Abstract
Impact of canopy management practices were assessed on canopy architecture, yield, and fruit composition of Norton grapes. Combinations of shoot thinning (ST), shoot positioning (SP), and leaf removal (LR) were imposed. The highest total soluble solids and lowest acidity were recorded either in LR or its combination with ST+SP treatments. Malic acid and potassium concentrations were highest on control, ST, SP, ST+SP vines. Canopy characteristics measured by point quadrat analysis differed significantly between treatments with more leaf layer number and less percent gap in control vines and in vines without LR treatments. These same treatments also recorded highest percent interior leaves and clusters.
INTRODUCTION
‘Norton’ (Vitis aestivalis), also referred as ‘Cynthiana’, is a popular wine grape cultivar grown in the Eastern and Midwestern states of the United States. ‘Norton’ grapes are grown in an area of about 128 ha, which accounts for about 19.3% of the total area under grape cultivation in Missouri. These vines are very vigorous and have a procumbent growth habit. It produces medium to full-bodied dry red wine with some fruit tones. But, it has some unusual fruit composition characteristics, which make it difficult to grow and turn into a premium wine. Growing and making wine from these grapes in a similar manner as Vitis vinifera grapes will seldom result in a premium wine (CitationMain & Morris, 2004). It is high in titratable acidity (up to 15 g/L), malate (up to 6g/L), and potassium (up to 6 g/L) coupled with high juice pH (>3.5). It has poor color in warmer years, aggressive seed tannins, small clusters, small berry size, and low juice yield. Despite these challenging features, with proper management practices, an excellent wine can be made from ‘Norton’ grapes (CitationMain, 2005). Although malic acid contributes greatly to the acidity of musts, it possesses an aggressive, less desirable taste than tartaric acid. Canopy management practices, like partial defoliation, could increase total soluble solids and reduce acidity, malic acid, pH, and potassium levels in fruit (CitationWolf et al., 1986; CitationKliewer & Bledsoe, 1987), although some workers have not observed any effects on fruit composition (CitationKoblet, 1984, CitationWilliams et al., 1987). Most of the ‘Norton’ grape vineyards generally suffer from excessive vegetative growth, which results in disturbances of source sink relationships, an increase in canopy density, and an inferior canopy microclimate for the efficient photosynthetic activity of leaves.
Canopy management practices in wine grape cultivation have been developed with an aim of optimizing sunlight interception, photosynthetic capacity, and fruit microclimate to improve fruit yield and wine quality especially in vigorous and robust growing cultivars with dense canopies. For wine making, significant benefits have been harnessed from comprehensive approaches to control shoot vigor through the use of different methods of pruning, deficit irrigation techniques, rootstocks, and other canopy management practices (CitationSmart, 1985).
Canopy management practices along with balanced pruning, training, and trellising is primarily focused on altering canopy components. The cluster microclimate during fruit development is also changed, mostly in favor of improved light distribution in the canopy (CitationMeyers & Vanden Heuvel, 2008). Altering the physical appearance of canopy by judicious canopy management practices also has physiological implications that virtually always comprise a change in source-sink relationships in the grapevine and a simultaneous improvement in photosynthetic activity and the translocation of photosynthates from leaves to sinks, such as berries (CitationJohnson et al., 1982; CitationHunter & Visser, 1988; CitationCandolfi-Vasconcelos & Koblet 1990 CitationHunter et al., 1995; CitationKoblet et al., 1996). Integrating the canopy management practices, which are necessary for controlling vigor, and the accumulation of secondary metabolites, which determine wine quality in ‘Norton’, remains a challenge with its distinct fruit composition.
Sunlight intensity received at different zones in the vine canopy is known to strongly influence fruit composition, such as sugars, acids, and other secondary metabolites involved in wine aroma including phenolics (CitationDowney et al., 2006). Accordingly, many viticultural treatments associated with canopy management are intended to manipulate photosynthetic photon flux (PPF) of the fruiting zone or the distribution of photon flux across the total leaf area of the canopy to achieve metabolic effects. Point quadrat analysis (PQA) has been used for decades to measure and compare microclimatic indicators of a canopy, including canopy consistency, leaf area density, cluster exposure, and leaf area source-sink balance (CitationSmart & Robinson, 1991).
In most grape cultivars during fruit ripening, changes occur in the berry causing a marked reduction in the conversion of glucose and fructose to malic acid and a cessation of tartaric acid biosynthesis. Tartaric acid per berry remains approximately constant, because it is not broken down, but malic acid declines in concentration as it is utilized as substrate in respiration (CitationHardy, 1968). But in some of the cultivars like Norton the concentration of malic acid is too high at the ripening stage, which poses a severe problem for maintaining desirable juice pH during wine making. Viticultural research on ‘Norton’ grapes with respect to the influence of canopy management practices and canopy microclimate on canopy characteristics and fruit composition is very limited (CitationMain & Morris, 2004, Citation2008; CitationStriegler et al., 2009; CitationMorris & Main, 2010). Hence, the present experiment was conducted with the objective of examining the influence of different canopy management practices on canopy characteristics and fruit composition of ‘Norton’ grapes trained to a high unilateral cordon system.
