Abstract
This article discusses some of the approaches we have tested for managing the bacterial pathogen Xanthomonas fragariae in infected strawberry nursery stock. X. fragariae causes angular leaf spot in strawberry and is transmitted to production fields almost exclusively through infected nursery stock. Of the methods that we have investigated over the past several years, a modified heat treatment has proven to be very effective at reducing systemic infections in propagation material, but cultivar selection affected the outcome. Surface-sterilizing treatments also had an effect on angular leaf spot. We tried procedures from dipping plants in a solution of 10% chlorine bleach to the use of UV-C radiation to reduce the severity of angular leaf spots in the field. Lastly, a sanitation-type treatment, namely removing or trimming remnant leaf and petiole tissue from nursery-trimmed plants, was found to have a significant impact on angular leaf spot. No one method completely eliminated X. fragariae from the planting stock, but there is good indication that a strategic combination of control practices that includes heat treatment should help to reduce significantly the initial amount of bacteria introduced into a field.
Keywords:
INTRODUCTION
Angular leaf spot (ALS) is a common disease of strawberry caused by the bacterium Xanthomonas fragariae (CitationKennedy and King, 1962; CitationMaas et al., 1995). The disease most notably affects the foliage and the calyx (causing “black cap” [or “brown cap”]). However, the pathogen can move systemically within the plant's vascular system and infect additional leaf tissue and crown tissue as well as developing daughter plants (CitationHildebrand et al., 1967; CitationMilholland et al., 1996). Plants with infected crowns are presumably less productive and may suffer vascular collapse if heavily infected. The pathogen is transmitted to production fields almost exclusively through infected nursery stock. This creates problems for nurseries that export plants to Europe and Mexico because of quarantine restrictions. For example, the European and Mediterranean Plant Protection Organization lists X. fragariae as an A2 quarantine pathogen (i.e., a pathogen that is absent from the majority of European countries, but has the potential to establish there). Nurseries wishing to export plants to Europe must maintain certain phytosanitary standards. Specifically, planting material must be derived from mother plants certified free of X. fragariae and production sites should be documented free from ALS for the past five growing seasons (CitationSmith et al., 1992). Nurseries currently manage ALS through a combination of rouging diseased plants, foliar application of copper bactericides, or (in worst-case scenarios) the abandonment of infected fields.
Thermal treatment using hot water (HW) immersions or hot air (HA) have been shown to be effective at killing or reducing systemic pathogens in propagation material for crops such as apple, cherry, and grape (CitationBurr et al., 1989; CitationHall et al., 2002; CitationKeck et al., 1995). CitationBuchner (1991) developed a HW treatment for strawberry where plants were submerged directly in a hot water bath for 5 to 7 min at 48–49°C. Although effective for control of some insect pests and nemotodes, this treatment was insufficient for killing X. fragariae because the exposure time was too short (CitationHerder and Turechek, 2006; CitationTurechek and Peres, 2009). Furthermore, it is believed that hot water treatment used for control of other pests has contributed to the recent increase of ALS in field production because bacteria released into the bath water from infected plant batches contaminate uninfected batches. We recently modified the thermal treatment of CitationBuchner (1991) to be effective against X. fragariae while simultaneously reducing the risk of spread between batches (CitationTurechek and Peres, 2009). This was accomplished by sealing plants in plastic bags prior to the HW treatment of either 48°C for 2 hr or 44°C for 4 hr. These treatments were selected because they were shown to be sufficient for killing X. fragariae in liquid suspension and cause minimum damage to the plants.
In general, the study above provided “proof of concept” but revealed areas where improvement was needed. Primarily, it was uncertain how to scale-up the procedure for commercial use where thousands of plants must be treated routinely to meet production demand. In our earlier study, plants were treated in small batches (∼20–25 plants). This was partially due to the design of the study, but also because it was not possible to treat large batches of bagged plants in water baths in a manner that ensured uniform heating throughout the bag. In a follow-up study, we found that the temperature within the center of a larger bag of ∼300 plants increased only slightly when exposed to the treatments listed above (unpublished data). Lastly, although the reduction in ALS was substantial, some bacteria survived the thermal treatment (CitationTurechek and Peres, 2009), so additional treatment (in the field) may be needed when conditions favor disease.
