Abstract
The fruits of six Ficus species, including F. auriculata Lour., F. maclellandii King., F. hirta Vahl., F. nervosa Heyne ex. Roth., F. racemosa Linn., and F. semicordata Buch-Ham.ex. Roth, are traditionally consumed by local people in North East India. The fruits were studied for their total phenolic content (TPC) and total flavonoid content (TFC). The 90% ethanolic extract of the fruits were screened for potential antioxidant capacity by employing different in vitro assays like ABTS•+ (2,2-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid), FRAP (ferric reducing antioxidant power), DPPH• (2,2′-diphenyl-1-picryl hydrazyl) scavenging activity, hydrogen peroxide radical scavenging activity, hydroxyl radical scavenging activity, chelating power, and reducing power properties. Extract from F. maclellandii and F. racemosa showed higher antioxidant activity in all of the systems than other species. It was concluded that these two species are promising sources of natural dietary antioxidants. According to statistical analysis, the TPC and TFC appear to be responsible for the excellent antioxidant capacity of the extract and all of the extract exhibited dose-dependent antioxidant activity.
INTRODUCTION
Ficus is a tropical, deciduous, evergreen tree genus with more than 800 species. Bark, root, leaves, fruit, and latex of this plant are frequently used for the treatment of various illnesses. Ficus produces a unique fruit, which is actually an inverted flower. Ficus species are a rich source of polyphenolic compounds, flavanoids which are responsible for strong antioxidant properties that help in prevention and therapy of various oxidative stress-related diseases, such as neurodegenerative and hepatic diseases (Sirisha et al., Citation2010).
From the literature it was found that the antioxidant profile of most species belonging to Ficus genus has remained unexamined and lacks extensive documentation. The Ficus species belongs to the Moraceae family. Stem barks of Ficus auriculata Lour. have been used for treatment of diarrhea, dysentery, cuts, and wounds. Ficus hirta Vahl has been used for edible purposes. Stem latex is used for wounds (Manandhar, Citation1990) and stem bark is boiled and its gel is used for fever (Manandhar, Citation1998). Ficus maclellandii King is used as folk medicine (Sirisha et al., Citation2010). Ficus nervosa Heyne ex. Roth is used as Chinese traditional medicines for treating inflammation, cancer, and pain, including diabetes mellitus (Raj et al., Citation2011). Ficus racemosa Linn. is a popular medicinal plant in India, which has long been used in Ayurveda, the ancient system of Indian medicine, for various diseases/disorders, including diabetes, liver disorders, diarrhea, inflammatory conditions, hemorrhoids, respiratory, and urinary diseases. F. racemosa Linn. is pharmacologically studied for various activities, including antidiabetic, antipyretic, anti-inflammatory, hepatoprotective, and antimicrobial activities. Ficus semicordata Buch-Ham.ex. Roth is used as animal fodder and human food. Other uses include use of a bath made from the bark to cure leprosy (the fruit and bark is a cure for leprosy), its latex is drank to cure fever, raw fruits are eaten for diarrhea, young fruit juice is applied to the forehead to relieve headache, and young twigs are fed to cattle for facilitating the discharge of placenta (Sirisha et al., Citation2010).
In recent years, researchers have tried to isolate powerful and nontoxic natural antioxidant from edible plants not only to prevent autoxidation and lipid peroxidation, but also to replace synthetic antioxidants. So, a comparative study was done on evaluation of a potential antioxidant and its correlation with total phenolic content (TPC) and total flavonoid content (TFC) in the fruits of F. racemosa, F. auriculata, F. maclellandii, F. hirta, F. nervosa, and F. semicordata. The main objectives of this study were (i) to determine and compare the TPC and TFC of six Ficus species, (ii) to examine the effective concentration at 50% inhibition/scavenging in different methods, and (iii) effect of scavenging activity (%) with concentration of the extract.
MATERIALS AND METHODS
The plant materials were collected from Arunachal Pradesh, India. The voucher specimens were deposited in the herbarium of the Forestry Department, NERIST, Nirjuli, Arunachal Pradesh.
