ABSTRACT
Naringin, a bitter bioflavonoid of orange, widely reported for health promoting benefits is also responsible for reduced acceptability of many orange products where peels are integral components. The current study quantified naringin concentrations present in three types of oranges viz., Nagpur (Citrus reticulata Blanco), Kinnow (Citrus nobilis × Citrus deliciosa), and Mandarin (Citrus reticulata) spectrophotometrically and by high performance liquid chromatograph. The oranges were subjected to repeated blanching at a mild blanching temperature of 65 °C, subjected to osmodehydration and the loss of naringin monitored during processing as well as during extended storage of up to 6 months at room temperature. A wide range of physicochemical parameters were monitored at monthly intervals during the entire period of storage. Blanching reduced the naringin content by 50% and storage further decreased it to 3–10 mg/100g for all of the cultivars studied. In terms of physicochemical parameters and sensory analysis, Kinnow and Mandarin cultivars are better suited for osmotic dehydration.
KEYWORDS:
Introduction
Citrus fruits are one of the most important fruit crops of the world and are primarily consumed as juice in developed countries and as fresh fruits in the developing countries. Either way, a significant quantity of protective and health promoting bioactive compounds present in the peels is wasted, some of which is also incidentally responsible for the bitterness occasionally present in citrus family fruit juices. The bioflavonoids, hesperidin and naringin, present in citrus fruits have been reported to exhibit biological and pharmacological properties like anti-inflammatory, anti-carcinogenic, lipid lowering, and antioxidant activities (Bok et al., Citation1999; Choi et al., Citation2001). Hesperedin and naringin have also been shown to play an important role in preventing the progression of hyperglycemia (Jung et al., Citation2004). Naringin has been reported to serve as a potential therapeutic agent to treat wear-debris-associated osteolysis (Li et al., Citation2014) and osteoporosis (Wei et al., Citation2007).
Some of these bioflavonoids are bitter to the taste and their presence in fruit juices, which is sometimes inevitable, as a result of contamination during the juice extraction process, lowers their consumer acceptability. Hence, debittering of citrus fruits to remove some of these bioflavonoids is an important processing step required to make palatable citrus products. Physical separation techniques like resin adsorption, adsorption on activated carbon (Barmore et al., Citation1986; Hernandez et al., Citation1992; Manlan et al., Citation1990; Puri et al., Citation1996a; Shaw and Buslig, Citation1986), or enzymatic hydrolysis (Po Chien et al., Citation2001; Puri et al., Citation1996b; Soares and Hotchkiss, Citation1998; Tsen and Yu, Citation1991) have been widely used by the industry. These methods suited for citrus juices, require an additional filtration step to remove carbon if activated carbon is used, tend to remove essential nutrients from juices while the use of enzymes adds on to the cost of the final product. Further, these methods are not universally applicable to all citrus products; for example, these methods have limited application for the osmotic dehydration of oranges wherein orange peel forms an integral part of the preserved product. Methods to convert these flavonone glycosides of citrus fruits responsible for bitterness, to their chalcone derivatives, which are many times sweeter than saccharin, have been reported in the literature (Horowitz et al., Citation1968). However, these methods involve the use of organic and inorganic solvents for the chemical conversion reaction, which are not acceptable for osmodehydrated products, as these harsh chemicals tend to destroy the integrity of the final product.
The present article explores repeated blanching in reducing the bitterness contributed by naringin in osmodehydrated oranges. Osmotic dehydration involves the removal and replacement of water in perishable fresh produce with osmotic solutions, such as salts, alcohols, starch solutions, and low molecular weight saccharides like sucrose, glucose, fructose, etc., followed by dehydration where moisture content is further reduced to make the product shelf stable. This process has certain advantages, such as inhibiting enzymes responsible for browning, reducing the damage to flavor, color, nutrients, and bioactive compounds, which could result if high thermal treatments are employed. These osmodehydrated products can be stored at room temperature and, hence, is an environmental friendly processing technique, as there is no requirement for refrigerated storage or transport. Osmotic dehydration, as applied to fresh fruits and vegetables, reduces their weight by 50% (Rastogi and Raghavarao, Citation1997), increases shelf life with little loss of aroma and texture (Petrotos and Lazarides Citation2001), and has high sensory resemblance to the fresh produce.
