (CitationAharoni et al., 2000; CitationDavis et al., 2010; CitationFolta et al., 2010; CitationPontaroli et al., 2009). Elegant studies examined molecular processes in strawberry fruits relevant to important traits, like flavor (CitationAharoni et al., 2000, 2004) and softening (CitationPombo et al., 2009; CitationRosli et al., 2004; CitationSpolaore et al., 2003; CitationWoolley et al., 2001). This experimental progress was achieved in the absence of whole genome information, meaning that any molecular laboratory endeavor had to start with procuring gene sequence. This primary characterization alone could take weeks to months, and in some cases it was not possible at all. The new millennium brought with it new means to capture substantial genome sequence through methods that were relatively inexpensive, and sequencing the strawberry genome shifted to high on the priority list for the strawberry research community. At the North American Strawberry Symposium in 2007, it was a lofty yet desperately desired objective.
In the era of new high-throughput sequencing, application of these technologies to generate strawberry genome sequence information made perfect sense. Strawberry is among the smallest plant genomes, certainly among plants directly related to valuable crops. The diploid strawberry genome is composed of approximately 240 million base pairs of DNA (Mb), quite small when compared to other crops, such as rice (∼430 Mb), tomato (950 Mb), onion (∼15,000 Mb), or pine (21,658 Mb). A small genome means less genetic real estate to decipher, so in this sense the strawberry genome was an extremely attractive technology target. However, strawberry lacked fundamental resources that would enable a successful genome sequencing effort, such as dense linkage maps, physical maps, and large-sequenced genomic fragments from BAC libraries. The only resource approaching a hard genomic sequence starting point was the report of 1% of the Fragaria vesca genome (a diploid species), from 30–50 kb pieces from Sanger sequencing (CitationDavis et al., 2010; CitationPontaroli et al., 2009). The sequences were present in fosmids, bacterial plasmids engineered to contain large pieces of strawberry DNA. The fosmids sequenced were selected randomly in about half the cases (CitationPontaroli et al., 2009), while the other half was comprised of fosmids bearing sequences likely to influence flowering time, disease resistance, fruit development, or other traits important to horticulture (CitationDavis et al., 2010). While having 1% of the genome in hand was an important step in understanding the fundamentals of strawberry genome structure and gene arrangement, the lack of hard physical maps made a larger effort less promising. On the other hand, peach and apple, strawberry relatives in the family Rosaceae, possessed well developed maps that would allow assembly and orientation of next-gen sequencing scaffolds. These resources made the likelihood of success much higher in these species, so sequencing resources and interest were diverted away from strawberry and over to its valuable tree crop relatives.
Time, technology, and a bold proposal would ultimately conspire to raise interest in strawberry genome sequencing. At the 2008 Plant-Animal Genome meeting in San Diego, California, a group of rosaceous crop researchers met for an annual meeting. Those in attendance heard updates on the status of apple and peach sequencing. Late in the session, Drs. Vladimir Shulaev and Richard Veilleux of Virginia Tech proposed a challenge to those in the room. Shulaev and Veilleux had forged an arrangement with Virginia Tech Bioinformatics Institute to sequence the diploid strawberry (Fragaria vesca) using a newly-acquired piece of equipment, the Roche-454 Sequencing Platform (CitationMargulies et al., 2005). According to the plan, VBI would provide the service at a discounted rate in conjunction with Roche-454, the company supplying the reagents. For Roche-454 it was an opportunity to test the mettle of their technology, showing that their short-read sequencing platform and software could generate large assemblies of contiguous sequence from plant genomes. Shulaev and Veilleux were looking for buy-in from a hungry strawberry genomics community. Stopping just short of passing a hat around the table for contributions, the quest for a strawberry sequence was set in motion. A formal attempt to sequence the strawberry genome would be made with a core of informatics expertise, a new technology, a community of diverse talents—and absolutely no single source of substantial funding.