MATERIALS AND METHODS
This experiment was conducted in a 10-year-old ‘Norton’ vineyard located at Huntsdale (Boone County), Missouri during the fruiting season of 2009–2010. The vines were on their own roots and were trained to unilateral high cordon system with a spacing of 9 × 11 ft. The soil type in the experimental vineyard was Menfro silt loam (60004), well drained, no zone of moisture saturation or root restrictive layers with high available water capacity. The row orientation was north to south and shoots on cordons were trained on both east and west side of the canopy. During both of the years, vines were individually balance pruned during the dormant season to facilitate collection of vegetative data. Pruning was done utilizing a 50+10 formula standardized for ‘Norton’ grapes for the region. Each treatment was replicated six times with three vines per replication.
Canopy Management Practices
Seven different canopy management treatments were imposed on vines including control vines. The details of canopy management practices are as follows: Shoot thinning (ST), shoot positioning (SP), leaf removal (LR), ST+SP, ST+LR, SP+LR, ST+SP+LR, control. Shoot thinning was performed at the time of flowering by selecting unfruitful or weak shoots arising from the same node. Shoot positioning was performed manually by positioning the vertically growing shoots to a downward orientation twice; one immediately after fruit set and again a week later. Leaf removal was done after fruit set by removing in a 15 cm zone of leaves from the base of the shoot, thus exposing clusters. Leaf removal was done only on the east side of the canopy to avoid exposing clusters to western sunlight, particularly at mid day.
Point Quadrat Analysis and Photosynthetic Photon Flux Measurement
Point quadrat analysis was performed at the time of veraison by inserting a thin metal rod into the fruiting zone along the transverse axis of the canopy row (Smart & Robinson, 1991). Two-vine panels were designated and a tape measure was used as a guide for insertion, which were made at 20-cm intervals along the length of each two vine panels at the height of the fruiting wire, resulting in about 30 insertions per panel. Canopy characters were calculated as explained by CitationMeyers and Vanden Heuvel (2008) PPF (photosynthetic photo flux) was measured with an AccuPAR LP-80 photosynthetically active radiation sensor (Decagon Devices, Pullman, WA, USA). For the ambient measurements, the ceptometer sensor bar was oriented parallel to the ground, with the sensors facing directly upward toward the sky. Canopy components were analyzed using the software Enhanced Point Quadrat Analysis (EPQA) and Calibrated Exposure Mapping (CEM) Tools version 1.6.2 (CitationMeyers & Vanden Heuvel, 2008)
Yield Components and Analysis of Basic Fruit Composition
Fruit harvesting was done separately on both sides of the canopy (east and west sides) to compare the influence of canopy side on fruit composition. Ten sample clusters from each treatment replicate were harvested and placed in plastic bags and transported to the laboratory in a refrigerated truck maintained at a temperature of 4°C. One set of fresh samples was used for analysis of basic fruit composition parameters, such as total soluble solids (TSS), juice pH, and titratable acidity (TA). A second set of samples was stored in sealed plastic bags at −17°C for one month before analyzing for anthocyanins, tannins, and phenols by spectrophotometer following a protocol developed by Australian Grape and Wine Research Institute and for analysis of organic acids (malic acid, tartaric acid, citric acid, and lactic acid) and sugars (glucose and fructose) by high performance liquid chromatography (HPLC).
Spectrometric Analysis of Tannins, Anthocyanins, and Sugars
Frozen berries were removed from cold storage and thawed overnight under refrigerated conditions (4°C) and approximately 100 berries were weighed and homogenized in a Retsch (Düsseldorf, Germany) Grinder at a speed of 8000 rpm for 20 s. One gram of homogenate was taken in 10-ml plastic centrifuge tubes and 10 ml of 50% (v/v) aqueous ethanol was added and the mixture was agitated for 1 h at 400 rpm. Then the mixture was thereafter centrifuged at 1800 rpm for 10 min. The supernatant (extract) was used for estimation of anthocyanins, total phenols, and tannins.
For analysis of anthocyanins and total phenols, about 200 μL of extract was transferred to acrylic cuvettes and 3.8 mL of 1.0 M HCl was added and covered with paraffin film and mixed by inverting. The mixture was incubated for 3 h at room temperature and the color was measured at 520 nm for anthocyanins and 280 nm for total phenols.
For analysis of tannins, the methyl cellulose polymer tannin binding assay was performed, wherein 0.04% methyl cellulose was added to 1 mL of the extract and left for 3–4 min for the reaction to take place. Later, saturated ammonium sulfate was added and volume was made up to 10 ml. The reaction mixture was incubated for 10 min at room temperature, centrifuged for 5 min at 4000 rpm, and the absorbance of supernatant was read at 280 nm. Blank reactions were carried out in a similar manner without methyl cellulose and both the readings were used for calculating tannin concentration.
HPLC Analysis of Organic Acids and Sugars
Fruit samples stored at −17°C were kept for overnight thawing under refrigerated conditions (4°C) and on the day of extraction the samples were heated in a water bath at 70°C and were pressed to extract juice. The juice samples were filtered through double layer cheese cloth, centrifuged at 4000 rpm for 3 min, and supernatant was collected. The samples were diluted 50 times with distilled water and were filtered through nylon filters (200 μ) and kept in sample vials.