The current work studied the effect of repeated blanching in reducing the bitterness of orange peels during the osmotic dehydration of orange. The major flavonone, naringin, responsible for bitterness was monitored during processing and the storage losses determined. The product was stabilized in terms of microbial and chemical quality.
Materials and methods
Chemicals
Naringin (4,5,7-trihydroxyflavonone 7-rhamnoglucoside, 95%, high performance liquid chromatograph (HPLC)) and Hesperedin were obtained from Sigma Aldrich (St. Louis, MO, USA). Acetonitrile, water, and glacial acetic acid (HPLC grades) were obtained from Ranbaxy Fine Chemicals Limited (New Delhi, India). Stock solutions (1 mg/ml) of naringin were prepared in warm distilled water and hesperedin in Dimethyl sulfoxide (DMSO, Sigma Aldrich, St. Louis, MO, USA).
Fresh orange fruits were obtained from the local market in Mysore, India. Three different types of oranges categorized as Nagpur type (Citrus reticulata Blanco), Kinnow type (Citrus nobilis × Citrus deliciosa), and Mandarin type (Citrus reticulata) were studied. The oranges were thoroughly washed. The washed oranges were cut into thin slices or rings (3–4 mm thick) including the peel and subjected to repeated blanching at 65 °C. After blanching, the slices were dipped in a hot sugar solution (75–80 °Brix) for 2–3 h and dried in a cabinet dryer (Kilburn Industries, Chennai, India) at 50–60 °C. The osmodehydrated oranges were stored at 28 ± 5 °C for a period of 180 days. Storage was done in an insulated wooden closed cabinet and temperature was monitored periodically using a thermometer. Naringin contents in fresh oranges with peel, orange slices after each blanching treatment, and the final osmodehydrated slices were analyzed spectrophotometrically and also by HPLC.
Spectrophotometric determination of naringin
Naringin concentration was determined spectrophotometrically by the Davis test (Davis, Citation1947). Initially, 2–5 g of fresh orange with peel or osmodehydrated orange slices were crushed with a mortar and pestle, mascerated well, centrifuged at 5000×g for 10 min, and the supernatant taken for analysis. Further, 25 ml of diethylene glycol were added to 0.5 ml of the sample supernatant. The contents were mixed thoroughly; 0.5 ml of 4 N NaOH was added and allowed to stand for 10 min. The absorbance was read at 420 nm against a reagent blank. Naringin concentration (mg/ml) was calculated from sample absorbance based on a linear regression equation of the standard curve of absorbance at 420 nm against concentration of naringin standards.
Determination of naringin by HPLC
Sample preparation
To begin, 20–25 g of the fresh or osmodehydrated orange including the peel was macerated in a pestle and mortar mixed with a known quantity of warm water, and centrifuged at 5000 rpm for 10 min. The collected supernatant was filtered through a 0.45-µm nylon membrane and used for HPLC analysis.
HPLC analysis
The HPLC system used for detection and quantification consisted of a Waters 515 HPLC pump, pump control module II, Rheodyne sample injector, 4.6 mm × 250 mm Waters Spherisorb 5 µm ODS2 C18 column, 2489 UV/VIS dual wavelength detector (Waters Corporation, Milford, MA, USA). The mobile phase consisted of 79.5 parts of water, 20 parts of acetonitrile, and 0.5 parts of glacial acetic acid. A 20-µl sample was analyzed using an isocratic elution at a flow rate of 1.0 ml/min (Rouseff, Citation1988). The detection was done at 280 nm by comparison of retention time with a standard naringin solution.
Stock solution (1000 µg mL–1) of standard was prepared in the mobile phase. This was diluted with water to make 100, 200, 300, and 400 µg mL–1 standard solutions as required. Quantification was done from sample absorbance based on a linear regression equation of the standard curve of absorbance peak area at 280 nm against concentration of naringin and hesperedin standards under identical conditions.
