Abstract
Wine quality is determined by the stage of harvest of grapes. In order to understand the effect of ripening stages on the berry aroma and antioxidant quality, berries were grouped into three categories, namely: (a) full ripe (before skin shriveling); (b) partially shriveled; and (c) 75% shriveled berries. Total terpenoids, alcohols, hydrocarbons, radical scavenging capacity (DPPH), and total anthocyanins were higher in grapes harvested at full ripe stage, when compared to other stages. Therefore, for better aroma and antioxidants in wines, grapes should be harvested at full ripe (no shriveled berries) stage instead of overripe shriveled berry stages.
INTRODUCTION
Wine quality is determined by the combination of various volatile aroma compounds present in grapes (CitationPalomo et al., 2006). Terpenes, alcohols, aldehydes, and esters are the major volatile groups of grape aroma. Among them, terpenes are the primary group responsible for the typical flavor of grape wines. Ratio of alcohols to aldehydes is also reported to be one of the quality parameters of wine aroma (CitationKalua & Boss, 2009). Wine aroma depends on the volatile composition of berries, volatiles produced during crushing, fermentation, and aging (CitationSchreier & Jennings, 1979). The period of skin contact during fermentation is one of the important factors responsible for the aroma intensity of wines, since most of the aroma compounds are present in the skin of berries (CitationVersini & Sartori, 1981). Berries accumulate aromatic compounds during maturation and ripening. It has been reported that the grapes have both bound and free terpenoids. Relative quantities of free and bound terpenes vary with the cultivar and maturity of berries (CitationPark et al., 1991). Some of the terpenes have been reported to decrease in overripe grapes (CitationBayonove & Cordonnier, 1970, Citation1971). In ‘Shiraz’ grapes, an advanced stage of ripening was reported to have lower terpenoids (CitationHernandez-Orte et al., 2010). Anthocyanin content of berries was found to have a good correlation with wine quality (CitationBarnett, 2004). In spite of the importance of aroma for the quality of wine, grapes are being harvested at overripe shriveled stages for higher total soluble solids. However, this may reduce the quality of wine by losing many important volatile flavor compounds like terpenes, alcohols, and also anthocyanins. Therefore, to understand the effect of different ripening stages on the berry volatile aroma and antioxidant quality, volatile flavor profile, anthocyanin, total phenols, flavonoids, and antioxidant capacity were estimated at three stages of ripening in the cultivar Shiraz.
MATERIALS AND METHODS
Plant Material and Sample Preparation
‘Shiraz’ grapes were harvested from 8-year-old vines grown in Grover's vineyard located at Doddaballapur (13° 171 31″ N and 77° 321 35″ E, elevation of 880 MSL), Bangalore rural district of Karnataka, India.
Whole berries from harvested bunches were segregated into three different stages: (a) fully ripe berries with no skin shriveling (stage-I); (b) fully ripe with partially shriveled berries (stage-II), and (c) over ripe with 75% shriveled berries (stage-III). About 300 g of berries were separated from the clusters, pooled, and homogenized in a blender for 1 min. A known quantity of homogenized fruits was transferred immediately to a conical flask containing acidic methanol (99:1, Methanol: HCl) solution and incubated for 48 h under dark at room temperature for further analysis of anthocyanins, phenols, flavonoids, and antioxidant capacity. All of the biochemical analyses were done in triplicate.
Total Anthocyanin Content
Total anthocyanin was estimated by measuring the absorbance of the extract at 540 nm (CitationFuleki, 1969). The total amount of anthocyanins present in the sample was expressed as milligrams of cyanidin hydrochloride equivalents per 100 g dry weight.
Total Phenols
Total phenols were estimated following the Folin-Ciocalteu method (CitationSingleton and Rossi, 1965). Acidic methanol extract was mixed with Folin-Ciocalteu Reagent (Merck Co. Ltd., Darmstadt, Germany), and the color was developed using 20% sodium carbonate reagent. Intensity of color developed was read by measuring the absorbance at 700 nm using a spectrophotometer (Beckman DU64, Beckman Instruments International, SA, Nyon, Switzerland). Results were expressed as milligrams of gallic acid equivalents per 100 g dry weight.