Financial support and intellectual contributions eventually trickled in from around the globe and the amount of raw sequence grew exponentially. Industry, government, and university labs brought funds to the effort. Many researchers joined from other plant systems, seeing the utility of a strawberry genome in their own studies. The project gained the attention of recognized computational experts that looked at strawberry as a new frontier to apply their expertise. Other scientists sought to guide writing and submission for publication. Over the next 2 years and countless conference calls, a draft sequence of the strawberry genome was submitted for publication, finding favor at Nature Genetics (CitationShulaev et al., 2011).
It was interesting to observe the spectrum of responses heard upon final word of manuscript acceptance. While computational biologists exclaimed, “Cheers! Now we're done!” the biologists in the consortium ignited with, “Cheers! Now we can begin!” The varied response represents two unique perspectives on a published genome. It is analogous to the disparity in attitude between the workers that plan and build the playground and the children that want to play in it. Many of us took off our work hats—now it was our time to play.
The F. vesca Sequencing Strategy
The cultivated strawberry is known as Fragaria × ananassa. The “×” reminds us that the modern commercial strawberry is a hybrid of two wild species, one from North America and one from Chile in South America. These two species first crossed in the royal botanical gardens in Versailles, France in the mid-1700s, making the dessert strawberry a relatively recent species (CitationDarrow, 1966). F. × ananassa does not contain a simple genome (CitationBringhurst et al., 1966; CitationRousseau-Gueutin et al., 2008; CitationSenanayake and Bringhurst, 1967). It is the fortunate victim of a chance trick of the plant world—polyploidy (for review: CitationWendel, 2000). Whereas the cells of diploid organisms contain two copies of every gene (typically one maternal and one paternal), polyploids contain additional copies, residing on an entirely separate set of chromosomes. These may occur from doubling of genetic materials or possibly from abnormal production of gametes resulting in atypical doses of genetic material passed from pollen or ovule (CitationIslam, 1960). F. × ananassa contains a complex genome (reviewed in CitationFolta and Davis, 2006), composed of four subgenomes (one subgenome would be diploid, four subgenomes result in an octoploid). This arrangement makes sequencing and assembly of the cultivated strawberry almost impossible (at least at this point), due to constraints of the sequencing and assembly strategy (next section). Instead, a simpler relative of the octoploid strawberry, F. vesca, was chosen. F. vesca contains a simple, diploid genome, but more importantly it shares a close common ancestor with at least one of the four subgenomes of octoploid strawberry. In this way, F. vesca makes an appropriate selection for sequencing as many sequences will be identical, while the differences will tell us something about strawberry history and evolution.
The diploid strawberry was sequenced using “next generation” sequencing technology. The concept is simple. Using new technology it is easy and inexpensive to obtain short runs of DNA sequence, on the order of 30 to 500 bases. These short runs of sequence (reads) are gathered by the millions. The reads are then compared to each other computationally, aligning them and stacking them by shared sequence to derive a consensus sequence. An analogy of this process is shown in Imagine that someone wanted to resolve the sequence of the letters of the alphabet—but it was unknown. A next-generation sequencing approach would produce a few letters at a time in the correct order (; ABCD, CDEF, TUVW, etc). When many of the short sequences are compared and aligned against each other () it becomes possible to determine an entire consensus sequence from end-to-end () with great confidence.
FIGURE 1 The theory of next-generation, short-read sequencing, and assembly as applied to the alphabet: Relatively short runs of letters are obtained where the letters appear in the correct order (A). The letters are computationally aligned based on common features (B). A consensus sequence is derived (C). The same process was used to sequence the strawberry genome, only using millions of fragments that were between 76 and ∼400 bp in length.