HPLC Conditions
The mobile phase consisted of 0.045 N H2SO4 and 6% acetonitirile in deionized water with a flow rate of 0.5 ml/min. A Biorad ion exclusion column [Aminex HPX 87H (300 × 7.8 mm), Life Science Research, Hercules, CA, USA] was used for the analysis. A UV 9q detector at 210 nm was used for organic acids and refractive index 6A detector was used for estimation of sugars. The total run time was 30 min. A standard curve was developed using citric acid, tartatic acid, malic acid, lactic acid, glucose, and fructose as standards at the concentration of 1 mg/mL.
Statistical Analysis
Data were analyzed using SAS statistical software (Version 9.1; SAS Institute, Cary, NC, USA). Tukey's studentized range test was used to separate means between treatments.
RESULTS
Yield and Basic Berry Composition
Canopy side influenced some yield and cluster characteristics, such as number of clusters, cluster weight, juice TA, and pH (). More clusters were observed on the west side of the canopy while the highest cluster weight (139 g) was recorded on the east side of the canopy. TSS did not differ between canopy sides and no differences were recorded with respect to berry weight between canopy sides.
TABLE 1 Yield Parameters and Basic Fruit Composition of ‘Norton’ Grapes in Response to Canopy Management Practices, Huntsdale, MO, USA
Yield and cluster numbers differed among treatments, whereby the highest cluster numbers were observed in the SP+LR treatment, and fewer clusters were observed on vines that received shoot thinning treatments either alone or in combination with other management practices due to reduced number of shoots (). Similar trends were noted with respect to yield per vine. Few differences were recorded for all other yield and basic fruit composition variables, such as berry weight, cluster weight, TSS, TA, juice pH, and juice potassium. Although no differences were observed for juice potassium content, it tended to be lowest on vines with ST+SP+LR treatments (1399 ppm) and highest on ST, SP, and control vines (1509 to 1554 ppm).
Analysis of Biochemical Composition
Canopy side influenced concentrations of citric acid, malic acid, anthocyanin, and phenol concentrations (). The highest citric acid (0.52 g/L) and malic acid (5.35 g/L) concentrations were observed in fruits on the east side. In contrast, the highest anthocyanins (3.45 mg/g) and phenols (3.24 AU/g) were also observed on the east side of the canopy.
TABLE 2 Concentration of Organic Acids, Sugars, Anthocyanins, Tannins, and Phenols in ‘Norton’ Grapes as Influenced by Canopy Management Practices, Huntsdale, MO, USA
Canopy management practices likewise impacted biochemical composition (). Concentrations of citric acid, malic acid, and tartaric acid differed among treatments with the least citric acid observed in the LR treatment alone or in combination with other treatments. Malic acid concentrations also differed among treatments with the least (3.92 g/L) in the LR treatment followed by the SP + LR (3.98 g/L) and ST + SP + LR (4.17 g/L) treatments. The highest malic acid levels were found in the control (7.82 g/L), followed by those on ST + SP (6.29 g/L). No significant differences could be observed with respect to tartaric acid concentrations. Differences were observed in fructose concentrations whereby vines that received LR either alone or in combination with other treatments (ST + LR, SP + LR, ST + SP + LR) ranged from 116.67 to 120.11 g/L, while the least fructose was recorded in shoot thinned (110.57 g/L) and control (112.65g/L) vines.
Concentration of anthocyanins differed between treatments, with either control vines or vines that received ST + SP + LR treatments having lowest anthocyanin concentrations (2.97 to 2.99 mg/g) followed by ST vines (3.01 mg/g). The highest anthocyanin concentration was recorded in the LR treatment (3.65 mg/g), which was similar to the ST + LR and SP + LR treatments. Canopy management practices did not influence the concentrations of glucose, tannins, and phenols.
Canopy Assessment
There were observed differences in canopy characteristics analyzed through PQA (). The largest canopy gaps (33.33%) were recorded in vines that received ST+SP+LR treatments followed by other treatments with a combination of LR. No gaps could be recorded either in control vines or vines which received ST or ST + SP treatments. Leaf layer number was highest on control vines (4.91) followed by SP (3.88) vines. The lowest leaf layer number was recorded in ST + SP + LR vines (1.59) followed by LR, ST + LR, and SP + LR vines. Only 1.51 to 8.86% of the ambient photosynthetically active radiation (PAR) was received inside the canopy at fruit zone in control vines or ST, SP, ST + SP vines, while vines with LR, ST + SR, SP + LR, and ST + SP + LR treatments received about 37.03 to 69.54% of the ambient PAR at fruit zone inside the canopy. The other canopy component factors, with enhanced point quadrat analysis, are shown in .