The mature fruits were collected and dried under shade to obtain a dry sample. The dry samples were powdered in a Willy Mill (Mumbai, India) to 250 micron size and used for solvent extraction. The dried fruits of each plant were extracted with 90% ethanol. The solvent was removed by a rotary evaporator (Buchi, Zurich, Switzerland) and taken for evaluation of antioxidant activities, TPC, TFC, peroxide scavenging, hydrogen peroxide, hydroxyl radical scavenging, chelating power, and reducing power assay.
Ascorbic acid, aluminum chloride, 2,2′-diphenyl-1-picryl hydrazyl (DPPH), gallic acid, 2,4,6-tripyridyl-s-triazine (TPTZ), ferric chloride, potassium persulphate, Folin-Ciocalteu phenol reagent, 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), and ferrozine were obtained from Sigma-Aldrich (Buchs, Switzerland). All other reagents and chemicals of analytical grade were procured from Merck (Mumbai, India) and local sources of India, and milli-Q quality water was used.
Determination of Total Phenolic Content (TPC)
TPC was determined by the Folin-Ciocalteu method (Singleton and Rossi, Citation1965) with slight modification. Briefly, 100 μl of 0.2 mg/ml extract was mixed with 900 μl of distilled water and 1 ml of Folin-Ciocalteu reagent. After 5 min, 2 ml of saturated sodium carbonate (75 gm/l) and 2 ml of water were added. The absorbance of the resulting blue colored solution was measured at 765 nm after incubation at 30°C for 1.5 h with intermittent shaking. Quantitative measurement was performed based on a standard calibration curve of gallic acid in 80% methanol. The TPC was expressed as gallic acid equivalent (GAE) in milligram per gram of dry sample.
Determination of Total Flavonoid Content (TFC)
TFC was measured by the colorimetric method (Park et al., Citation2008). Initially, 5 mg of each dry extract was dissolved in 10 ml of 80% aqueous ME. In a 10-ml test tube, 0.3 ml of extract, 3.4 ml of 30% ME, 0.15 ml of 0.5 M NaNO2, and 0.15 ml of 0.3M AlCl3.6H2O were added and mixed. After 5 min, 1 ml of 1M NaOH was added. The absorbance was measured at 506 nm. The standard curve for TFC was made using rutin as the standard solution (0–100 mg/l) under the same procedure as above.
DPPH Radical Scavenging Activity Assay
Antioxidant activity was evaluated by the DPPH method (Aoshima et al., Citation2004), in which the donation capacity of the extracts was measured by bleaching of the purple-colored solution of the DPPH radical. Briefly, to 100 μl of 0.2 mg/ml sample extract or standard, 2.9 ml of 0.1 mM DPPH methanolic solution was added. The mixture was shaken vigorously and kept at room temperature for 30 min. Then the discoloration of DPPH was measured against blank at 517 nm. The scavenging activity was estimated based on the percentage of DPPH radical scavenged using the following equation:
The antiradical activity was expressed as EC50 (μg/ml), the concentration required to cause 50% DPPH inhibition. Ascorbic acid was used as a positive control in all assays.
Ferric Reducing Antioxidant Power (FRAP) Assay
FRAP was determined according to the method described by Iris et al. (Citation1999) with some modifications. Briefly, 3.0 ml of FRAP reagent was added to the appropriate concentration of sample extract (few samples were diluted further to get into the standard range) and after incubation for 4 min at room temperature the absorbance was measured at 593 nm.
ABTS Radical Scavenging Activity
The ABTS radical cation scavenging activity was measured according to the method developed by Re et al. (Citation1999) with slight modification. In brief, ABTS solution (7 mM) was reacted with potassium persulfate (2.45 mM) solution and kept overnight in the dark to yield a blue-green colored solution containing ABTS radical cation. Prior to use in the assay, the resulting blue-green ABTS•+ solution was diluted with 50% ME for an initial absorbance of about 0.700 ± 0.02 at 734 nm, with the temperature control set at 30°C. Free radical scavenging activity was assessed by mixing 100 μl of test sample with 2.9 ml of ABTS radical solution microcuvette. The decrease in absorbance was measured exactly 1 min after mixing the solution, then up to 6 min. The final absorbance was noted.