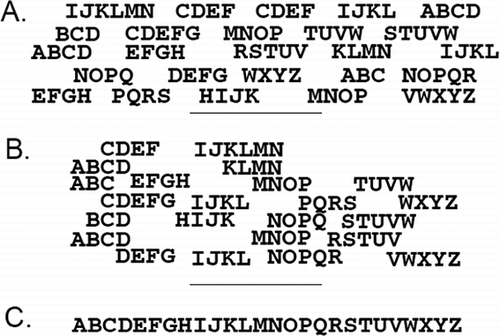
This method is quite suitable for use on F. vesca, a plant with a small and simple genome. Whereas many genomes are cluttered with repeated information, strawberry parks its valuable genetic information into discrete parcels that could be sequenced and assembled. The median scaffold (large contiguous genomic sequence) size was over 1.3 million bases long, demonstrating that the small runs produced via short read sequencing could be constructed into substantial assemblies of contiguous strawberry genes. Innovative methods were then used to order and orient the scaffolds using the Fragaria diploid linkage map (CitationSargent et al., 2011). This feat was a substantial accomplishment as it allowed the long scaffolds to be ordered in a manner that reflects their true physical position relative to one another, in essence, assembling them into their actual positions on the chromosomes.
The Parts of the Strawberry-Making Machine
Any sequenced genome is simply a parts list. It is a comprehensive accounting of the components that make up the genetic basis of the organism and the elements that control their expression and activity. Strawberry is no different. Successful sequencing and assembly of the strawberry genome now provides researchers with a solid starting point to begin functional inquiry as to how the various genetic elements influence each other and work together to affect the biology of seeds, plants, and ultimately the fruit product in the clamshell that entices the consumer's senses.
The strawberry plant may be thought of as a factory, a factory that takes water, sunlight, carbon dioxide, and a pinch of minerals to assemble a desirable product. If you want to understand the product and how to make it better, cheaper, or faster, you need to understand the mechanics of the factory at a nuts-and-bolts level. This level of understanding comes quite quickly if you have the blueprints. Blueprints show you how parts are assembled and interact. Unfortunately, blueprint-level resolution of the strawberry is still decades in the future. But we do have that parts list. The parts list contains all of the raw information about the plant; it is the research community's job to experimentally link the parts together into pathways, networks, and webs that ultimately will build that needed blueprint.
The Visible Genome
As presented, the diploid strawberry genome is not just a long string of letters that define the genes of strawberry. Instead, it is viewable in an annotated and dynamic format that is freely available to any interested user on the internet, at www.strawberrygenome.org At this website, one can explore the genome, either on substantial pieces of chromosomes or one gene at a time. Most importantly, the genome browser hosted by Plant and Food Research in New Zealand provides a means to compare strawberry gene sequences to those of grape, tomato, or Arabidopsis. It shows where genes are predicted to occur, even if there is no previous evidence of such a gene's function, providing new areas for research into strawberry-specific sequences. The genome browser also allows a user to visualize if a gene is expressed, as evidenced by the existing RNA transcripts that correspond to the gene sequence. Many other features of the genome sequence may be observed, such as the presence of potential molecular markers and comparisons to other strawberry species. These tools permit anyone to explore strawberry gene space, and compare the parts of strawberry to those of other important species.
Major Categories of Important Parts
The strawberry genome contains genes that are similar to genes identified in other plants, such as Arabidopsis or rice, that are known to be involved in processes interesting to the farmer and/or the biologist. These are enumerated in detail in the Supplemental Materials presented by CitationShulaev et al. (2011). Of the major classes of genes uncovered are those related to flavors and aromas (CitationAharoni et al., 2000, 2004; CitationLunkenbein et al., 2006; CitationRaab et al., 2006). Many of these have been previously characterized, such as quinone oxidoreductase, alcohol dehydogenases, alcohol acyl transferases, O-methyltransferases, and various terpene synthases. While previous research has implicated these genes in the production of specific flavor compounds, examination of genome sequence reveals multiple family members. These various gene versions may catalyze production of other flavor compounds or possibly are expressed in only particular parts of the fruit or plant.