Physical parameters
The moisture content of samples was obtained according to the AOAC method (AOAC, Citation1990). The water activity (aw) of samples was determined with a dew point hygrometer at 25 °C (Aqualab series 3TE, Decagon Devices Inc., Pullman, WA, USA).
Chemical analysis
Acidity, pH, vitamin C, reducing sugar, total sugars, carotenoids, and naringin contents in fresh orange slices as well as after storage (28 ± 5 °C) for a period of 180 days were measured at monthly intervals. Storage was done at room temperature in an insulated wooden closed cabinet and temperature was monitored periodically using a thermometer.
Initially, 10 g of the sample was macerated with 100 ml of distilled water and pH measured using a pH meter (Cyber Scan, Eutech Instruments, India; Accuracy ± 0.01). A 10-ml aliquot of the macerated sample was titrated with 0.1N NaOH, to a visual end point using phenolphthalein indicator and total acidity expressed as percentage citric acid per 100g of sample. Vitamin C and sugars were determined by HPLC with a short extraction and clean up (Jagannath et al., Citation2012a, Citation2012b) carotenoids were estimated spectrophotometrically based on the molar absorptivity value of 2500 for a 1% solution as described earlier (Jagannath et al., Citation2014).
Statistical data analysis
All of the experiments were done in triplicate and values were expressed in terms of mean and standard deviations. The data were subjected to one way analysis of variance (ANOVA) and significant differences between means (P < 0.05) were determined by Duncan’s Multiple Range Test (DMRT). Stastitica 7.1 (Stat Soft Inc., Oklahoma City, OK, USA) was used for data analysis.
Sensory analysis
The sensory panel consisted of 50 volunteers who were trained for the sensory attributes in a preparatory session. Sensory analysis of the osmodehydrated orange samples immediately after osmodehydration and after 180 days of storage was conducted using the following descriptors: physical integrity, stickiness, bitter taste, sweet taste, flavor retention, color retention, overall acceptability, and evaluated using 100-mm graphical nonstructured abscissas with the description of extreme points.
Microbiological analysis
Total bacterial counts and coliform, yeast, and mold counts were determined by serially diluting the osmodehydrated orange samples at the end of 180 days storage in 0.85% physiological saline (w/v) and pour plating on plate count agar, Mac Conkey agar, and potato dextrose agar, respectively (Himedia Laboratories, Mumbai, India). The plates were incubated at 37 °C for the bacteria and 30 °C for the yeast and mold counts and the number of colonies counted after 48 h. The microbial counts were expressed as mean colony forming units (cfu) per ml of the sample.
Results and discussion
Naringin quantities were measured both spectrophotometrically and by HPLC in the current study. Davis test overestimated the naringin concentration as this test determines total citrus flavonoids and is not specific to naringin. The test relies on the production of a yellow color on the addition of alkali in the presence of diethylene glycol and materials other than naringin like narirutin and its hydrolytic products, which are present in citrus fruits, can give this color under the test conditions. The results of HPLC-based detection are presented here.
Distribution of naringin content and its loss during blanching
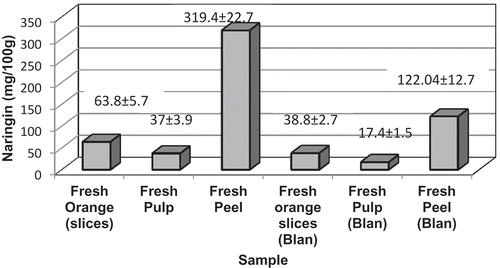
The naringin contents of fresh oranges and after repeated blanching is shown in . The results are the mean of all three cultivars studied. The naringin contents for individual cultivars are shown in . Fresh peel has the maximum content of naringin amounting to about 319 ± 22.7 mg/100g, while pulp has the least (37 ± 3.9 mg/100g) (see ). Fresh orange including peel 15% and pulp 85%, as is the case for osmotic dehydrated orange slice, has a moderate amount (63.8 ± 5.7mg/100g). Among the cultivars processed, the slices of mandarin type had higher naringin content 71.8 ± 0.8 mg/100g compared to the other two cultivars, viz. Nagpur and Kinnow (). Levaj et al. (Citation2009) determined flavonoids in pulp and peel of mandarin fruits and reported naringin levels of 1.8 mg/100g pulp, 10.6 mg/100g peel for Satsuma and 4.3 mg/100g pulp, 13.5 mg/100g peel for Clementine cultivars, which is less than the levels found in the current study. In another study Gorinstein et al. (Citation2006) reported naringin concentration of 52.5 ± 3.5 mg/100g pulp in certain Israeli cultivars of citrus. Yusof et al., (Citation1990) studied naringin concentrations in different parts of pummelo (p) and rough lime (rl) cultivars of citrus fruits and reported higher levels in peel and membrane [517 µg/g (p); 3910 ug/g (rl)] than in juice [220 µg/g (p); 98 µg/g (rl)].