Hydrogen Peroxide Scavenging Activity
Hydrogen peroxide scavenging activity was measured according to a slightly modified method of Zhao et al. (Citation2006). First, 1 ml H2O2 (0.1 mM) and 1 ml of 100 μg/ml concentration of the extracts or standard antioxidants were mixed with 100 μl ammonium molybdate (3%), 10 ml H2SO4 (2 M), and 7 ml KI (1.8 M). The mixed solution was titrated with Na2S2O3 (5 mM) until the yellow color disappeared. The percentage scavenging effect was calculated as:
Hydroxyl Radical Scavenging Assay
Hydroxyl radical scavenging activity of extracts was assayed by the method developed by Halliwell and Gutteridge (Citation1981). The reaction mixture contained 500 ml of 2-deoxyribose (2.8 mM) in phosphate buffer (50 mM, pH 7.4), 200 ml of premixed ferric chloride (100 mM) and EDTA (100 mM) solution (1:1; v/v), and 100 ml of hydrogen peroxide (200 mM) without or with the extract solution (100 ml). The reaction was triggered by adding 100 ml of 300 mM ascorbate and incubated for 1 h at 37°C. A solution of TBA in 1 ml (1%; w/v) of 50 mM NaOH and 1 ml of 2.8% (w/v; aqueous solution) TCA was added. The mixture was heated for 15 min on a boiling water bath and then cooled. The absorbance was measured at 532 nm.
Chelating Power
The ability of the extract to chelate iron (II) was estimated according to the method of Dastmalchi et al. (Citation2008). The extracts were dissolved with ME to prepare various sample solutions at 8, 6, 4, 2, and 1 mg/ml and 500 and 250 μg/ml. An aliquot of each extract (200 μl) was mixed with 100 μl of FeCl2·2H2O (2.0 mM/l) and 900 μl of ME. After 5 min incubation, the reaction was initiated by the addition of 400 μl of ferrozine (5.0 mM/l). After 10 min incubation, the absorbance at 562 nm was recorded.
Reducing Power Assay
The method of Oyaizu (Citation1986) was used to assess the reducing power of different extracts. A total of 2 ml of different extracts of concentration 10 mg/ml was mixed with 2 ml of 0.2 M phosphate buffer (pH 6.6) and 2 ml of 1% potassium ferricyanide [K3Fe(CN)6] and incubated in a water bath at 50°C for 20 min. Then, 2 ml of 10% trichloroacetic acid was added to the mixture, which was centrifuged at 650 g for 10 min. The supernatant (2 ml) was then mixed with 2 ml distilled water and 0.4 ml of 0.1% ferric chloride solution. After 10 min, the intensity of the blue-green colored solution was measured at 700 nm. The EC50 value (μg/ml) is the extract concentration at which the absorbance was 0.5 (a.u.) for the reducing power and was calculated from the graph of absorbance at 700 nm against extract concentration.
Statistical Analysis
All assays were carried out in triplicate and the results are expressed as means ± standard deviation (n = 3). The one way ANOVA test was used to analyze the differences among EC50 of various extracts for different antioxidant assays with least significance difference (LSD) P < 0.01 as a level of significance. The EC50 values were calculated using the Origin (Version 8.0) software.
RESULTS AND DISCUSSION
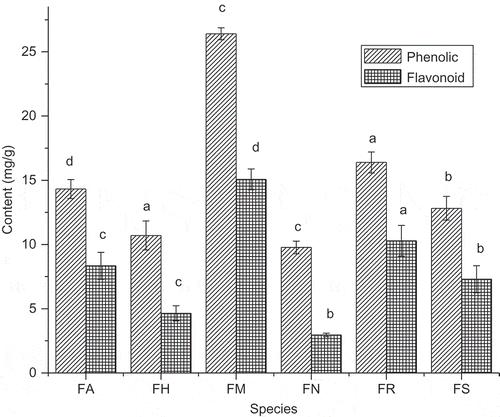
indicated the extractable TPC and TFC levels in the fruit samples. It was found that the F. maclellandii had the highest TPC (26.41 mg GAE/g) and TFC (15.07 mg RE/g), while F. nervosa had the lowest TPC (09.77 mg GAE/g) and TFC (02.96 mg RE/g). The TPC ranged from 9.77–26.41 mg GAE/g and TFC ranged from 2.96–15.07 mg RE/g. The result was comparable as reported by Shi et al. (2011) for the leaves of F. virens var. sublanceolata, F. auriculata, F. vasculosa, F. callosa, F. virens var. verins, F. racemosa, and F. oligodon. Phenolic acid and flavonoid have been reported to be the main phytochemicals responsible for the antioxidant capacity of fruits and vegetables (Bahramikia et al., Citation2009). Polyphenols and flavonoids are the groups of compounds naturally present in most edible fruits and vegetables. Flavonoids from vegetable or green leaf are currently widely studied as components that had potential to provide multiple health benefits.
