ABSTRACT
Macrophomina crown and root rot has become a significant soil-borne disease issue in California. For many locations in the state, the disease is associated with fields that are no longer pre-plant, flat field fumigated with methyl bromide + chloropicrin. Inoculation experiments indicated that some differences in strawberry cultivar susceptibility to Macrophomina phaseolina were seen a short time after the inoculation, but as disease progressed such differences did not persist. Preliminary characterization studies of Macrophomina phaseolina isolates from strawberry indicated that such isolates may have a host preference for strawberry. Macrophomina phaseolina isolates from watermelon, thyme, and apple failed to cause disease in strawberry. Five cover crop species, which can be rotated with strawberry, did not develop disease when inoculated with strawberry isolates. In preliminary analysis using simple sequence repeat markers, isolates obtained from strawberry formed a separate group compared to isolates recovered from other known Macrophomina phaseolina hosts.
KEYWORDS:
Introduction
Strawberry (Fragaria × ananassa) is one of California’s many important, high-value fruit crops. In 2013, California grew approximately 16,795 ha of strawberries, which accounted for 71% of the United States crop. The value of this 2013 crop was approximately $2.2 billion, representing 87% of the United States total value for strawberry (USDA, Citation2014). Most of the state’s crop is grown on the central to south central coastal counties stretching from Santa Cruz County in the north and moving south through Monterey, San Luis Obispo, Santa Barbara, and Ventura counties; 14,235 ha were produced in these coastal counties in 2013, which represented 85% of the state acreage. Historically the typical system for conventional California strawberry production consists of a pre-plant fumigation treatment with a combination of methyl bromide + chloropicrin, which is injected into the soil and then covered with plastic tarps. Following this treatment, the field soil is formed into beds where strawberry transplants are placed and grown for only one season. This system has successfully dealt with a range of weed, soil insect, and soil-borne pathogen issues.
Beginning at least as early as 2005 and continuing through 2014, strawberry growers throughout California reported an increasing problem with collapsing strawberry plants. Symptoms consisted of wilting of foliage, plant stunting, and necrosis of older leaves, with the central youngest leaves often remaining green and alive. Internal vascular and cortical crown tissues were dark to orange brown. Plants eventually collapsed and died, especially when they were subjected to environmental stresses or were bearing a heavy load of fruit. The fungus Macrophomina phaseolina was consistently isolated from symptomatic plants and was confirmed to be the cause of the dieback in California (Koike, Citation2008). By 2014, Macrophomina crown rot was confirmed in all major coastal strawberry counties in California as well as in a few smaller production areas in the state’s interior valleys. Most of the affected fields no longer received the annual methyl bromide + chloropicrin pre-plant treatment, so disease development has been associated with this change in disease management practices. The objectives of this research were to investigate susceptibility of strawberry cultivars and cover crops to M. phaseolina, examine susceptibility of strawberry to non-strawberry M. phaseolina isolates, and characterize M. phaseolina isolates recovered from California strawberry.
Materials and methods
Strawberry cultivar susceptibility to Macrophomina phaseolina
To investigate the relative susceptibility of strawberry cultivars to M. phaseolina, a common toothpick inoculation method used in Macrophomina studies was employed (Koike, Citation2008; Mertely et al., Citation2005; Mihail, Citation1992). Inoculum was prepared by placing sterilized, round, wooden toothpicks on the surface of potato dextrose agar in 100-mm petri plates and then inoculating the plates with agar plugs from an actively growing culture of isolate 08-11, recovered from diseased strawberry plants from Ventura County. Cultures were incubated at 23 °C for 10 days to allow for fungal and microsclerotial development onto the toothpicks. In November, commercial transplants of five cultivars were potted singly into 3.78-liter nursery pots filled with potting mix (Sunshine no. 4) and grown in a shadehouse in Salinas, CA for 5 months. To inoculate the plants, an entry wound was made by jabbing the tip of a sterile dissecting needle 0.6 cm deep into the main crown of each plant; a single colonized toothpick was then inserted into the wound. Ten plants per cultivar were inoculated, with each plant being considered a replication. For controls, 10 plants per cultivar were inoculated with sterile toothpicks. All plants were maintained in a shadehouse having ambient temperatures (22 °C to 26 °C) representative of the growing conditions for strawberry in Monterey County. Disease severity was rated at 4- and 8-week periods by using the following 5-point scale: 1 = no symptoms; 2 = only lower older leaves were wilted, chlorotic, and/or necrotic; 3 = less than 50% of the foliage was wilted, chlorotic, and/or necrotic and included symptomatic younger foliage; 4 = more than 50% of the foliage was wilted, chlorotic, and/or necrotic; 5 = complete plant collapse and death. Statistical analyses (analysis of variance and Fisher’s protected least significant difference [LSD]) were conducted using Statgraphics Centurion XVI (Warrenton, VA, USA).