TABLE 3 Canopy Characteristics of ‘Norton’ Vines as Influenced by Canopy Management Practices as Analyzed by Enhanced Point Quadrat Analysis, Huntsdale, MO, USA
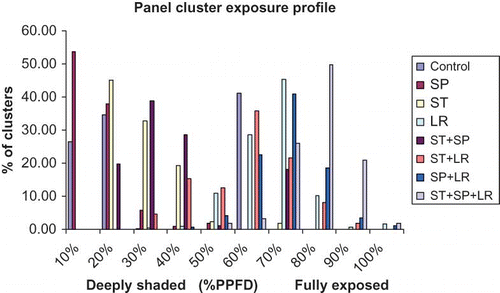
Panel cluster exposure profile
It is evident from that about 30–50% of the clusters were situated in vine canopy which received less than 40% of PPFD (photosynthetic photon flux density) on vines which received treatments, such as SP, ST, and ST + SP including control vines, indicating less exposure of clusters to sunlight in the range of 10–20% of PPFD. However, the percentage of clusters that were exposed to >50% of sunlight were situated on vines that received treatments, such as LR, ST + LR, SP + LR, and ST + SP + LR. On these vines, maximum percent of clusters were well exposed to sunlight and were not situated in a deeper canopy layer.
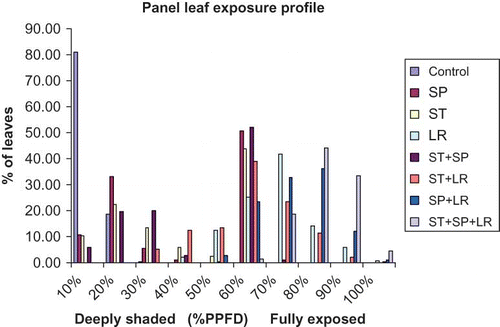
Panel leaf exposure profile
indicates that the maximum percent of leaves were situated in the canopy zone, which received only 20–30% of PPFD on vines that received ST, SP, and ST + SP treatments including control vines. However, on vines that received LR, ST + LR, SP + LR, and ST + SP + LR treatments, maximum percent of leaves were situated in the canopy zone, which received more than 50–60% of PPFD, indicating less percent of leaves in the shaded zone, which received less than 10–20% of PPFD.
DISCUSSION
Canopy management includes a range of techniques that can be applied in a vineyard to alter the position or amount of leaves, shoots, and fruits in space to harness maximum benefits of microclimate. These practices include shoot thinning, leaf removal, shoot vigor control, and improved training systems. The main objective of our study was to improve the fruit composition in terms of reducing malic acid and potassium content in juice, which in turn reduces juice pH to solve the problems encountered during wine making from ‘Norton’ grapes.
Adequate fruit exposure to sunlight promotes wine quality, though the effect of leaf and fruit exposure could not be separated experimentally. Shaded berries accumulated more potassium, malic acid, tartaric acid, and sometimes anthocyanin contents (CitationKliewer and Smart, 1989).
Most of the yield and fruit characteristics, including basic fruit composition parameters, did not differ among canopy management treatments except for the number of clusters and yield per vine. Increased number of clusters and yield per vine was recorded on vines that did not receive ST treatment, which indicates a positive relationship between numbers of shoots and clusters per vine. Increased yield on those treatments might also be attributed to more leaf area. More clusters were recorded on the west side of the canopy in most of the treatments. This might be due to an asymmetrical canopy architecture created by wind driven shoots from east to west, which resulted in a higher number of shoots on the west side of the canopy, thus producing more clusters as discussed by CitationTarara et al. (2005). The increased cluster size in shaded canopies, as also reported by CitationCrippen and Morrison (1986) and CitationReynolds et al. (1996), may be due to lower temperatures and reduced transpiration in the shaded fruit zone. The increased cluster weight in ST and control vines compared to LR and other treatments is similar to findings of CitationHaselgrove et al. (2000). According to CitationRistic et al. (2007), shading reduced the size of the berry by 20% while total concentration of anthocyanins was not affected by shading, but their individual composition was affected in ‘Cabernet Sauvignon’. Both shaded and normal berries had the same brix indicating no compromise of photosynthesis as foliage was not shaded, while in contrast, TA and pH were higher in shaded berries suggesting increased malic and potassium levels. Similar findings of increased TA in shaded conditions and reduced TA in partially defoliated vines was observed by CitationWolf et al. (1986) and CitationKliewer and Lider (1970). In contrast, reduced TSS and increased acidity and juice potassium from those vines explains the importance of exposed canopy to harness sufficient sunlight into the canopy. In ST, SP, ST + SP treatments including control vines more leaf layer number and less canopy gaps were observed (). Those vines received less sunlight inside the canopy, thereby increasing maximum percentage of interior clusters () and leaves () to be positioned in canopy zone with less light intensity and more shade.
Importance of canopy/cluster exposure to optimum sunlight is evident with respect to most of the biochemical composition, which differed significantly between treatments. In grapevine canopies, depending on canopy management practices, leaves and bunches can develop in zones varying from heavily shaded to fully exposed clusters. Generally, the berries that develop in open canopies have high sugar concentrations, improved acid metabolism, and increased concentrations of berry phenolics including anthocyanins (CitationGladstones, 1992). Although it is worth noting that leaf removal decreased fruit weight in ‘Gewurztraniber’, ‘Seyval’, ‘Cabernet Sauvignon’, ‘Sangiovese’, and ‘Trebbiano’ grapes, many investigators found that sunlight exposed fruits are generally rich in total soluble solids and reduced titratable acidity, compared to non-exposed or canopy shaded berries (CitationKliewer & Lakso, 1968; CitationFerree et al., 2004; CitationKliewer & Dokoozlian, 2005 CitationSantesteban & Royo, 2006; CitationMain & Morris, 2004). But, in contrast, some workers found that defoliation had no effect on soluble solids and titratable acidity (CitationVasconcelos & Castagnoli, 2000; CitationHowell et al., 1994; CitationPoni et al., 2006). In our study, though we did not record significantly different soluble solid concentration among treatments, the TSS was highest on vines that were exposed to sunlight through LR treatments. The increased TSS in vines that received LR and combinations might be either due to remobilization of stored carbohydrates, an increase in photosynthetic activity of remaining leaves and improvement in the light microclimate of the remaining leaves, and an increase in sink strength (CitationKliewer & Antcliff, 1970), and increases fruit temperature and changes in the pattern of assimilate movement (CitationBledsoe et al., 1988).