Table 1. Physicochemical parameters of fresh and osmodehydrated orange.
Effect of blanching and losses in blanch water
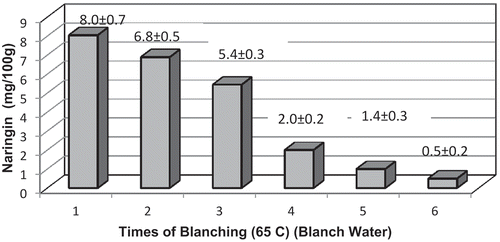
Repeated blanching was able to reduce the naringin quantities in peel and pulp, as well as in fresh orange rings used for osmodehydration (). Multiple blanching steps were able to reduce the bitter naringin part by 50% for fresh orange slices, peel, and pulp. shows the loss of naringin in the blanch water during successive blanching steps (1–6) for orange slices. As much as 25 mg/100g, i.e., 40–50% of naringin present in orange can be removed by repeated blanching. Naringin is soluble in hot water and processors tend to give a warm water wash to remove naringin, the bitterest compound present in orange. Pulley, as early as in the year 1936, noted the solubility of naringin to increase rapidly from 42.21 g L–1 at 65 °C to 108.24 g L–1 at 75 °C (Pulley, Citation1936). Hot water blanching also results in water inflow in the fruit and leaching of solutes including bitter flavanone glycosides, as shown recently by Zid et al. (Citation2015) for Citrus aurantium peels. Further, this outflow of flavanone glycosides has been shown by the same researchers to be dependent on the blanching variables like time and temperature. The loss of naringin has been shown to increase from 14% at 5 min to 52% at 60 min of treatment at 85 °C with higher blanching temperatures showing greater outflow of flavanones (Zid et al., Citation2015). However, higher temperatures tend to weaken the tissue structure of both peels and albedo, which makes the tissues lose integrity during the subsequent steps of osmotic dehydration and drying as employed in the present study. Hence, repeated blanching at a relatively milder temperature of 65 °C was employed in the current study.
HPLC separation
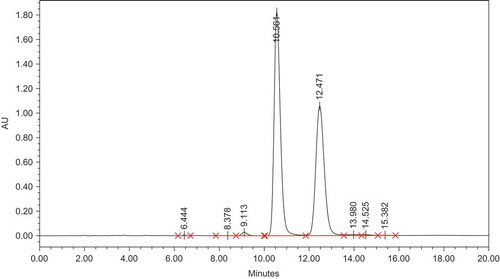
shows the typical chromatogram showing the separation of the primary flavanoids, naringin and hesperedin, in fresh orange slices. The retention time for naringin and hesperedin were 10.56 ± 0.30 and 12.47 ± 0.15 min, respectively, in the present study. Naringin is responsible for bitterness and is the main flavonoid present, while hesperedin concentrations range from 15–25 mg/100g in fresh orange and is a minor component especially in Nagpur and Kinnow cultivars of orange studied. The Mandarin cultivar had a higher concentration, i.e., 25–40 mg/100g of hesperedin. Gorinstein et al. (Citation2006) using a diode-array detector reported a retention time of 32.76 and 33.2 min for naringin and hesperidin, respectively, while Musmade et al. (Citation2014) reported a retention time of 7.46 ± 0.50 min, using a photo diode array detector. Different analytical conditions like flow rate, column type length, width, mobile phase, and their ratios tend to influence the retention times. Hence, the retention times were confirmed injecting standard solutions of naringin and hesperedin.