Many genes associated with softening and cellulose degradation (CitationDotto et al., 2006; CitationJimenez-Bermudez et al., 2002; CitationRosli et al., 2004, Citation2009; CitationWoolley et al., 2001), such as pectate lyases, β-galactosidases, chitinases, and β-glucanases, are among those found in the genome. Most importantly, these genes also appear to be found in families of closely-related members, suggesting that various members may have discrete roles that may change throughout development. Other genes affecting fruit quality likely play a role in fruit expansion in response to auxin secreted from the achenes (CitationManning, 1994; CitationNam et al., 1999). Still, others likely control pigmentation and the development of secondary compounds that make fruits attractive to the eye and possibly provide benefits to human health (CitationSeeram, 2008).
Another class of genes is known to encode proteins with roles in plant defense to pests or pathogens (CitationDong, 2004; CitationTor et al., 2009). The familiar gene sequences, such as Npr1, the Pathogenesis Related gene family, various WRKY-motif factors, and NB-LRR proteins, are often found in families where individuals may direct resistance to various strawberry diseases. Genes playing a role in hormone synthesis and sensing (GID1, TIR1), as well as those involved in light sensing (cryptochromes, phytochromes) and flowering (Constans, Flowering Locus T) are present, showing parallels with other plant systems. Functional tests in plants will permit description of the roles of these genes in strawberry and allow scientists to compare and contrast these genes with those described in other systems.
While the function of many of the genes predicted may be easily inferred from what is known in other plant systems, some predicted strawberry genes defy intuitive functional annotation. When compared against other plant genes, these sequences report back as “unknown,” “hypothetical,” or “predicted” with no described function. Others are unique to strawberry; the genes that make strawberry a strawberry. Now that they have been identified they can be tested using transgenic technologies, defining their specific roles in strawberry biology.
How Will the Sequence Impact Consumers?
There is an undeniable demand for perfect, large, fresh, strawberry fruits available throughout the year. The first gains that will come from a sequenced genome will be from identification of molecular markers, easily traceable DNA patterns that associate with a trait of interest, complementing the current set available in strawberry (CitationWhitaker, 2011). For example, it is possible to amplify a short piece of DNA in plants that tend to be resistant to anthracnose fruit rot, caused by Colletotrichum acutatum (CitationLerceteau-Kohler et al., 2005). Plants containing this fragment are resistant to the disease, whereas susceptible plants fail to permit amplification. In other words, this small stretch of DNA is likely to be physically proximal to a gene or a chromosome segment that confers resistance. The distillation of the genome sequence provides researchers with a starting point to identify genes potentially linked to an important horticultural process. The same genes may be examined for other germplasm for variation that can be used and easily traced in breeding. In the future, such markers will be developed for resistance to other diseases like Verticillium wilt, photoperiodic flowering habits, or flavor compound content.
The advantage to using markers is that seedlings may be screened to test for candidates likely to possess a series of markers consistent with favorable horticultural qualities. Right now a strawberry breeder may screen tens of thousands of plants to find just one that has a favorable combination of high yield, large fruits, resistance to disease and pests, insensitivity to abiotic stress, and, to some extent, acceptable flavor. Such searches require acres of manicured fields, complete with mechanisms to fertilize, water, and protect plants. Finding a new variety is labor intensive and expensive—in dollars and time.
Imagine if a breeder could make a cross between two elite strawberry plants and then screen the offspring at the seedling stage. A single snippet from a juvenile leaf would provide enough DNA to screen for hundreds of markers associated with favorable traits. Such technologies might assist a breeder to narrow 10,000 seedlings down to the 100 most likely to exhibit improved traits. Not only would this save tremendous resources in labor, fuel, water, land, and time, it also would permit a breeder to test larger numbers of candidates known to possess a higher likelihood of becoming a new elite variety. This process of identifying the genetic variants that may be elevated to useful molecular markers is greatly facilitated by capture of a whole genome sequence.
So Now What?
The derivation of a strawberry genome sequence enables accelerated study on two levels—findings that will benefit farmers and consumers, and findings that will advance our understanding of processes that control plant growth and development. Whether it is fruit size, disease resistance/susceptibility, fertilizer requirements, stress tolerance, or fruit flavor, every trait in a strawberry is shaped by contributions from at least one gene, but usually many genes. The challenge before the strawberry research community is to now connect the 34,809 genes in the new genome sequence with the traits that they support. While obtaining and describing the strawberry genome took over 70 scientists a few years, even a cursory characterization of strawberry's many genes will easily consume the next decade. This ambitious timeline is predicated on increasing interest from a wider plant-science community, as expertise from physiology, biochemistry, and molecular biology will need to be pooled to exploit this new resource.