Influence of leaf removal performed at different stages of berry development was studied by different workers. Leaf removal is usually performed in the fruit zone during vegetative season between fruit set and ripening (CitationPoni et al., 2006). If it is done at the veraison stage, it affects synthesis of primary and secondary metabolites and this effect is directly related to leaf layer number, photosynthetic rate, and canopy surface area. Several experiments have shown increased sugars, flavor, and flavonoids and decreased acidity when leaf removal was done at the veraison stage (CitationPercival et al., 1994; CitationPoni et al., 2006; CitationZoecklein et al., 1992, Citation1998). The leaf removal may affect source sink relations, modifying the activity of light-induced enzymes, such as PAL, and to better microclimate conditions (Dokoozlian & CitationKliewer, 1995; CitationHaselgrove et al., 2000). In contrast, leaf removal at veraison on plants with low canopy density does not affect grape sugar, acidity, or color (CitationReynolds et al., 1986). In ‘Norton’ grapes, no information is available on the stage at which the leaf removal operation should to be performed. Standardizing the time of leaf removal may be one of the areas of research to be conducted to study its impact on fruit quality.
Reduced anthocyanin concentration was recorded on both control vines and on vines that received ST + SP + LR treatment. Vines in the control treatment recorded the maximum number of leaf layers (4.7) with absolutely zero light interception inside the canopy. It is likely that both light and berry temperature (either in excess or reduced quantity) may be the factor in the accumulation of anthocyanins. This is in accordance with reduced anthocyanins in both shaded berries and fully exposed clusters in ‘Shiraz’ berries reported by CitationHaselgrove et al. (2000), where fully exposed clusters recorded relatively higher berry temperature due to more exposure to sunlight, which might have reduced anthocyanin accumulation or increased degradation. The increased anthocyanin accumulation in clusters on vines that received LR, ST+LR, and SP+LR treatments suggest that if light conditions within the canopy are such that bunches/cluster zone receives sufficient sunlight of moderate intensity, then light is not necessarily a limiting factor for anthocyanin accumulation (CitationKeller & Hrazdina, 1998). However, these effects may also be temperature dependent as explained by CitationMabrouk and Sinoquet (1998). Enzymes involved in the anthocyanin biosynthesis pathway exhibit an optimum temperature range for activity. CitationKeller and Hrazdina (1998) suggested that the breakdown of anthocyanins may be caused by glycosidase and peroxidase activity in grape skin vacuoles.
Point quadrat analysis (PQA) was first described by CitationSmart (1982) to characterize canopy components, such as distribution of leaves and fruits in space, thereby promoting quantitative description of canopy. Though ST reduced the number of shoots in the present study, the vigorous nature of retained shoots and extensive lateral growth and leaf production on lateral shoots must have increased intense shade in the canopy, which is obvious by leaf layer numbers and the reduced percentage of PAR received in the canopy. All the canopy management practices either LR alone or in combination of ST and SP might have increased the light conditions in the canopy, especially in the cluster zone. The leaf thinning at cluster zone resulted in a canopy that allowed sunlight to penetrate in such a way that the interior leaves and bunches received diffused sunlight in the interior leaf layer, which became more photosynthetically active than in the treatments where dense canopy with more number of leaf layers, which might prevent sunlight reaching interior leaves. Concentrations of citric and malic acid were highest on vines that received treatments, such as ST, SP, and ST + SP, including control vines. However, the concentration of tartaric acid was lowest on vines that received the same treatments and were highest on vines that received LR, ST + LR, SP + LR, and ST + SP + LR treatments.
This is in confirmation to studies of Carbonneau and Huglin (1982) and CitationSmart (1983), who also recorded more malic acid and less tartaric acid concentration in clusters that were situated in shaded canopies. Tartaric acid and malic acid generally account for 69–92% of all organic acids in grapes. Malic acid levels vary greatly as the berries develop and mature, whereas tartaric acid levels vary considerably less (CitationKliewer, 1965, Citation1967). CitationLakso and Kliewer (1975), in their studies on influence of temperature on malic acid metabolism in grape berries, reported the simultaneous action of malic acid producing enzyme (PEP carboxylase and malic dehydrogenase) and malic acid degrading enzyme (malic enzyme) systems at different temperatures, the greatest tendency for malic acid accumulation was at 20–25°C. They could also observe greater loss and lesser recovery of PEP carboxylase over the 4 h period at 40°C compared to loss and recovery of malic enzyme (malate degradation activity), which they explained through lower levels of malic acid observed in grapes at a higher temperature. Sun exposed grape berries have also been found to reduce malic acid in the field as observed by CitationKliewer and Lider (1968). The reduced malic acid concentration on LR, ST + LR, SP + LR, and ST + SP + LR treated vines might be due to increased berry temperature, which might have decreased the activity of malic acid synthesizing enzymes as explained above.