Storage stability of osmodehydrated oranges
The variations in the physicochemical parameters of fresh and osmodehydrated oranges and the effect of extended storage on these parameters at room temperature are shown in .
There were no significant varietal differences in the moisture, water activity, pH, and acidity (). Based on the final moisture content, these osmodehydrated oranges can be classified under intermediate moisture foods having moisture content at various stages of storage in the range of 15% to 30%. The water activity was low enough to prevent the growth of major spoilage organisms leading to good microbiological quality even after 6 months of storage at room temperature (data not shown). Bacteria, yeast molds, and coliforms were absent at the end of 180 days of storage. Osmodehydrated products show good storage stability ranging from 6 months to 1 year owing to the low water activity and high sugar concentration. Osmotic dehydration studies have shown good storage stability of up to 6 months at room temperature for other fruits like papaya (Ahemed and Choudhary, Citation1995), banana (Gaspartero et al., Citation2003), and mango slices (Sagar and Khurdiya, Citation1999).
Vitamin C content was higher in the Kinnow and Mandarin cultivars (54.0 ± 0.5 and 49.0 ± 0.8) compared to the Nagpur cultivar (34.0 ± 1.7). These two cultivars also showed better retention of vitamin C at the end of the 6-month storage period. Kinnow and Mandarin cultivars also had higher contents of reducing sugar and total sugars as compared to the Nagpur cultivar. Fresh citrus fruits contain carbohydrates in the form of sucrose, glucose, and fructose. The osmotic process infused sucrose, which gradually decreased by 25–30% by the end of the 6-month storage period.
The carotenoid contents in the fresh oranges were 22.6 ± 1.48, 34.3 ± 3.34, and 42.6 ± 2.44 mg/100g for Nagpur, Kinnow, and Mandarin cultivars, respectively, which decreased after the osmodehydration process and subsequent storage (). A complex mixture of carotenoids, such as β-cryptoxanthine and β-citraurin, with high tinctorial properties are responsible for the color of orange fruits (Spiegel-Roy and Goldschmidt, Citation1996). The heat driven disintegration of chromoplasts can lead to the dissolution of carotenes in the cellular lipids of the orange peel during blanching (Artes et al., Citation2002) and this process could have extended beyond processing during the storage periods as well.
Naringin concentrations were also reduced during storage by 10–30% depending on the cultivar with final levels being less than 10 mg/100g at the end of 6 months of storage at room temperature.
The results of the sensory profile analysis of osmodehydrated samples at the end of 6 months of storage is presented in . The orange samples were evaluated on the following descriptors, viz. physical integrity, stickiness, bitter taste, sweet taste, flavor retention, color retention, and overall acceptability. Kinnow and Mandarin cultivars showed better overall acceptability as compared to the Nagpur cultivar. The Nagpur cultivar has a hollow center and hence scored low in terms of physical integrity, since the slices tend to disintegrate during the processing.
Figure 4. Sensory profile analysis of Nagpur (□, solid line), Kinnow (Δ, dotted line), and Mandarin (×, dashed) varieties of osmodehydrated oranges after 180 days of storage.
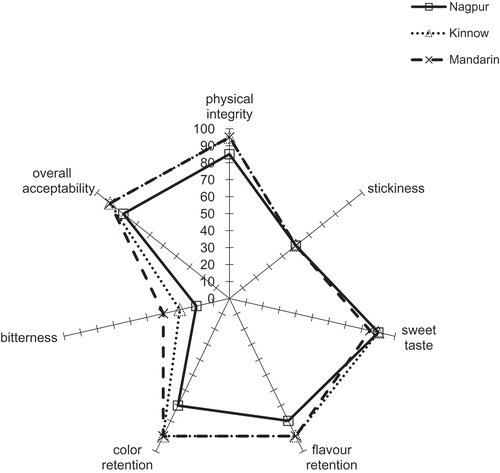
In terms of physicochemical parameters and sensory analysis, Kinnow and Mandarin cultivars of orange are better suited for osmotic dehydration; however, the Nagpur cultivar had lower concentrations of naringin at the end of 6 months of storage.