FIGURE 1 Levels of total phenolic content and flavonoid content present in fruit of the six Ficus species from North East India. Values are the mean of triplicate determinations ± SD. FA: Ficus auriculata; FB: Ficus maclellandii; FH: Ficus hirta; FN: Ficus nervosa; FR: Ficus racemosa; FS: Ficus semicordata; AA: ascorbic acid. The bars with different lowercases are significantly different (P < 0.05).
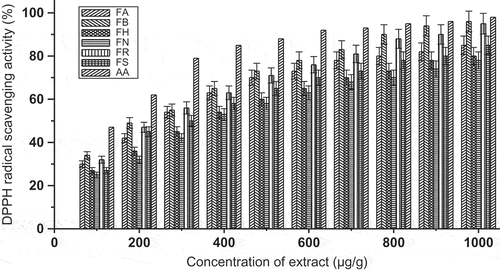
In this study, F. maclellandii extract showed higher antioxidant capacity compared to other Ficus species (). It is generally observed that the DPPH radical-scavenging effect increased as the concentration of the solvent extract increased, to a certain limit, and then leveled off, even with further increases in the concentration (P < 0.01). The study revealed that the extract of F. maclellandii has significant scavenging activities, acting possibly as primary antioxidant. The lower EC50 value means higher scavenging effect. As shown in , F. maclellandii and F. racemosa had lower EC50 values than other species. It suggested that these two species had higher scavenging radical activity than other species. The ascorbic acid had shown a significant scavenging effect. The result was comparable as reported by Shi et al. (2011). The radical scavenging activity (%) with respect to concentration of the extract is shown in . The DPPH scavenging effect was found significant in F. maclellandii. The result was found to be similar to reported data for Ficus carica and Ficus microcarpa L. species (Ao et al., Citation2008).
TABLE 1 Antioxidant Effect (EC50) on DPPH Radicals, FRAP Assay, ABTS Radicals, Hydrogen Peroxide Radicals, Hydroxyl Radicals, Chelating Power, and Reducing Power of Ethanol Extracts of Ficus Species from North East Indiaz
FIGURE 2 DPPH scavenging activity (%) with variation of concentration of extract of Ficus species. FA: Ficus auriculata; FB: Ficus maclellandii; FH: Ficus hirta; FN: Ficus nervosa; FR: Ficus racemosa; FS: Ficus semicordata; AA: ascorbic acid. Values are the mean of triplicate determinations ± SD.
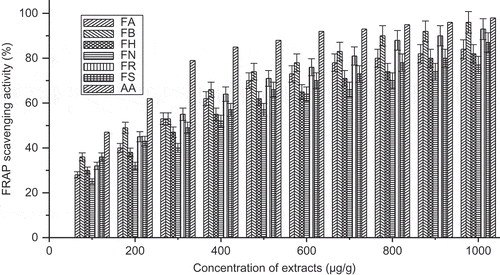
In FRAP assay, it was found that F. maclellandii exhibited higher FRAP compared to other Ficus species. The result indicated that a phenolic compound of the species was responsible for reduction of Fe3+ to Fe2+. The EC50 value ranged from 206.5–382.3 μg/ml of the sample in . The FRAP activity (%) with change of concentration of extract was shown in . It was found that the inhibition activity was higher in F. maclellandii and F. racemosa compared to other species. The result was comparable as reported by Shi et al. (2011).
FIGURE 3 FRAP scavenging activity (%) with variation of concentration of extract of Ficus species. FA: Ficus auriculata; FB: Ficus maclellandii; FH: Ficus hirta; FN: Ficus nervosa; FR: Ficus racemosa; FS: Ficus semicordata; AA: ascorbic acid. Values are the mean of triplicate determinations ± SD.