Total Flavonoids
Methanol extract (1 mL) was mixed with 0.3 mL of 5% NaNO2 followed by 0.3 mL of 10% AlCl3. After 1 min, 3.4 mL of 4N NaOH was added and diluted to 10 mL with double distilled water and mixed thoroughly (CitationChun et al., 2003). The absorbance of the pink mixture was read at 510 nm and expressed as mg of catechin equivalents per 100 g dry weight.
Ferric Reducing Antioxidant Potential (FRAP)
Antioxidant capacity was measured as FRAP using a modified method of CitationBenzie and Strain (1996). At low pH, reduction of ferric tripyridyltriazine (FeIII-TPTZ) complex to the ferrous form by the antioxidants present in the sample results in an intense blue color that was measured at 593 nm to estimate the antioxidant capacity. The FRAP assay mixture, containing 200 μL of the extract and 1.8 mL of FRAP reagent, was incubated at room temperature for 40 min and the absorbance was measured at 593 nm. The standard curve was prepared using ascorbic acid as an antioxidant. Antioxidant capacity was expressed as milligrams ascorbic acid equivalent antioxidant capacity per 100 g dry weight.
Diphenyl Picryl Hydrazyl (DPPH) Radical Scavenging Ability
Radical scavenging ability was measured with DPPH radical assay (CitationShivashankara et al., 2010). Acidic methanol extract (0.2 mL) was mixed with 0.3 mL of 100 mM acetate buffer (pH 5.5) and 0.25 mL of ethanolic DPPH (0.5 mM) solution. The reduction in color due to scavenging of DPPH radicals by the antioxidants was estimated by reading absorbance at 517 nm. Radical scavenging ability was expressed as ascorbic acid equivalents.
Extraction of Volatile Aroma Compounds
Volatile flavors were extracted from the berries using the liquid:liquid solvent extraction method (CitationOng et al., 2006; CitationSerkan et al., 2006). Whole berries from harvested bunches were segregated into three different stages, as mentioned earlier, based on the extent of skin shriveling. A known quantity (15 g) of the berries were homogenized for 1 min at 4°C and the homogenate was diluted with distilled water (1:4) and 0.5 g of NaCl was added before extraction for complete recovery of aromas; the solution was then mixed with dichloromethane solvent and the mixture was incubated at 4°C for 24 h with constant stirring on a magnetic stirrer. The dichloromethane layer was separated by centrifugation and the traces of water were removed by drying over anhydrous Na2SO4, filtered through 0.2 micron filter, and concentrated to 1 ml using vacuum evaporation prior to gas chromatography (GC) injection. A known quantity of dodecanol (10 μg) was added at the time of extraction to each sample as an internal standard for quantification.
Gas Chromatography
Gas chromatography/flame ionisation detector (GC/FID) analysis was carried out using a Varian-3800 gas chromatograph system on a VF-5 column (Varian, USA), 30 m × 0.25 mm i.d., 0.25 μm film thickness. The carrier gas was helium with a flow rate of 1 ml min−1; injector and detector temperatures were 250 and 270°C, respectively, and the temperature program for the column was as follows: initially 60°C for 6 min, followed by a linear increment at 3°C/min to 200°C, hold for 3 min, then to 220°C at an increment of 10°C/min, and maintain the same temperature for 8 min. Initially the injection mode was split (1:20) followed by a splitless mode after 10 min. For the qualitative identification of volatile substances and comparative variation of retention time and index, the following standard (ethyl acetate, propanol, hexanol, 1-octene-3-ol) were co-chromatographed.