It should not be a problem to generate substantial interest in the strawberry system. As funding agencies focus on translational systems to advance findings from models to plants of agricultural import, diploid strawberry plugs the chasm between the model plant Arabidopsis thaliana and large-format rosaceous tree crops. The diploid strawberry has many attractive advantages over other experimental plant systems:
| • | It may be grown, seed to seed in 10-cm pots. | ||||
| • | It may be propagated sexually as well as by branch crowns or runners. | ||||
| • | There is substantial transcriptome coverage. | ||||
| • | It is a perennial, unlike other models. | ||||
| • | Strawberry cycles from seed to seed in 12–16 weeks. | ||||
| • | Construction of transgenic lines is possible. | ||||
| • | It is possible to suppress transcripts or overexpress them, allowing for loss- and gain-of-function studies. | ||||
| • | Hundreds of plants may be grown on a single greenhouse bench. | ||||
| • | It has a tiny, sequenced genome! | ||||
New genetic and genomic tools and resources are being devised and will greatly enhance the resources available for scientific inquiry. Such resources include collections of mutants:
| • | Production of T-DNA insertion lines: Almost random disruptions in genes can be identified by a few simple steps to help researchers identify plants lacking function in genes of interest (CitationOosumi et al., 2010; Ruiz-Rojas et al. 2011). | ||||
A generation of activation-tagged lines or plants containing transgenes that activate expression from proximal genes will allow researchers to identify genes associated with an important process by turning on their expression inappropriately, possibly beaconing their activity.
| • | EMS populations: Several are in development. These plants feature random chemically-induced mutations throughout the genome, producing plants that feature abnormalities in important traits. These traits may be dissected back to the gene using genetics and/or sequencing techniques. | ||||
Lines that are homozygous for most genes:
| • | Recombinant-inbred lines for notable crosses: These lines will help to identify genes associated with traits of interest that are segregating in established populations. | ||||
| • | Inbred lines for transgenic use: These lines allow the production of transgenic plants in backgrounds that are genetically homogenous and stable, allowing more reliable and consistent presentation of phenotypes (e.g., CitationSlovin et al., 2009). | ||||
These are just a few of the important new plant genetic resources developed in the past few years. All of these inherent qualities and enabling resources truly render strawberry as a superior system to analyze processes that may further illuminate our understanding of plant biology. More importantly, the strawberry is a transitional system into all species within the Rosaceae family. Its rapid growth, large seed set, transformability, and small stature are all traits shared between strawberry and Arabidopsis, the white lab mouse of the plant world. Studies in strawberry may rapidly advance the understanding of processes in apples, pears, roses, cherries, peaches, and other valuable crops that require more time and space to make research gains. Strawberry should serve as an important intermediate in many research schemes, and may make lofty research ideas a bit more tractable and less risky than working solely in tree crops or brambles.
Strawberry also adds to the understanding of how fruits grow and develop. Strawberry is an unusual fruit. In fact, botanically speaking it is not a fruit at all, but rather an accessory fruit. The valuable red “berry” is really an enlarged, fleshy receptacle, dotted with achenes that are the true fruits. How is this “berry” different developmentally from tomatoes, grapes, kiwis, or other important berries? How do the genes and gene expression patterns differ between raspberries, roses, peaches, and apples? The successful addition of a strawberry sequence to the body of sequenced genomes provides another important node of comparisons from an unusual plant that bears an unusual fruit.