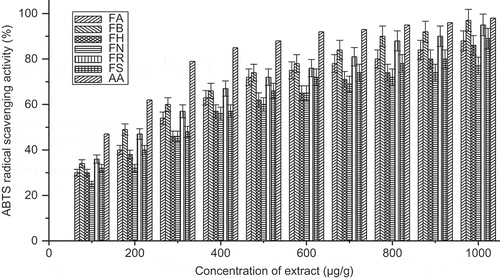
ABTS+· is a well known method widely used to determine antioxidant activity. The lower value EC50 of F. maclellandii and F. racemosa suggested that the ABTS+· radical scavenging effect of these species were more effective than the other species. The ABTS+· radical scavenging effect (%) with concentration of the extract was shown in . The ABTS+· radical scavenging effect can be arranged as follows with decreasing order: F. maclellandii > F. racemosa > F. auriculata > F. semicordata > F. hirta > F. nervosa. The data were comparable as reported (Shi et al., 2011; Chawla et al., Citation2012).
FIGURE 4 ABTS scavenging activity (%) with variation of concentration of extract of Ficus species. FA: Ficus auriculata; FB: Ficus maclellandii; FH: Ficus hirta; FN: Ficus nervosa; FR: Ficus racemosa; FS: Ficus semicordata; AA: ascorbic acid. Values are the mean of triplicate determinations ± SD.
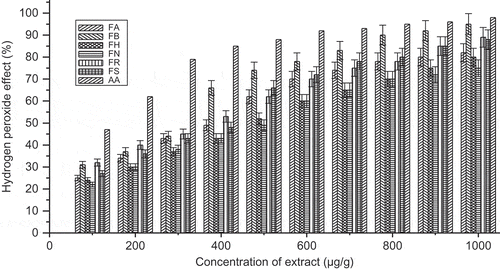
The peroxide scavenging activity was found to be higher in F maclellandii and F. racemosa than the other Ficus species. The hydrogen peroxide radical scavenging activity can be arranged as follows with decreasing order: F. maclellandii > F. racemosa > F. auriculata > F. semicordata > F. hirta > F. nervosa. The hydrogen peroxide radical scavenging activity was evaluated for ascorbic acid as referenced in this assay. The hydrogen peroxide radical scavenging activity (%) with concentration of the extract was shown in . The result was similar as reported (Sirisha et al., Citation2010; Manocha et al., Citation2011).
FIGURE 5 Hydrogen peroxide scavenging activity (%) with variation of concentration of extract of Ficus species. FA: Ficus auriculata; FB: Ficus maclellandii; FH: Ficus hirta; FN: Ficus nervosa; FR: Ficus racemosa; FS: Ficus semicordata; AA: ascorbic acid. Values are the mean of triplicate determinations ± SD.
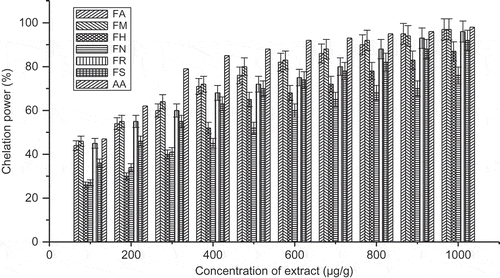
Hydroxyl radical scavenging activity of an extract is directly related to the antioxidant activities. The EC50 value of all the species was found to be >400 μg/ml of the species. However, the F. maclellandii had a lower EC50 value than the other species. Hydroxyl radical scavenging activity can be arranged as follows with decreasing order: F. maclellandii > F. racemosa > F. auriculata > F. semicordata > F. hirta > F. nervosa. The hydroxyl radical scavenging effect (%) with concentration of the extract was shown in . All of the extracts had high levels of ferrous ion chelating activity. The chelating activity increased with the concentration of each extract. A higher absorbance of the reaction mixture indicated greater reducing power. The chelating power can be arranged as follows with decreasing order: F. maclellandii > F. racemosa > F. auriculata > F. semicordata > F. hirta > F. nervosa (). The EC50 value found in F. maclellandii and F. racemosa were comparatively lower than the other Ficus species. It suggested that these species had potential chelating power. The EC50 value of chelating power ranged 160.4–426.6 μg/ml for the extract. The iron chelating data measured at different concentrations suggested that ferrous ion chelating effects of all extract of Ficus species would be somewhat beneficial to protect against oxidative damage.