Gas Chromatography/Mass Spectrometry (GC/MS)
GC-MS analysis was performed on a Varian-3800 gas chromatograph coupled with Varian 4000 GC-MS/MS mass selective detector. Volatile compounds were separated on a VF-5MS (Varian) column ( 30 m × 0.25 mm i.d. with 0.25 μm film thickness) by applying the same temperature program and injection mode as described above for GC-FID analysis. The mass spectrometer was operated in the external electron ionization mode with the carrier gas helium 1 ml/min, injector temperature 260°C, trap temperature 220°C, ion source-heating at 230°C, and transfer line temperature 250°C; EI-mode was 70 eV with the full scan-range of 50–450 amu.
The tentative identification of peaks was carried out based on retention index, and quantification of compounds was carried out by comparing between the FID peak area of known quantity of internal standard and the individual components. Volatile compounds were identified by comparing the retention index, which was determined by using the homologous series of n-alkanes (C5 to C32) (CitationJennings & Shibamoto, 1980; CitationKovats, 1965) and by comparing the mass spectra data with the libraries available (Wiley and NIST-2007).
RESULTS AND DISCUSSION
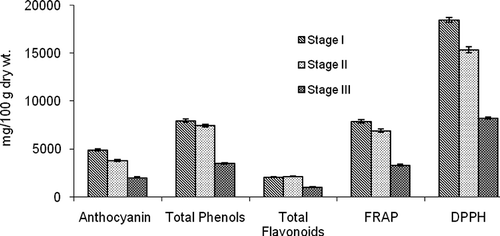
‘Shiraz’ wine grapes were harvested at the fully ripened stage and the berries were separated into three stages as mentioned earlier to understand the total antioxidant quality as well as volatile aroma changes of berries. Data on anthocyanins and polyphenols indicated that () all of the antioxidant parameters, including total antioxidant capacity and radical scavenging ability, decreased significantly in stage-III (75% shriveled berries) when compared to stage-I (full ripe berries with no shriveling). However, total polyphenols, flavonoids, and antioxidant capacity did not decrease in stage-II of ripening. Total anthocyanins, which are the indicators of color development, were highest in stage-I of ripening and decreased later. Total polyphenols, flavonoids, and FRAP were similar between stages-I and II but decreased in stage-III. Results clearly indicated that the antioxidant quality of ‘Shiraz’ grapes were high in the ripening stages-I and -II. Higher total phenols and tannins have been reported to positively correlate with the sensory grades of wine (CitationMercurio et al., 2010). Therefore, to maintain better antioxidant quality of wine, grapes should be harvested at stage-I of ripening (full ripe before shriveling of skins).
FIGURE 1 Antioxidant capacity, radical scavenging ability, total anthocyanins, phenols, and flavonoids in Stage I (fully ripe berries before skin shriveling), Stage II (partially shriveled berries), and Stage III (75% shriveled berries) of ripening.
Apart from the color and polyphenols, another important quality parameter for flavor is volatile aroma. Volatile aroma consists of a mixture of terpenoids, esters, alcohols, acids, aldehyde, norisoprenoids, methoxypyrazines, etc. The combination of these aroma compounds are variety specific. Variation in the combination or proportion of these compounds changes the flavor of the wines. Among the aroma compounds, linalool, terpeneol, geraniol, nerol, ionone, demascenone, and hexenol were found to be the major compounds at the fully ripe stage in ‘Shiraz’ grapes (CitationHernandez-Orte et al., 2010). A reduction in the sum of terpenoids, norisoprenoids, volatile phenols, and vanillin compounds in overripe stages was also reported by the same authors.
A significant reduction in the concentration of hydrocarbons, terpenoids, and alcohols was observed in ‘Shiraz’ grapes in stage-III of ripening (). However, acids and esters increased from stage-I to stage-IIII. Variations in the relative percentages of individual compounds indicate the change in the overall odor perception of the aroma. Relative percentages of important volatiles, like terpenoids, alcohols, aldehydes, hydrocarbons, and ketones, decreased in the ripening stage-III. On other hand, percentage of acids increased significantly in the same stage. These changes may affect the wine quality adversely since terpenoids have been associated with better wine flavor (CitationFenoll et al., 2009). This was further seen by the lower alcohol/aldehyde ratios in the stage-III berries, which is often considered as one of the quality parameters for wine grapes.