Conclusions
The present work has shown how repeated blanching can reduce naringin, the primary component of bitterness, in osmodehydrated oranges. The developed product had a long shelf life of 6 months at room temperature with good sensory acceptability. The process developed allows local farmers to handle their surplus of orange produce and convert them into shelf-stable products with nutritional and health promoting qualities as the peels, an integral part of osmodehydrated oranges, possess bioflavonoids and also contribute to the daily dietary fiber [non-starch polysaccharide (NSP)] intake.
Literature cited
- Ahemed, J., and D.R. Choudhary. 1995. Osmotic dehydration of papaya. Indian Food Packer 49:5–11.
- Artes, F., M.I. Minguez, and D. Hornero. 2002. Analysing changes in fruit pigments, p. 248–282. In: Colour in Food: Improving Quality. Woodhead Publishing Limited, Cambridge, England.
- Association of Official Analytical Chemists (AOAC). 1990. Official Methods of Analysis of the Association of Official Analytical Chemists. 15th ed. Washington: Association of Official Analytical Chemists, Washington, DC.
- Barmore, C.R., J.F. Fisher, P.J. Fellers, and R.L. Rouseff. 1986. Reduction of bitterness and tartness in grapefruit juice with Florisil. J. Food Sci. 51:415–416.
- Bok, S.H., S.H. Lee, Y.B. Park, K.H. Bae, K.H. Son, T.S. Jeong, and M.S. Choi. 1999. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and Acyl CoA, cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J. Nutr. 129:1182–1185.
- Choi, M.S., K.M. Do, Y.B. Park, S.M. Jeon, T.S. Jeong, Y.K. Lee, M.K. Lee, and S.H. Bok. 2001. Effect of naringin supplementation on cholesterol metabolism and antioxidant status in rats fed high cholesterol with different levels of vitamin E. Ann. Nutr. Metab. 45:193–201.
- Davis, W.B. 1947. Determination of flavanones in citrus fruits. Anal. Chem. 19:476–478.
- Gaspartero, O.C.P., P.D.L. Silva, and E. Gertrudes. 2003. Study of conservation of banana by osmotic dehydration and drying in a conventional dryer. J. Chem. Eng. 3:25–29.
- Gorinstein, S., D. Huang, H. Leontowicz, M. Leontowicz, K. Yamamoto, R. Soliva-Fortuny, O. Martin Bellosa, A.L. Martinez Ayala, and S. Trakhtenberg. 2006. Determination of naringin and hesperidin in citrus fruit by high-performance liquid chromatography, the antioxidant potential of citrus fruit. Acta Chromatogr. 17:108–124.
- Hernandez, E., R. Couture, R. Rouseff, C.S. Chen, and S. Barros. 1992. Evaluation of ultrafiltration and adsorption to debitter grape fruit juice and grapefruit pulp wash. J. Food Sci. 57:664–666.
- Horowitz, R.M., and B. Gentili. 1968. Conversion of Naringin to Neohesperidin and Neohesperidin dihydrochalcone. U.S. Patent 3375242.
- Jagannath, A., M. Kumar, and P.S. Raju. 2014. Nisin based stabilization of novel fruit and vegetable functional juices containing bacterial cellulose at ambient temperature. J. Food Sci. Technol. 51:1218–1222.
- Jagannath, A., P.S. Raju, and A.S. Bawa. 2012a. Controlled lactic fermentative stabilization of ascorbic acid in amaranthus paste. LWT–Food Sci. Technol. 48:297–301.
- Jagannath, A., P.S. Raju, and A.S. Bawa. 2012b. A two step controlled lactic fermentation of cabbage for improved chemical and microbiological qualities. J. Food Qual. 35:13–20.
- Jung, J.U., M.-K. Lee, K.-S. Jeong, and M.-S. Choi. 2004. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 134:2499–2503.