CONCLUSIONS AND PERSPECTIVES
From the perspective of a scientist that was intimately intertwined in the strawberry genome process from the day that Drs. Veilleux and Shulaev presented their proposal to the day that the final proofs magically materialized on the computer monitor, the whole genome sequence represents a starting point much more than an end. This sentiment is shared across the strawberry research community as well as the industry it serves. The draft sequence is a significant step that will enable researchers from other plant systems to lend their expertise to answering questions in strawberry. With this resource, there will be increased interest in the strawberry as a crop as well as an experimental system to study plant biology. Together, the enabling technology of a genome sequence will put a better product on the grocery store shelf and expand the scope of plant biology textbooks.
ACKNOWLEDGMENTS
This work represents the extensive contributions made by members of the International Strawberry Sequencing Consortium—financial, intellectual, and experimental. Whole-genome sequencing of strawberry would not have been possible without the primary vision and actions of Vladimir Shulaev and Richard Veilleux, in conjunction with Virginia Bioinformatics Institute at Virginia Tech.
LITERATURE CITED
- Aharoni , A. , Giri , A.P. , Verstappen , F.W. , Bertea , C.M. , Sevenier , R. , Sun , Z. , Jongsma , M.A. , Schwab , W. and Bouwmeester , H.J. 2004 . Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species . Plant Cell. , 16 : 3110 – 3131 .
- Aharoni , A. , Keizer , L.C. , Bouwmeester , H.J. , Sun , Z. , Alvarez-Huerta , M. , Verhoeven , H.A. , Blaas , J. , van Houwelingen , A.M. , De Vos , R.C. , van der Voet , H. , Jansen , R.C. , Guis , M. , Mol , J. , Davis , R.W. , Schena , M. , van Tunen , A.J. and O'Connell , A.P. 2000 . Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays . Plant Cell. , 12 : 647 – 662 .
- Bringhurst , R.S. , Senanaya , Y.D. and Kahn , D.A. 1966 . Fragaria polyploids chromatographic analysis . Amer. J. Bot. , 53 : 638 abstr
- Darrow , G. 1966 . The strawberry: History breeding and physiology , New York : Holt, Rinehart and Winston .
- Davis , T.M. , Shields , M.E. , Zhang , Q. , Tombolato-Terzic , D. , Bennetzen , J.L. , Pontaroli , A.C. , Wang , H. , Yao , Q. , SanMiguel , P. and Folta , K.M. 2010 . An examination of targeted gene neighborhoods in strawberry . BMC Plant Biol. , 10 : 81
- Davis , T.M. and Yu , H. 1997 . A linkage map of the diploid strawberry, Fragaria vesca . J. Heredity , 88 : 215 – 221 .
- Dong , X. 2004 . NPR1, all things considered . Curr. Opin. Plant Biol. , 7 : 547 – 552 .
- Dotto , M.C. , Martinez , G.A. and Civello , P.M. 2006 . Expression of expansin genes in strawberry varieties with contrasting fruit firmness . Plant Physiol. Biochem. , 44 : 301 – 307 .
- Folta , K.M. , Clancy , M.A. , Chamala , S. , Barbazuk , W.B. , Dhingra , A. , Brunings , A. , Gomide , L. , Kulathinal , R.J. , Peres , N. and Davis , T.M. 2010 . A transcript accounting from diverse tissues of a cultivated strawberry (Fragaria × ananassa) . Plant Genome , 3 : 99 – 105 .
- Folta , K.M. and Davis , T.M. 2006 . Strawberry genes and genomics . Crit. Rev. Plant Sci. , 25 : 399 – 415 .
- Haymes , K.M. and Davis , T.M. 1998 . Agrobacterium mediated transformation of ‘Alpine’ Fragaria vesca, and transmission of transgenes to R1 progeny . Plant Cell Rep. , 17 : 279 – 283 .
- Islam , A.S. 1960 . Possible role for unreduced gametes in the origin of polyploid Fragaria . Biologia , 6 : 189 – 192 .
- Jimenez-Bermudez , S. , Redondo-Nevado , J. , Munoz-Blanco , J. , Caballero , J.L. , Lopez-Aranda , J.M. , Valpuesta , V. , Pliego-Alfaro , F. , Quesada , M.A. and Mercado , J.A. 2002 . Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene . Plant Physiol. , 128 : 751 – 759 .