Since the initial study, we have experimented with radio frequency (RF) energy as the heating source. RF energy has been used as a thermal treatment for post-harvest insect control in storage products, primarily dried nuts and fruits, and more recently, pulse crops (chickpea, lentils, and pea) (CitationWang et al., 2001, Citation2002, Citation2007a, Citation2007b, Citation2010). Electromagnetic energy at radio frequencies of 10–100 MHz interacts directly with materials containing polar molecules and charged ions to generate heat volumetrically as compared to conventional methods (such as HW treatment) in which the heated outer layers transfer heat to the inner layers at a rate dependent upon various factors. Moreover, RF energy has higher penetration depth in agricultural commodities than microwave frequencies because of its longer wavelength.
In addition to RF heating, we have experimented with ultraviolet C radiation (aka. shortwave or germicidal UV). UV-C treatments are used in a variety of settings requiring sterilization, including air and water purification systems (such as re-circulating watering systems in greenhouses), sterilization of laboratory equipment, and food and beverage protection. UV-C lamps generally emit UV-C at a peak intensity of 254 nm, which breaks the molecular bonds in DNA. When concentrated in a closed environment, UV-C is lethal to nearly all microorganisms given sufficient exposure, essentially a product of the intensity and the duration of exposure to the radiation.
Lastly, in our routine evaluations of plants for ALS, we noticed that the source of bacteria usually (but not always) was a small leaf near the base of the plant that had not been removed during the leaf-pruning process at the nursery. We hypothesized that ALS incidence could be reduced if this remnant leaf and petiole tissue was trimmed from the plants prior to planting. Or in lieu of pruning the remnant tissue, which is a time-consuming process, the plants could be subjected to a 10% chlorine dip to eradicate the pathogen from the remaining tissue.
The following article reports on the results from a series of field trials where RF, UV-C, chlorine dips, and/or leaf-trimming were evaluated for the efficacy against ALS of strawberry.
MATERIALS AND METHODS
The Effects of RF Heating and UV-C Radiation
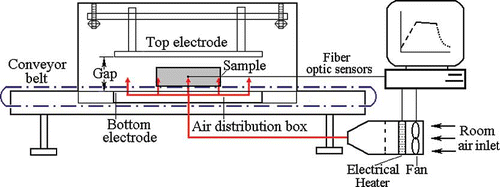
Strawberry plants of the cultivars Strawberry Festival and Ventana were shipped to Washington State University (Pullman, WA, USA) from Lassen Canyon Nursery (Redding, CA, USA) in Oct. 2009. The plants were dug fresh in California and the leaves were trimmed at the nursery. Six treatments were evaluated for the ability to reduce the incidence of ALS and their effect on plant health: 1) 1 hr exposure to 48°C (via RF), 2) 2 hr exposure to 48°C (via RF), 3) 30 min exposure to UV-C, 4) 1 hr exposure to UV-C, 5) 1 hr exposure to 48°C (via RF) + 1 hr exposure to UV-C, 6) 2 hr exposure to 48°C (via RF) + 1 hr exposure to UV-C, and 7) untreated control. For each cultivar, 48 plastic bags were each packed with five bare-root strawberry plants. Each bag was placed on a conveyer belt that was in contact with the bottom plate electrode of a 6 kW, 27 MHz pilot–scale RF system (COMBI 6–S, Strayfield International, Wokingham, UK) combined with a customized auxiliary hot air (HA) system (a 5.6-kW electrical strip heater and a blower fan) after achieving the desired HA temperature (48°C) inside the RF cavity (). The HA system provided 48°C forced hot air into the RF cavity through an air distribution box under the bottom electrode. RF power was turned on for 3 to 4 min to raise the central temperature of strawberry samples from 23°C (room temperature) to 48°C with an electrode gap of 19 cm. After the plants reached the desired temperature, the bag of plants was transferred