TABLE 1 Concentration of Different Groups of Volatiles and Their Relative Percentage in ‘Shiraz’ Berry Skins at Three Stages of Ripening
Among the hydrocarbons, pentadecane and napthalene compounds were more in stage-I and decreased in the stage-III berries (). The relative percentages of napthalene and pentadcane were 1.73 and 2.26% in stage-I berries and 0.486 and 0.41%, respectively, in stage-III berries. The major tepenoid compound, menthene, decreased from 4.65 to
TABLE 2 Quantity and Relative Percentages of Volatile Constituents in ‘Shiraz’ Grapes at Three Stages of Ripening
Benzyl alcohol and 1-propoxy-2-propanol were the major alcohols in stage-I berries (4.43 and 5.22%, respectively). However, in stage-III the relative percentage was drastically reduced to 0.8 and 0.9%, respectively. Acids do not have a significant contribution to the volatile aroma flavor. However, if their content increases drastically they can be a source of off flavor. Data on acids indicated that the relative percentage of total acids increased from 16.66% in stage-I to 46.66% in stage-III berries. Hexadecanoic acid increased significantly from nil to 17.9% from stage-I to stage-III berries. Octadecanoic, syringic, and dodecanoic acids also increased drastically in the shriveled berries. These acids may contriblute to the development of off flavors in wine.
Esters contribute fruity aromas to the wine, some of which may be produced during wine preparation but the majority are coming from the berries. Methyl syringate and methyl octadecadienoate are the major esters in all three stages of ripening. Methyl linoleate was significantly higher in stage-III berries (4.21%) when compared to stage-I (0.42%).
Vinylphenols and vanillic compounds were reported to be the major groups in ‘Shiraz’ grapes grown in Spain (CitationHernandez-Orte et al., 2010). However, esters, acids, and alcohols were found to be the major compounds in our grapes, whereas vanillic compounds and phenols were found in lower quantities. This difference may be due to geographical location as well as variations in the bound and free aroma compounds in the berry skins.
CONCLUSIONS
Results clearly indicated that total antioxidant capacity, phenols, flavonoids and anthocyanins, alcohols, terpenoids, hydrocarbons, and aldehydes were more in the full ripe berries before skin shriveling (stage-I) compared to overripe 75% shriveled berries (stage-III). Therefore, harvesting before skin shriveling begins may yield better wine in terms of flavor and antioxidant quality.
ACKNOWLEDGMENTS
This study was taken up under the “ICAR Network Project on Impact, Adaptation and Vulnerability of Indian Agriculture to Climate Change.” We are also thankful to the Director, IIHR for providing the facilities.
LITERATURE CITED
- Barnett , T. 2004 . Berry color as a wine quality indicator at Brown Brothers winery . Wines & Vines , 85 : 38 – 41 .
- Bayonove , C. and Cordonnier , R. 1970 . Recherches sur l'ar6me du muscat. 1. Evolution des constituants volatils an cours de la maturation du Muscat d'Alexandrie . Ann. Technol. Agr. , 19 : 79 – 93 .
- Bayonove , C. and Cordonnier , R. 1971 . Recherches surl'ar6me du muscat. 111. Etude de la fraction terpénique . Ann. Technol. Agr. , 20 : 347 – 355 .
- Benzie , I.F.F. and Strain , J.J. 1996 . The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power” the FRAP assay . Anal. Biochem. , 239 : 70 – 76 .
- Chun , O.K. , Kim , D.O. , Moon , H.Y. , Kang , H.G. and Lee , C.Y. 2003 . Contribution of individual polyphenolics to total antioxidant capacity of plums . J. Agr. Food Chem. , 51 : 7240 – 7245 .