- Lerceteau-Kohler , E. , Guerin , G. and Denoyes-Rothan , B. 2005 . Identification of SCAR markers linked to Rca 2 anthracnose resistance gene and their assessment in strawberry germ plasm . Theor. Appl. Genet. , 111 : 862 – 870 .
- Lunkenbein , S. , Salentijn , E.M.J. , Coiner , H.A. , Boone , M.J. , Krens , F.A. and Schwab , W. 2006 . Up- and down-regulation of Fragaria x ananassa O-methyltransferase: Impacts on furanone and phenylpropanoid metabolism . J. Expt. Bot. , 57 : 2445 – 2453 .
- Manning , K. 1994 . Changes in gene-expression during strawberry fruit ripening and their regulation by auxin . Planta , 194 : 62 – 68 .
- Margulies , M. , Egholm , M. , Altman , W.E. , Attiya , S. , Bader , J.S. , Bemben , L.A. and Rothberg , J.M. 2005 . Genome sequencing in microfabricated high-density picolitre reactors . Nature , 437 : 376 – 380 . …
- Mezzetti , B. and Costantini , E. 2006 . Strawberry (Fragaria x ananassa) . Methods Mol. Biol. , 344 : 287 – 295 .
- Nam , Y.W. , Tichit , L. , Leperlier , M. , Cuerq , B. , Marty , I. and Lelievre , J.M. 1999 . Isolation and characterization of mRNAs differentially expressed during ripening of wild strawberry (Fragaria vesca L.) fruits . Plant Mol. Biol. , 39 : 629 – 636 .
- Nehra , N.S. , Chibbar , R.N. , Kartha , K.K. , Datla , R.S.S. , Crosby , W.L. and Stushnoff , C. 1990 . Genetic-transformation of strawberry by Agrobacterium tumefaciens using a leaf disk regeneration system . Plant Cell Rep. , 9 : 293 – 298 .
- Oosumi , T. , Gruszewski , H.A. , Blischak , L.A. , Baxter , A.J. , Wadl , P.A. , Shuman , J.L. , Veilleux , R.E. and Shulaev , V. 2006 . High-efficiency transformation of the diploid strawberry (Fragaria vesca) for functional genomics . Planta , 223 : 1219 – 1230 .
- Oosumi , T. , Ruiz-Rojas , J.J. , Veilleux , R.E. , Dickerman , A. and Shulaev , V. 2010 . Implementing reverse genetics in Rosaceae: Analysis of T-DNA flanking sequences of insertional mutant lines in the diploid strawberry, Fragaria vesca. Physiol . Plant. , 140 : 1 – 9 .
- Pombo , M.A. , Dotto , M.C. , Martinez , G.A. and Civello , P.M. 2009 . UV-C irradiation delays strawberry fruit softening and modifies the expression of genes involved in cell wall degradation . Postharvest Biol. Tech. , 51 : 41 – 148 .
- Pontaroli , A. , Rogers , R. , Zhang , Q. , Davis , T. , Folta , K. , San Miguel , P. and Bennetzen , J. 2009 . Gene content and distribution in the nuclear genome of Fragaria vesca . Plant Gen. , 2 : 93 – 101 .
- Raab , T. , Lopez-Raez , J.A. , Klein , D. , Caballero , J.L. , Moyano , E. , Schwab , W. and Munoz-Blanco , J. 2006 . FaQR, required for the biosynthesis of the strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone, encodes an enone oxidoreductase . Plant Cell , 18 : 1023 – 1037 .
- Rosli , H.G. , Civello , P.M. and Martinez , G.A. 2004 . Changes in cell wall composition of three Fragaria x ananassa cultivars with different softening rate during ripening . Plant Physiol. Biochem. , 42 : 823 – 831 .
- Rosli , H.G. , Civello , P.M. and Martinez , G.A. 2009 . Alpha-L-arabinofuranosidase from strawberry fruit: Cloning of three cDNAs, characterization of their expression and analysis of enzymatic activity in cultivars with contrasting firmness . Plant Physiol. Biochem. , 47 : 272 – 281 .