immediately to an oven at 48°C and held for 1 or 2 hr depending upon the treatment. After incubation, samples were transferred immediately to a refrigerator (4°C) for cooling. The 48 bags of five plants each were then grouped in sets of four to create a replicate of 20 plants. There were three 20-plant replicates for each treatment. All plants were shipped overnight to the USDA-ARS-US Horticultural Research Laboratory (USHRL) Subtropical Plant Pathology Unit, Fort Pierce, FL. Upon arrival to USHRL, plants selected for UV treatment were treated for either 30 min or 1 hr and, following treatment, all plants were immediately packed and driven to the University of Florida's Gulf Coast Research and Education Center (GCREC) in Wimauma, FL where they were planted the same day (21 Oct. 2009) into methyl-bromide:chloropicrin (50:50)-fumigated soil in plastic-mulched raised beds. The treated plants were irrigated for several hours per day by overhead sprinklers for 10 to 12 days to aid establishment, then irrigated and fertilized through drip tape as needed for the duration of the study. Plants were evaluated for survival and ALS incidence on 18 Nov. 2009. The presence of ALS was assessed on every leaf on all plants.
FIGURE 1 Schematic view of the pilot-scale 6 kW, 27.12 MHz radio frequency (RF) unit showing the two-pair plate electrodes, conveyor belt, and the hot air system (CitationWang et al., 2010) (color figure available online).
The Effects of Heat Treatment, Leaf-Trimming, and Chlorine Dips
An experiment was designed to evaluate the individual and combined effects of hot water treatment (= Heat), leaf and petiole removal (= Trim), and chlorine dips (= Dip) on ALS development. The experiment was conducted twice. In both experimental trials, plants of cultivar Strawberry Festival were obtained and shipped from Lassen Canyon Nursery to USHRL where they were subjected to their treatments. The treated plants were planted at GCREC for the first experiment and at USHRL for the second experiment. In each experiment, eight treatments in four replicates, where each replicate was a group of 20 plants, were evaluated for the ability to reduce the incidence of ALS and their effect on plant health. The eight treatments were: 1) Heat, 2) Trim, 3) Dip, 4) Trim & Heat, 5) Trim & Dip, 6) Heat & Dip, 7) Trim & Heat & Dip, and 8) untreated control. In plants selected to receive the Trim treatment, any remaining leaf and petiole tissue was removed using small scissors prior to any other treatment. Plants selected for Heat treatment were first grouped in sets of 20 (i.e., experimental unit), the groups of 20 plants were sealed in separate 177 × 305 mm, 3 mil. sterile blender plastic bags (Twirl ‘Em Sampling Bags, Labplas, Quebec, Canada) and then sealed in a second plastic bag before being submerged in a water bath for 4 hr at 44°C. A separate water bath was used for the individual replications. The double-bagging was added for protection to ensure that the plants would remain dry for the duration of the treatment. Finally, those plants slated for dipping were arranged in groups of 20 prior to being submerged in 10% chlorine bleach solution for 5 min. At GCREC, treated plants were planted on 19 Nov. 2010 in 1,3-dichloropropene:chloropicrin (Telone C-35, Dow AgroSciences, Indianapolis, IN, USA)-fumigated soil in plastic-mulched, raised beds and were irrigated for several hours per day by overhead sprinklers for 10 to 12 days to aid establishment. The plants were rated on 23 Dec. 2010 and again on 20 Jan. 2011. At Fort Pierce, plants were planted in raised beds on 22 Nov. 2010 that were prepared prior to planting using anaerobic soil disinfestation (CitationButler et al., 2009). The plants were rated on 3 Jan. and again on 12 Jan. 2011. The presence of ALS was assessed on every leaf on all plants.