Fructose was the dominant sugar in ripe berries and differed significantly among treatments, which is in accordance with the findings of CitationHunter et al. (1995). The concentration of fructose was highest on vines that received either LR treatment alone or in combination with ST and SP. The increased fructose and reduced malic and citric acid concentration on vines that received LR treatment in combination with ST and SP is explained by the canopy cluster exposure profile and the canopy leaf exposure profile. The importance of canopy exposure (both leaf and cluster) to sunlight and adverse effect of more shaded canopy is explained by several workers in the past, which holds true for this present study also. Grapevine leaves are strong absorbers of sunlight (solar radiation) in the 400–700 nm zone, which is popularly called photosynthetically active radiation (PAR). As only about 6% of light in this wave band is transmitted by a leaf, light levels at the center of the dense canopies are very low, often less than 1% of the above canopy value (CitationSmart, 1985). Light encountered in shade conditions has altered quality as well as quantity with important physiological implication for leaves and fruit shade conditions. Shade can be avoided by reducing leaf area and increasing the proportion of canopy gaps (CitationSmart, 1988). Excess canopy gap could also result in a waste of sunlight falling on the floor of the vineyard rather than on the grapevine canopy.
CONCLUSIONS
Management practices that create a canopy architecture where clusters receive sufficient sunlight for anthocyanin synthesis, but where berries are protected from berry heating, would seem appropriate for the production of fruit with optimal levels of anthocyanins for vines grown in hot regions. Our results confirm the general concept that an optimal canopy microclimate is necessary for cultivation of ‘Norton’ grapes and this can be achieved through canopy management practices, such as leaf removal, shoot positioning, and shoot thinning. The optimization of these three practices is necessary to expose the cluster zone and leaf surface to sufficient sunlight. Intensive shade or excess exposure in the canopy is not ideal as both the conditions have adverse effects on fruit composition either in terms of acids, sugars, or accumulation of anthocyanin pigments. Considering the vigorous nature of ‘Norton’ grapes, adopting a divided canopy system of trellising, such as the Geneva Double Curtain, could open the canopy and reduce shade by optimizing the number of leaf layers and canopy gap percentage, which may improve overall fruit composition of ‘Norton’ grapes in terms of acids and pH. Further studies on standardizing the time at which leaf removal should be done will help in improving overall fruit composition of ‘Norton’ grapes.
LITERATURE CITED
- Bledsoe , A.M. , Kliewer , W.M. and Marois , J.J. 1988 . Effects of timing and severity of leaf removal on yield and fruit composition of Sauvignon Blanc grapevines . Amer. J. Enol. Viticult. , 39 : 49 – 54 .
- Candolfi-Vasconcelos , M.C. and Koblet , W. 1990 . Yield, fruit quality, bud fertility and starch reserves of the wood as a function of leaf removal . Vitis vinifera—Evidence of compensation and stress recovering. Vitis , 29 : 199 – 221 .
- Carbonneau , A. and Huglin , P. 1982 . “ Adaptation of training systems to French regions ” . In Grape and Wine Centennial Symposium Proceedings , Edited by: Webb , A.D. 376 – 385 . Davis , CA : University of California, Davis .
- Crippen , D.D. and Morrison , J.C. 1986 . The effects of sun exposure on the compositional development of Cabernet Sauvignon berries. Amer . J. Enol. Viticult. , 38 : 235 – 242 .
- Dokoozlian , N.K. and Kliewer , W.M. 1995 . The light environment within grapevine canopies. II. Influence of leaf area density on fruit zone light environment and some canopy assessment parameters . Amer. J. Enol. Viticult. , 46 : 219 – 226 .
- Downey , M.O. , Dokoozlian , N.K. and Krstic , M.P. 2006 . Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research . Amer. J. Enol. Viticult. , 57 : 257 – 268 .
- Ferree , D.C. , Scurlock , D.M. , Steiner , T. and Gallander , J. 2004 . ‘Chambourcin’ grapevine response to crop level and canopy shade at bloom . J. Amer. Pomol. Soc. , 58 : 135 – 141 .
- Gladstones , J. 1992 . Viticulture and Environment , 310 Adelaide , , South Australia : Winetitles .
- Hardy , J.P. 1968 . Metabolism of sugars and organic acids in immature grape berries . Plant Physiol. , 43 : 224 – 228 .
- Haselgrove , L. , Botting , D. , Van Heeswijck , R. , øi , P.B. H , Dry , P.R. , Ford , C. and Iland , P.G. 2000 . Canopy microclimate and berry composition: The effect of bunch exposure on the phenolic composition of Vitis vinifera L. cv. Shiraz grape berries . Aust. J. Grape Wine Res. , 6 : 141 – 149 .
- Howell , G.S. , Candolfi-Vasconcelos , M.C. and Koblet , W. 1994 . Response of Pinot noir grapevine growth, yield, and fruit composition to defoliation the previous growing season . Amer. J. Enol. Viticult. , 45 : 188 – 191 .