- Rousseau-Gueutin , M. , Lerceteau-Kohler , E. , Barrot , L. , Sargent , D.J. , Monfort , A. , Simpson , D. , Arus , P. , Guerin , G. and Denoyes-Rothan , B. 2008 . Comparative genetic mapping between octoploid and diploid fragaria species reveals a high level of colinearity between their genomes and the essentially disomic behavior of the cultivated octoploid strawberry . Genetics , 179 : 2045 – 2060 .
- Ruiz-Rojas , J.J. , Sargent , D.J. , Shulaev , V. , Dickerman , A.W. , Pattison , J. , Holt , S.H. , Ciordia , A. and Veilleux , R.E. 2010 . SNP discovery and genetic mapping of T-DNA insertional mutants in Fragaria vesca L . Theoret. Appl. Genet. , 121 : 449 – 463 .
- Sargent , D.J. , Kuchta , P. , Lopez Girona , E. , Zhang , H. , Davis , T.M. , Celton , J-M. and Simpson , D.W. 2011 . Simple sequence repeat marker development and mapping targeted to previously unmapped regions of the strawberry genome sequence . Plant Gen. , 4 : 165 – 177 . …
- Sargent , D.J. , Battey , N.H. , Wilkinson , M.J. and Simpson , D.W. 2006 . The detection of QTL associated with vegetative and reproductive traits in diploid Fragaria: A preliminary study . Acta Hort. , 708 : 471 – 474 .
- Sargent , D.J. , Davis , T.M. , Tobutt , K.R. , Wilkinson , M.J. , Battey , N.H. and Simpson , D.W. 2004 . A genetic linkage map of microsatellite, gene-specific and morphological markers in diploid Fragaria . Theoret. Appl. Genet. , 109 : 1385 – 1391 .
- Seeram , N.P. 2008 . Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease . J Agric. Food Chem. , 56 : 627 – 629 .
- Senanayake , Y.D.A. and Bringhurst , R.S. 1967 . Origin of Fragaria polyploids . I. Cytological analysis. Amer. J. Bot. , 54 : 221 – 228 .
- Shulaev , V. , Sargent , D.J. , Crowhurst , R.N. , Mockler , T.C. , Folkerts , O. , Delcher , A.L. and Folta , K.M. 2011 . The genome of woodland strawberry (Fragaria vesca) . Nat. Genet. , 43 : 109 – 116 . …
- Slovin , J.P. , Schmitt , K. and Folta , K.M. 2009 . An inbred line of the diploid strawberry Fragaria vesca f. semperflorens for testing gene function . BMC Plant Meth. , 5 : 15
- Spigler , R.B. , Lewers , K.S. , Johnson , A.L. and Ashman , T.L. 2010 . Comparative mapping reveals autosomal origin of sex chromosome in octoploid Fragaria virginiana . J. Hered. , 101 (Suppl. 1):S107–S117 )
- Spolaore , S. , Trainotti , L. , Pavanello , A. and Casadoro , G. 2003 . Isolation and promoter analysis of two genes encoding different endo-beta-1,4-glucanases in the non-climacteric strawberry . J. Exp. Bot. , 54 : 271 – 277 .
- Tor , M. , Lotze , M.T. and Holton , N. 2009 . Receptor-mediated signalling in plants: Molecular patterns and programmes . J. Exp. Bot. , 60 : 3645 – 3654 .
- Wendel , J.F. 2000 . Genome evolution in polyploids . Plant Mol. Biol. , 42 : 225 – 249 .
- Whitaker , V.M. 2011 . Applications of molecular markers in strawberry . J. Berry Res. , 1 : 115 – 127 .
- Woolley , L.C. , James , D.J. and Manning , K. 2001 . Purification and properties of an endo-beta-1,4-glucanase from strawberry and down-regulation of the corresponding gene, cel1 . Planta 214 , : 11 – 21 .