Data Analysis
The proportion of leaves developing ALS was transformed by first applying the Haldane transformation to the raw data [i.e., y = (0.5 + x)/(n + 1)] and then applying the arcsine transformation [i.e., arcsin(√y)] to the Haldane-corrected proportion (y), where x is the number of symptomatic leaves and n is the total number of leaves assessed in replicate plots. For the RF/UV trial, the number of plants surviving treatment was transformed via the natural logarithm. For the Heat/Trim/Dip trials, the Haldane-corrected proportion of stunted plants was calculated by dividing the total number of stunted plants (plus 0.5) by the number of surviving plants (plus 1), and then applying the arcsine transformation to the Haldane-corrected proportion. The transformed responses were analyzed in a generalized linear mixed model (GLMM) treating ‘cultivar’ (RF trial only) and ‘treatment’ as factors and ‘block’ as a random effect, and specifying an identity link function and Gaussian (normal) error distribution. Treatments were compared to the untreated control using Dunnett's multiple comparison procedure. The software package MINITAB (v. 13, Minitab Inc., State College, PA, USA) was used to conduct the analysis.
RESULTS
The Effect of RF Heating and UV-C Radiation
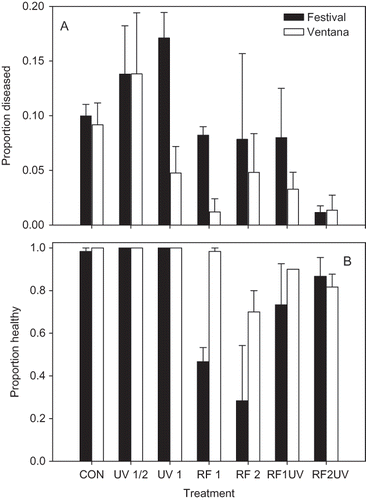
There was significant cultivar (P = 0.001) and treatment effect (P = 0.003) on the incidence of ALS, but their interaction was not significant (). The combination of the 2 hr/48°C RF heat treatment with a 1 hr UV-C (RF2UV) exposure was the only treatment that was significantly lower than the untreated control according to Dunnett's test. There was also a significant cultivar (P = 0.007) and treatment effect (P < 0.001) on the proportion of plants surviving treatment (); only the 2 hr/48°C heat treatment (RF2) had significantly lower survival than the untreated control.
FIGURE 2 Proportion of leaves with angular leaf spot symptoms (A) and the proportion of plants out of 20 surviving the treatment (B) (i.e., not stunted or killed) in field experiments conducted in GCREC in Wimauma, FL. Plants were treated with UV-C radiation for 30 min or 1 hr (UV 1/2 and UV 1, respectively), or by radio frequency (RF) treatment to achieve a temperature of 48°C for 1 or 2 hr (RF1 and RF2, respectively), or by a combination of RF treatment for 1 or 2 hr plus UV-C for 1 hr (RF1UV and RF2UV, respectively). An untreated control was also included (CON). Each bar represents the proportion of three replicate plots of 20 plants each, along with the standard error. Filled bars represent cv. Strawberry Festival and open bars represent cv. Ventana.
The Effects of Heat Treatment, Leaf-Trimming, and Chlorine Dips
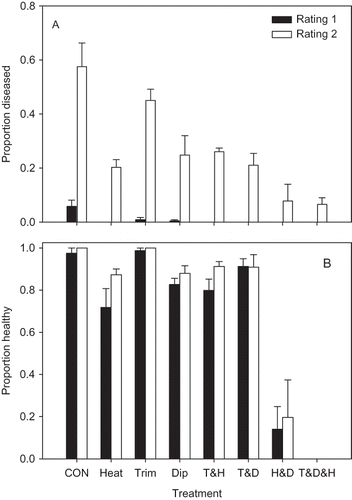
There was a significant treatment effect (P = 0.002) on the incidence of ALS for the trial conducted at GCREC for both the first and second ratings (). For the first rating, all treatments except for the Heat & Dip treatment had significantly lower ALS than the untreated control (P-value for H&D = 0.066); the high mortality in the Heat & Dip treatment inflated the standard error of the transformed proportion and affected the statistical test. For the second rating, all treatments had significantly lower ALS than the control except for the Trim treatment (P = 0.455). Plants exposed to the Heat & Dip and the Trim & Heat & Dip treatments were significantly stunted relative to the untreated control for both ratings, whereas the Heat treatment was significantly stunted for the first rating only ().