FIGURE 6 Hydroxy radical scavenging activity (%) with variation of concentration of extract of Ficus species. FA: Ficus auriculata; FB: Ficus maclellandii; FH: Ficus hirta; FN: Ficus nervosa; FR: Ficus racemosa; FS: Ficus semicordata; AA: ascorbic acid. Values are the mean of triplicate determinations ± SD.
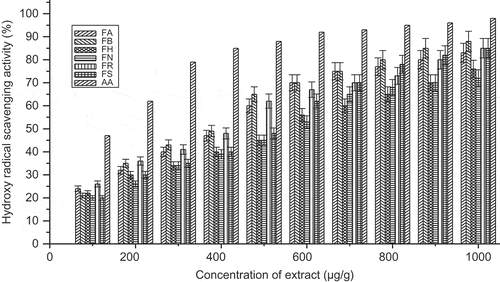
FIGURE 7 Chelating power (%) with variation of concentration of extract of Ficus species. FA: Ficus auriculata; FB: Ficus maclellandii; FH: Ficus hirta; FN: Ficus nervosa; FR: Ficus racemosa; FS: Ficus semicordata; AA: ascorbic acid. Values are the mean of triplicate determinations ± SD.
In the reducing power assay, the presence of reductants (antioxidants) in the fraction would result in the reduction of Fe3+/ferric cyanide complex to the ferrous form by donating an electron. Increasing absorbance at 700 nm indicated an increase in reducing ability. It was found that the reducing power increased with the concentration of each extract. The sequence for reducing power was F. maclellandii > F. racemosa > F. auriculata > F. semicordata > F. hirta > F. nervosa extract, respectively. The results were comparable as reported (Shi et al., 2011). Similar observations of antioxidant properties in terms of dose dependency(P < 0.01) and reducing power activity had been reported for solvent extracts of the aerial root of F. bengalensis L. and the stem bark of F. racemosa L. (Manian et al., Citation2008).
Phenolic compounds derived from plants are very important by virtue of their antioxidant activities by chelating redox-active metal ions, inactivating lipid free radical chains, and preventing hydro peroxide conversions into reactive oxyradicals. Significant amounts of phenolic compounds frequently occur in foods, such as fruits and vegetables, and are routinely consumed in our diet. Epidemiological and clinical studies have provided evidence of a potential role for flavonoids in lowering the risk of coronary heart disease, prevention cardiovascular diseases, and lung cancer as reported (Yao et al., Citation2004). Through correlation analysis for phytochemical contents with EC50 values of radical scavenging activity and/or antioxidant ability of extract, the phenolic content exhibited a significant positive correlation (P < 0.01) with DPPH, FRAP, ABTS radicals, hydrogen peroxide, hydroxyl radical, chelating power, and reducing power assay. This result suggested that the phenolic compounds contributed significantly to the antioxidant capacity of the investigated plant species. It was not specific to polyphenols, but many interfering compounds may react with the reagent, giving elevated apparent phenolic concentration (Prior et al., Citation2005). Moreover, various phenolic compounds respond differently in these assays, depending on the number of phenolic groups they had (Singleton and Rossi, Citation1965) and TPC did not incorporate necessarily all the antioxidants that may be present in an extract. Hence, this may explain the equivocal correlation between total phenolic content and antioxidant activity of different extracts.
Significant correlations were also observed between antioxidant capacities determined by different systems (P < 0.01), indicating that these methods have satisfactory correlation for the examination of antioxidant.
CONCLUSION
For the first time, the antioxidant effect of the edible young fruit of F. racemosa, F. auriculata, F. maclellandii, F. hirta, F. nervosa, and F. semicordata along with their TPC and TFC was evaluated. The results showed that the underutilized potential edible fruits of the six Ficus species possess abundant antioxidants at various concentrations, and the ethanol extract of F. maclellandii and F. racemosa showed considerable high antioxidant potential compared with other species.
Further research is essential to establish the antioxidant potential of some underexploited Ficus species, especially those being used traditionally for pharmacological or dietetic purposes. The nutrient content and antioxidant potential should be given preferential consideration for functional food or local special products development, which aims to meet the demands for pollutant free, health improving, and house special dishes of the resident.