- Fuleki , T. 1969 . The anthocyanins of strawberry, rubber and onion . J. Food Sci. , 34 : 365 – 369 .
- Fenoll , J. , Manso , A. , Hellín , P. , Ruiz , L. and Flores , P. 2009 . Changes in aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening . Food Chem. , 114 : 420 – 428 .
- Hernandez-Orte, P., N. Loscos, M. Suarez, J. Cacho, and V. Ferreira. 2010. Evolution of the aromatic potential during ripening of syrah grapes exposed to different irrigation strategies. 22 October 2011 http://www.oiv2010.ge/poster/posr.../p.i.01-33_%20coumincacio.pdf (http://www.oiv2010.ge/poster/posr.../p.i.01-33_%20coumincacio.pdf)
- Jennings , W. and Shibamoto , T. 1980 . Qualitative analysis of flavour and fragrance volatiles by glass capillary gas chromatography , 472 New York : Academic Press .
- Kovats , E. 1965 . Gas chromatographic characterization of organic substances in the retention index system . Adv. Chromat. , 1 : 229 – 247 .
- Kalua , C.M. and Boss , P.K. 2009 . Evolution of volatile compounds during the development of cabernet sauvignon grapes (Vitis vinifera L.) . J. Agr. Food Chem. , 57 : 3818 – 3830 .
- Mercurio , M.D. , Dambergs , R.G. , Cozzolino , D. , Herderich , M.J. and Smith , P.A. 2010 . Relationship between red wine grades and phenolics. 1. Tannin and total phenolics concentrations . J. Agr. Food Chem. , 58 : 12313 – 12319 .
- Ong , B.T. , Nazimah , S.A.H. , Osman , A. , Quek , S.Y. , Voon , Y.Y. , Hashim , D.M. , Chew , P.M. and Kong , Y.W. 2006 . Chemical and flavour changes in jackfruit (Artocarpus heterophyllus Lam.) cultivar J3 during ripening . Postharvest Biol. Technol. , 40 : 279 – 286 .
- Palomo , E.S. , Perez-Coello , M.S. , Diaz-Maroto , M.C. , Vinas , M.A.G. and Cabezuda , M.D. 2006 . Contribution of free and glycosidacally bound volatile aroma compounds to the aroma of muscat ‘a petit grains’ wines and effect of skin contact . Food Chem. , 95 : 275 – 289 .
- Park , S.K. , Morrison , J.C. , Adams , D.O. and Noble , A.C. 1991 . Distribution of free and glycosidically bound monoterpenes in the skin and mesocarp of Muscat of Alexandria grapes during development . J. Agr. Food Chem. , 39 : 514 – 518 .
- Schreier , P. and Jennings , W.G. 1979 . Flavor composition of wines. A review . Crit. Rev. Food Sci. Nutr. , 12 : 59 – 111 .
- Singleton , V.L. and Rossi , J.A. 1965 . A colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents . Amer. J. Enol. Viticult. , 16 : 144 – 158 .
- Serkan , S. , Canbas , A. , Cabaroglu , T. , Erten , H. and Günata , Z. 2006 . Aroma components of cv. Muscat of Bornova wines and influence of skin contact treatment . Food Chem. , 94 : 319 – 326 .
- Shivashankara , K.S. , Jalikop , S.H. and Roy , T.K. 2010 . Species variability for fruit antioxidant and radical scavenging abilities in mulberry . Intl. J. Fruit Sci. , 10 : 355 – 366 .
- Versini , G.S.I. and Sartori , G. 1981 . A capillary column gas chromatographic research into the terpene constitutents of Riesling Renano wine from Grentino Alto Adige. Their distribution within berries their passage into must and their presence in the wine according to different wine making procedures . Vino Ital. , XXIII : 189 – 211 .