FIGURE 3 Proportion of cv. Strawberry Festival leaves with symptoms of angular leaf spot (A), and the proportion of surviving plants rated as healthy (B) (i.e., not stunted or killed) in field experiments investigating the effects of heat treatment at 44°C for 4 hr (Heat), the removal of remnant leaf and petiole tissue (Trim), a 5 min dip in a 10% solution of chlorine bleach (Dip), and all possible combinations of the three treatments. An untreated control was also included (CON). Each bar represents the proportion of four replicate plots of 20 plants each, along with the standard error. Filled bars represent the first disease rating taken 35 days after planting, and the open bars represent the second disease rating taken 64 days after planting. The experiment was conducted at GCREC in Wimauma, FL.
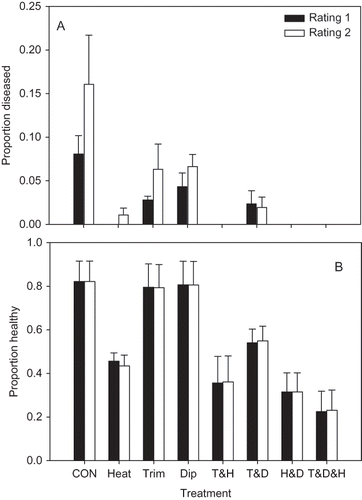
There was a significant treatment effect on the incidence of ALS for the trial conducted at USHRL for both the first (P = 0.021) and second (P < 0.000) ratings (). For the first rating, the Heat (P = 0.004) and the Trim & Heat (P = 0.008) treatments were the only two treatments that had significantly lower ALS than the untreated control; the high mortality associated with the other treatments with low disease affected the standard errors of the transformed proportions and the statistical test overall. For the second rating, all treatments except the Dip treatment (P = 0.078) had significantly lower ALS than the untreated control. The Heat & Dip, Trim & Heat, and the Trim & Heat & Dip treatments were significantly more stunted than the control for both ratings. The Heat treatment was significantly stunted at P = 0.07 for both ratings ().
FIGURE 4 Proportion of cv. Strawberry Festival leaves with symptoms of angular leaf spot (A) and the proportion of surviving plants rated as healthy (B) (i.e., not stunted or killed) in field experiments investigating the effects of heat treatment at 44°C for 4 hr (Heat), the removal of remnant leaf and petiole tissue (Trim), a 5-min dip in a 10% solution of chlorine bleach (Dip), and all possible combinations of the three treatments. An untreated control was also included (CON). Each bar represents the proportion or mean of four replicate plots of 20 plants each, along with the standard error. Filled bars represent the first disease rating taken 43 days after planting, and the open bars represent the second disease rating taken 53 days after planting. The experiment was conducted at USHRL in Fort Pierce, FL.