ACKNOWLEDGMENT
We are thankful to the Director, North East Institute of Science & Technology, Jorhat, Assam, for providing facilities and valuable advice.
LITERATURE CITED
- Ao, C.W., A.P. Li, A.A. Elzaawely, T.D. Xuan, and S. Tawata. 2008. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control 19:940–948.
- Aoshima, H., T. Hideaki, K. Hirofumi, and K. Yoshinobu. 2004. Aging of whiskey increases 1, 1-diphenyl-2-picryl hydrozyl radical scavenging activity. J. Agr. Food Chem. 52:5240–5244.
- Bahramikia, S., A. Ardestani, and R. Yazdanparast. 2009. Protective effect of four Iranian medicinal plants against free radical-mediated protein oxidation. Food Chem. 115:37–42.
- Chawla, A., R. Kaur, and A.K. Sharma. 2012. Ficus carica Linn.: A review on its pharmacognostic, phytochemical and pharmacological aspects. Int. J. Pharm. Phytopharmacol. Res 1(4):215–232
- Dastmalchi, K.D., H.J.D. Doeman, P.P. Oinonen, Y. Darwis, I. Laaso, and R. Hiltunen. 2008. Chemical composition and in vitro antioxidative activity of a lemon balm (Melissa officinalis L.) extract. LWT–Food Sci. & Tech. 41:391–400.
- Halliwell, B. and J.M.C. Gutteridge. 1981. Formation of thiobarbituric acid reactive substances from deoxyribose in the presence of iron salts: The role of superoxide and hydroxyl radicals. FEBS Lett. 128:347–352.
- Iris, F., F. Benzie, and J.J. Strain. 1999. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method Enzymol. 299:15–27.
- Manandhar, N.P. 1990. Traditional phytotherapy of Danuwar tribe of Kamlakhong in Sindhuli district, Nepal. Fitoterapia 61(4):325–332.
- Manandhar, N.P. 1998. Native phytotherapy among the Raute tribe of Dadeldhura district, Nepal. J. Ethnopharmacol. 60:199–206.
- Manian, R., Anusuya, N., Siddhuraju, P., and Manian, S. 2008. The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem. 107:1000–1007.
- Manocha, N., S.K. Chandra, V. Sharma, B. Sangameswaran, and M. Saluja. 2011. Anti-rheumatic and antioxidant activity of extract of stem bark of Ficus bengalensis. Res. J. Chem. Sci. 1(2):1–8.
- Oyaizu, M. 1986. Antioxidant activity of browning products of glucosamine fractionated by organic solvent and thin-layer chromatography. Nippon Shokulin Kogyo Gakkaishi 35:771–775.
- Park, Y.-S., S.T. Jung, S.G. Kang, B.K. Heo, P. Arancibia-Avila, F. Toledo, J. Drzewiecki, J. Namiesnik, and S. Gorinstein. 2008. Antioxidants and proteins in phosphor-treated kiwifruits. Food Chem. 107:640–648.
- Prior, R.L.; X. Wu, and K. Schaich. 2005. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agr. Food Chem. 53:4290–4302.
- Raj, R.A., A.S. Kumar, and S.M. Lakshmi. 2011. Antidiabetic activity of Ficus nervosa Heyne ex Roth in alloxan induced diabetic rats. Int. J. Exp. Pharma. 1(2):27–32.
- Re, R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C. Rice-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Bio. Med. 26:1231–1237.
- Singleton, V.L. and J.A. Rossi. 1965. Colorimetry of total phenols with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Viticul. 16:144–158.
- Sirisha, N., M. Sreenivasulu, K. Sangeeta, and C.M. Chetty. 2010. Antioxidant properties of Ficus species—A review. Int. J. Pharm. Tech. Res. 2(4):2174–2182.
- Yao, L.H., Y.M. Jiang, J. Shi, F.A. Tomas-Barberan, N. Dutta, R. Singanusong, and S.S. Chen. 2004. Flavonoids in food and their health benefits. Plant Food Hum. Nutr. 59:113–122.
- Zhao, G.R., Z.J. Xiang, T.X. Ye, Y.J. Yuan, and Z.X. Guo. 2006. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng. Food Chem. 99:767–774.