- http://www.missouriwine.org/images/pdfs/GrapeFactsBrochure.pdf (http://www.missouriwine.org/images/pdfs/GrapeFactsBrochure.pdf)
- http://www.thefreelibrary.com/Rapidly,+simultaneously+analyze+sugars+and+organic+acids+in+wine.-a0224281023 (http://www.thefreelibrary.com/Rapidly,+simultaneously+analyze+sugars+and+organic+acids+in+wine.-a0224281023)
- Hunter , J.J. and Visser , J.H. 1988 . The effect of partial defoliation, leaf position and developmental stage of the vine on the photosynthetic activity of Vitis vinifera L. cv. Cabernet Sauvignon . S. Afr. J. Enol. Viticult. , 9 : 9 – 15 .
- Hunter , J.J. , Ruffner , H.P. , Volschenk , C.G. and Le Roux , D.J. 1995 . Partial defoliation of Vitis vinifera cv. Cabernet Sauvignon/99 Richter: Effect on root growth, canopy efficiency, grape composition, and wine quality . Amer. J. Enol. Viticult. , 46 : 306 – 314 .
- Johnson , J.O. , Weaver , R.J. and Paige , D.F. 1982 . Differences in the mobilization of assimilates of Vitis vinifera L. grapevines as influenced by increased source strength . Amer. J. Enol. Viticult. , 33 : 207 – 213 .
- Keller , M. and Hrazdina , G. 1998 . Interaction of nitrogen availability during bloom and light intensity during veraison. II. Effects on anthocyanin and phenolic development during grape ripening . Amer. J. Enol. Viticult. , 49 : 341 – 349 .
- Kliewer , M.W. 1965 . Changes in concentration of malates, tartarates and total free acids in flowers and berries of Vitis vinifera . Amer. J. Enol. Viticult. , 16 : 92 – 100 .
- Kliewer , W.M. 1967 . Concentration of tartrates, malates, glucose and fructose in the fruits of the genus Vitis . Amer. J. Enol. Viticult. , 18 : 87 – 96 .
- Kliewer , W.M. and Bledsoe , A. 1987 . Influence of hedging and leaf removal on canopy microclimate, grape composition, and wine quality under California conditions . Acta Hort. , 206 : 157 – 168 .
- Kliewer , W.M. and Antcliff , A.J. 1970 . Influence of defoliation, leaf darkening and cluster shading on the growth and composition of sultana grapes . Amer. J. Enol. Viticult. , 21 : 26 – 36 .
- Kliewer , M.W. and Lakso , L.A. 1968 . Influence of cluster exposure to the sun on the composition of Thompson Seedless fruits . Amer. J. Enol. Viticult. , 119 : 175 – 184 .
- Kliewer , W.M. and Lider , L.A. 1968 . Influence of cluster exposure to the sun on the composition of Thompson Seedless fruit . Amer. J. Enol. Viticult. , 19 : 175 – 184 .
- Kliewer , W.M. and Lider , L.A. 1970 . Effects of day temperature and light intensity on growth and composition of Vitis vinifera L. fruits . J. Amer. Soc. Hort. Sci. , 95 : 766 – 769 .
- Kliewer , W.M. and Dokoozlian , N.K. 2005 . Leaf area/crop weight ratio of grapevines influence on fruit composition and wine quality . Amer. J. Enol. Viticult. , 56 : 170 – 181 .
- Kliewer , W.M. and Smart , R.E. 1989 . “ Canopy manipulation for optimizing vine microclimate, crop yield and composition of grapes ” . In Manipulation of fruiting , Edited by: Wright , C.J. 275 – 291 . London : Butterworth .
- Koblet , W. 1984 . “ Influence of light and temperature on vine performance in cool climates and application to vineyard ” . In Proc. Intl. Symp. on Cool Climate Viticult. and Enol , 139 – 157 . Eugene , OR, p : Oregon State University .
- Koblet , W. , Keller , M. and Candolfi-Vasconcelos , M.C. 1996 . Effects of training system, canopy management practices, crop load and rootstock on grapevine photosynthesis , Edited by: Poni , S. , Peterlunger , E. , Iacono , F. and Intrieri , C. 133 – 140 . Conegliano , , Italy : Proc. Workshop Strategies to Optimize Wine Grape Quality .
- Lakso , A.N. and Kliewer , W.M. 1975 . The influence of temperature on malic acid metabolism in grape berries . I. Enzyme responses. Plant Physiol. , 56 : 370 – 372 .
- Mabrouk , H. and Sinoquet , H. 1998 . Indices of light microclimate and canopy structure of grapevines determined by 3D digitising and image analysis, and their relationship to grape quality . Aust. J. Grape Wine Res. , 4 : 2 – 13 .
- Main , G. 2005 . Growing and vinting Cynthiana/Norton grapes . Proc. 24th Annu. Horticult. Ind. Show , Fort Smith, AR, 14–15 January, 2005. http://www.uark.edu/depts/ifse/grapeprog/articles/ahis05wg.pdf
- Main , G.L. and Morris , J.R. 2004 . Leaf-removal effects on Cynthiana yield, juice composition, and wine composition . Amer. J. Enol. Viticult. , 55 : 147 – 152 .