DISCUSSION
These trials further demonstrated that ALS can be significantly reduced or completely eliminated in small plot trials with the application of an RF heat treatment. Similar to hot-water-based heat treatments (CitationTurechek and Peres, 2009), there was some variability in plant response (i.e., stunting or death) to RF treatments indicating the need to run additional studies to improve the RF protocol before attempting to scale-up results for industrial implementation. Nonetheless, the development of an RF protocol for heat treatment has some advantages over HW treatment including: (1) commercial-scale units/systems can be purchased allowing nurseries to quickly adopt the technology; (2) the possibility of providing uniform heating and the ability to regulate the rate of heating allows the user to tailor thermal treatment for heat-sensitive cultivars; (3) because treatment occurs under dry conditions, the mixing and spread of pathogens between plant batches does not occur; (4) anaerobic respiration that occurs and adversely affects some plants when they are sealed in plastic bags and submerged in a water bath is not a factor with RF heating (unpublished data); and (5) UV-C treatment can easily be added to either the HA or RF chamber to increase what appears to be a positive survival response to UV-C treatment in RF-treated plants.
The non heating-based treatments (i.e., Trim, Dip, and UV-C) that were intended to target active infections and epiphytic bacteria on remnant leaf and petiole tissue gave mixed results. Conceptually, removal (Trim) or treatment (UV-C or Dip) of the remnant basal leaves and petioles from nursery-trimmed strawberry plants should reduce ALS since these tissues can harbor the pathogen. Indeed, plots having only the Trim or Dip treatments had lower ALS than control plots and is an indication that these remnant tissues serve as a significant source of inoculum. However, when the Trim and Dip treatments were combined with Heat, the level of control did not always improve relative to Heat treatment alone. This makes sense as it should be expected that heat treatment would be sufficient to kill bacteria in remnant tissue along with crown infections given that these tissues do not offer the same level of protection for the bacteria compared to those surviving in crown tissue. Since both the Trim and the Dip treatments offer some level of control, we need to determine if adding Trim or Dip treatments to a Heat treatment would shorten the time plants need to be exposed to heat treatment, potentially minimizing the overall damage that some plant cultivars suffer from a full heat treatment.
The most significant problem associated with heat treatment is the stunting and sometimes death of plants. Clearly, it would be ideal to have the ability to predict the level of crown infection either through routine field scouting or through the use of a diagnostic test (CitationTurechek et al., 2008) to determine if treatment is necessary and possibly avoid having to treat plants. Field-based methods to prevent the spread of bacteria in a nursery planting, such as the use of tunnels and/or drip irrigation, can be used to reduce the level of infection between rounds of propagation, but most of these practices are expensive to implement and also come at the cost of reduced establishment. So much so that the loss due to heat treatment can often be smaller than that of other practices. However, as was found in past research (CitationTurechek and Peres, 2009), flower buds are seriously affected by heat treatment so it is not an ideal treatment for the final round of propagation where plants are expected to have flower buds. Alternatives must be sought for this final round if bacteria are expected to be present. However, if heat treatment (or some combination of heat with another practice) is used routinely up to the final round of propagation, one would expect a disease-free planting (or at least a significant reduction in ALS) in the final round. It is unknown what minimum level of initial infection (if any) leads to an insignificant level of infection in the planting. This needs to be further investigated.
In summary, the need to scale-up heat treatment for use in commercial practice was one of the primary motivations for investigating the RF-based protocol and other treatments. Commercial-scale units are available for RF treatment which would allow nurseries to quickly adopt the technology. However, specific RF conditions to achieve maximum bacterial kill and minimal plant damage need to be identified.
LITERATURE CITED
- Buchner , R.P. 1991 . “ Hot water preplant dip for strawberry disease control ” . In The strawberry into the 21st century , Edited by: Dale , A. and Luby , J.L. Portland , , OR : Timber Press, Inc .
- Burr , T.J. , Ophel , K. , Katz , B.H. and Kerr , A. 1989 . Effect of hot water treatment on systemic Agrobacterium tumefaciens biovar 3 in dormant grape cuttings . Plant Dis. , 73 : 242 – 245 .
- Butler , D.M. , Rosskopf , E.N. , Kokalis-Burelle , N. , Muramoto , J. and Shennan , C. Field evaluation of anaerobic soil disinfestations in a bell pepper-eggplant double crop . Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, MBAO . pp. 43.1 – 43.4 .