- Levaj, B., V. Dragovic-Uzelac, D. BurscacKovacevic, and N. Krasnici. 2009. Determination of flavonoids in pulp and peel of mandarin fruits. Agric. Conspect. Sci. 74(3):221–225.
- Li, N., Z. Xu, P.H. Wooley, J. Zhang, and S.-Y. Yang. 2014. Therapeutic potentials of naringin on polymethylmethacrylate induced osteoclastogenesis and osteolysis, in vitro and in vivo assessments. Drug Des. Dev. Ther. 8:1–11.
- Manlan, M., R.F. Matthews, R.L. Rouseff, R.C. Littell, M.R. Marshall, H.A. Moye, and A.A. Teixeira. 1990. Evaluation of the properties of polystyrene divinyl benzene adsorbents for debittering grapefruit juice. J. Food Sci. 55:440–445.
- Musmade, K.P., M. Trilok, S.J. Dengale, K. Bhat, M.S. Reddy, P.B. Musmade, and N. Udupa. 2014. Development and validation of liquid chromatographic method for estimation of naringin in nanoformulation. J. Pharm. 2014:1–8.
- Petrotos, K.B., and Lazarides, H.N. 2001. Osmotic concentration of liquid foods. J. Food Eng. 49:201–206.
- Po Chien, J., F. Sheu, and T. Yuan Shyu. 2001. Monitoring enzymatic debittering in grapefruit juice by high performance liquid chromatography. J. Food Drug Anal. 9(2):115–120.
- Pulley, G.N. 1936. Solubility of naringin in water. Ind. Eng. Chem. Anal. Ed. 8(5):360.
- Puri, M., S.S. Marwaha, and R.M. Kothari. 1996a. Studies on the applicability of alginate-entrapped naringinase for the debittering of kinnow juice. Enzyme Microb. Technol. 18:281–285.
- Puri, M., S.S. Marwaha, R.M. Kothari, and J.F. Kennedy. 1996b. Biochemical basis of bitterness in citrus fruit juices and biotech approaches for debittering. Crit. Rev. Biotechnol. 16:145–155.
- Rastogi, N.K., and K. Raghavarao. 1997. Water and solute diffusion coefficients of carrot as a function of temperature and concentration during osmotic dehydration. J. Food Eng. 34:429–440.
- Rouseff, R.L. 1988. Liquid chromatographic determination of naringin as a detector of grapefruit juice in orange juice. J. Assoc. Off. Anal. Chem. 71:798–802.
- Sagar, V.S., and D.S. Khurdiya. 1999. Studies on dehydration of Dashehari mango slices. Indian Food Packer 53(1):5–9.
- Shaw, P.E., and B.S. Buslig. 1986. Selective removal of bitter compounds from grape fruit juice and from aqueous solution with cyclodextrin polymers and with Amberlite XAD-4. J. Agric. Food Chem. 34:837–840.
- Soares, N.F.F., and J.H. Hotchkiss. 1998. Naringinase immobilization in packaging films for reducing naringin concentration in grapefruit juice. J. Food Sci. 63:61–65.
- Spiegel-Roy, P., and E.E. Goldschmidt. 1996. Reproductive physiology: Flowering and fruiting, p. 70–125. In: The biology of citrus. Cambridge University Press, Cambridge, UK.
- Tsen, H.Y., and G.K. Yu. 1991. Limonin and naringin removal from grapefruit with naringinase entrapped in cellulose triacetate fibers. J. Food Sci. 56:31–34.
- Wei, M., Z. Yang, P. Li, Y. Zhang, and W.C. Sse. 2007. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am. J. Chin. Med. 35(4):663–667.
- Yusof, S., H.M. Ghazali, and G.S. King. 1990. Naringin content in local citrus fruits. Food Chem. 37:113–121.
- Zid, M.B., C. Dhuique-Mayer, S. Bellagha, C. Sanier, A. Collignan, A. Servent, and M. Dornier. 2015. Effects of blanching on flavanones and microstructure of Citrus aurantium peels. Food Bioprocess Technol. 8:2246–2255.