- Main , G.L. and Morris , J.R. 2008 . Impact of pruning methods on yield components and juice and wine composition of Cynthiana grapes . Amer. J. Enol. Viticult. , 59 : 179 – 187 .
- Meyers , J.M. and Vanden Heuvel , J.C. 2008 . Enhancing the precision and spatial acuity of point quadrat analysis via calibrated exposure mapping . Amer. J. Enol. Viticult. , 59 : 425 – 431 .
- Morris , J.R. and Main , G.L. 2010 . An investigation of training system, pruning severity, spur length and shoot positioning on Cynthiana/Norton grapes . Amer. J. Enol. Viticult. , 61 : 445 – 450 .
- Percival , D.C. , Fisher , K.H. and Sullivan , J.A. 1994 . Use fruit zone leaf removal with Vitis vinifera L. cv. Riesling grapevines. II. Effect on fruit composition, yield, and occurrence of bunch rot (Botrytis cinerea Pers.: Fr.) . Amer. J. Enol. Viticult. , 45 : 133 – 140 .
- Poni , S. , Casalini , L. , Bernizzoni , F. , Civardi , S. and Intrieri , C. 2006 . Effects of early defoliation on shoot photosynthesis, yield components, and grape composition . Amer. J. Enol. Viticult. , 57 : 397 – 407 .
- Reynolds , A.G. , Wardle , D.A. and Naylor , A.P. 1996 . Impact of training system, vine spacing, and basal leaf removal on Riesling. Vine performance, berry composition, canopy microclimate, and vineyard labor requirements . Amer. J. Enol. Viticult. , 47 : 63 – 76 .
- Reynolds , A.G. , Pool , R.M. and Mattick , L.R. 1986 . Influence of cluster exposure on fruit composition and wine quality of Seyval Blanc grapes . Vitis , 25 : 85 – 95 .
- Ristic , R. , Downey , M.O. , Iland , P.G. , Bindon , K. , Francis , I.L. , Herderich , M. and Robinson , S.P. 2007 . Exclusion of sunlight from Shiraz grapes alters wine colour, tannin and sensory properties . Aust. J. Grape Wine Res. , 13 : 53 – 65 .
- Santesteban , L.G. and Royo , J.B. 2006 . Water status, leaf area and fruit load influence on berry weight and sugar accumulation of cv. ‘Tempranillo’ under semi-arid conditions . Scientia Hort. , 109 : 60 – 65 .
- Smart , R.E. 1982 . Vine manipulation to improve wine grape quality , 362 – 375 . Davis, CA , , 18–21 June 1980. : Proc. Grape and Wine Centennial Symp .
- Smart , R.E. 1983 . Improving wine quality in the vineyard. I. Proc. of Vintage ’83 Seminar . Te Kauwhata Oenological and Viticultural Bul. , 36, 14 p
- Smart , R.E. 1985 . Principles of grapevine canopy microclimate manipulation with implications for yield and quality—A review. Amer . J. Enol. Viticult. , 35 : 230 – 239 .
- Smart , R.E. 1988 . Shoot spacing and canopy light microclimate . Amer. J. Enol. Viticult. , 39 : 325 – 333 .
- Smart , R.E. and Robinson , M. 1991 . Sunlight into wine: A handbook of wine grape canopy management, p , 88 Adelaide , , South Australia : Winetitles .
- Striegler , R.K. , Morris , J.R. , Harris , J.L. and Main , G.L. 2009 . Early response of Norton grapevines to selected rootstocks in Arkansas . Abstr. Am. J. Enol. Viticult. , 60 : 553
- Tarara , J.M. , Ferguson , J.C. , Hoheisel , G.A. and Perez Pena , J.E. 2005 . Asymmetrical canopy architecture due to prevailing wind direction and row orientation creates an imbalance in irradiance at the fruiting zone of grapevines . Agr. For. Meteorol. , 135 : 144 – 155 .
- Vasconcelos , M.C. and Castagnoli , S. 2000 . Leaf canopy structure and vine performance . Amer. J. Enol. Viticult. , 51 : 390 – 396 .
- Williams , L.E. , Biscay , P.J. and Smith , R.J. 1987 . Effect of interior canopy defoliation and potassium distribution in Thompson Seedless grapevines . Amer. J. Enol. Viticult. , 38 : 287 – 292 .
- Wolf , T.K. , Pool , R.M. and Mattick , L.R. 1986 . Responses of young Chardonnay grapevines to shoot tipping ethephon, and basal leaf removal . Amer. J. Enol. Viticult. , 37 : 263 – 268 .
- Zoecklein , B.W. , Wolf , T.K. , Duncan , N.D. , Judge , J.M. and Cook , M.K. 1992 . Effect of fruit zone leaf removal on yield, fruit composition, and rot incidence of Chardonnay and White Riesling (Vitis vinifera L.) grapes . Amer. J. Enol. Viticult. , 43 : 139 – 148 .
- Zoecklein , B.W. , Wolf , T.K. , Duncan , S.E. , Marcy , J.E. and Jasinski , Y. 1998 . Effect of fruit zone leaf removal on total gly coconjugates and conjugate fraction concentration of Riesling and Chardonnay (Vitis vinifera L.) grapes . Amer. J. Enol. Viticult. , 49 : 259 – 265 .