- Hall , T.W. , Heidenreich , M.C. , Cicciarelli , R. , Andersen , R.L. and Turechek , W.W. 2002 . Eradication of Pseudomonas syringae pv. syringae from sweet cherry budsticks . Phytopathology , 92 : S33
- Herder , K. and Turechek , W.W. 2006 . Evaluating hot-water treatment as means for eradicating Xanthomonas fragariae in strawberry nursery stock . Phytopathology , 96 : S47
- Hildebrand , D.C. , Schroth , M.N. and Wilhelm , S. 1967 . Systemic invasion of strawberry by Xanthomonas fragariae causing vascular collapse . Phytopathology , 57 : 1260 – 1261 .
- Kennedy , B.W. and King , T.H. 1962 . Angular leaf spot of strawberry caused by Xanthomonas fragariae sp . nov. Phytopathology , 52 : 873 – 875 .
- Keck , M. , Chartier , R. , Zislavsky , W. , Lecomte , P. and Paulin , J.P. 1995 . Heat treatment of plant propagation material for the control of fire blight . Plant Pathol. , 44 : 124 – 129 .
- Maas , J.L. , Pooler , M.R. and Galletta , G.J. 1995 . Bacterial angular leafspot disease of strawberry: Present status and prospects for control . Adv. Strawberry Res. , 14 : 18 – 24 .
- Milholland , R.D. , Ritchie , D.F. , Daykin , M.E. and Gutierrez , W.A. 1996 . Multiplication and translocation of Xanthomonas fragariae in strawberry . Adv. Strawberry Res. , 15 : 13 – 17 .
- Smith , I.M. , McNamar , D.G. , Scott , P.R. and Harris , K.M. , eds. 1992 . “ Xanthomonas fragariae ” . In Quarantine pests for Europe. Data Sheets on European Communities and for the European and Mediterranean Plant Protection Organization , 829 – 833 . Wallingford, Oxon , , UK : Cab International .
- Turechek , W.W. and Peres , N.A. 2009 . Heat treatment effects on strawberry plant survival and angular leaf spot, caused by Xanthomonas fragariae, in nursery production . Plant Dis. , 93 : 299 – 308 .
- Turechek , W.W. , Hartung , J.S. and McCallister , J. 2008 . Development and optimization of a real time detection assay for Xanthomonas fragariae in strawberry crown tissue with receiver operating characteristic (ROC) curve analysis . Phytopathology , 98 : 359 – 368 .
- Wang , S. , Ikediala , J.N. , Tang , J. , Hansen , J.D. , Mitcham , E.J. , Mao , R. and Swanson , B. 2001 . Radio frequency treatments to control codling moth in in-shell walnuts . Postharvest Biol. Technol. , 22 : 29 – 38 .
- Wang , S. , Monzon , M. , Johnson , J.A. , Mitcham , E.J. and Tang , J. 2007a . Industrial-scale radio frequency treatments for insect control in walnuts: I . Heating uniformity and energy efficiency. Postharvest Biol. Technol. , 45 : 240 – 246 .
- Wang , S. , Monzon , M. , Johnson , J.A. , Mitcham , E.J. and Tang , J. 2007b . Industrial-scale radio frequency treatments for insect control in walnuts: II . Insect mortality and product quality. Postharvest Biol. Technol. , 45 : 247 – 253 .
- Wang , S. , Tang , J. , Johnson , J.A. , Mitcham , E.J. , Hansen , J.D. , Cavalieri , R. , Bower , J. and Biasi , B. 2002 . Process protocols based on radio frequency energy to control field and storage pests in in-shell walnuts . Postharvest Biol. Technol. , 26 : 265 – 273 .
- Wang , S. , Tiwari , G. , Jiao , S. , Johnson , J.A. and Tang , J. 2010 . Developing postharvest disinfestations treatments for legumes using radio frequency energy . Biosystems Eng. , 105 : 341 – 349 .