Pathogenicity of diverse Macrophomina phaseolina isolates on strawberry
To determine if strawberry is susceptible to potentially diverse genotypes of M. phaseolina, toothpick inocula of 11 isolates were prepared as described above. Tests included the following: 6 isolates from strawberry; 3 isolates from watermelon (Citrullus lanatus); 1 isolate from thyme (Thymus vulgaris); and 1 isolate from apple (Malus domestica). All isolates originally were recovered from symptomatic plants grown on commercial farms in California. Ten plants of strawberry cultivar San Andreas were inoculated per isolate, with each plant being considered a replication. Crown inoculations were completed as described above. For controls, 10 plants were similarly inoculated with sterile toothpicks. All plants were maintained in a shadehouse having ambient temperatures (22 °C to 26 °C) representative of the growing conditions for strawberry in Monterey County. To simplify this screening, plants were evaluated for disease severity only after 6 weeks using a 4-point scale: 1 = no symptoms; 2 = only lower older leaves were wilted, chlorotic, and/or necrotic; 3 = more than 50% of the foliage was wilted, chlorotic, and/or necrotic; 4 = complete plant collapse and death. Statistical analyses (analysis of variance and Fisher’s protected least significant difference [LSD]) were conducted using Statgraphics Centurion XVI (Warrenton, VA, USA).
Susceptibility of cover crops to Macrophomina phaseolina isolated from strawberry
With the development of Macrophomina crown rot throughout California, growers were concerned about whether cover crops, planted in rotation between strawberry seasons, could host the pathogen and thereby augment pathogen inoculum or contribute to disease on strawberry. Therefore, two M. phaseolina isolates from strawberry (08-12 and 08-17) were used to inoculate a range of cover crops. To prepare inoculum, 200 ml of a 3:1 sand-cornmeal medium (Mihail, Citation1992) were placed into 500-ml Erlenmeyer flasks and 120 ml of distilled water were added. The flasks were sealed with foil and autoclaved two times (24 h between autoclaving). Agar plugs cut from actively growing colonies of M. phaseolina from either isolate were added to each flask. Inoculated flasks were maintained at 23 °C for 2 months. When flask cultures were fully colonized, as evidenced by the black color due to microsclerotia formation, flask contents were emptied onto trays and allowed to dry at 23 °C for 2 days.
The following cover crops were used in this study: fava bean (Vicia faba), mustard (Sinapis alba cv. Ida Gold), oat (Avena sativa cv. Cayuse), rye (Secale cereale cv. Merced), and common vetch (Vicia sativa). Strawberry (cv. Albion) was used as a positive control. All cover crop species were grown as transplants by placing seeds into transplant trays; two seeds were placed in each cell for fava bean while five seeds were used in each cell for the other cover crop species. Dormant strawberry plants were planted into shallow trays containing potting mix in order to allow the plants to break dormancy and grow roots and foliage. The number of plants used for inoculations was 18 (divided into nine sets of two plants per cell) of fava bean, 45 plants for the other cover crop species (divided into nine sets of five plants per cell), and 10 strawberry plants. All plants were inoculated by sprinkling 4 grams of the sand-cornmeal inoculum (8000 microsclerotia/gram) onto the root balls of each set and then misting the root balls with distilled water to secure the material to the roots. Inoculated plants were potted into either 10-cm (cover crops) or 15-cm diameter (strawberry) nursery pots. Control plants of strawberry and all cover crops were inoculated with sterile sand-cornmeal medium and planted in similar fashion. All plants were maintained in a greenhouse (24 °C to 26 °C). Plants were evaluated for disease symptoms after 6 weeks.
After disease evaluation, roots of all Macrophomina-inoculated plants were sampled and tested for the pathogen. For strawberry, 20 root pieces showing internal discoloration were collected for each isolate set, rinsed, surface-sterilized (0.1% NaOCl for 1 min), and placed into petri plates containing acidified (2% lactic acid/liter) cornmeal agar (LA-CMA). For all cover crops, 30 root pieces were collected and handled in the same way. Cover crop root pieces were selected if any discoloration or disease symptom was evident; if no roots showed any symptom or discoloration, randomly collected healthy roots were tested.
Preliminary molecular characterization of Macrophomina phaseolina isolated from strawberry
In a preliminary analysis to identify the best simple sequence repeat (SSR) markers for differentiating California isolates of Macrophomina obtained from a range of hosts, a total of 182 microsatellite loci described in Arias et al. (Citation2011) were evaluated. The procedures were as reported in Arias et al. (Citation2011) with fragment sizing and analysis done with GeneMapper (Applied Biosystems, Foster City, CA, USA; Ver. 4.1). Unweighted pair group method with arithmetic means (UPGMA) analysis was done with NTSYS (Exter Software, Setauket, NY, USA; Ver. 2.2) to show the relationships among the different genotypes.
Results
Strawberry cultivar susceptibility to Macrophomina phaseolina
After 2 weeks, inoculated strawberry plants began to show disease symptoms with the lower leaves turning yellow and wilting. By week 4, extensive dieback was evident on all plants inoculated with M. phaseolina and statistically significant differences (P = 0.05) were recorded. Four of the cultivars (Albion, Camarosa, Diamante, and Ventana) scored 3.9 or higher and only cv. Seascape had a disease severity rating lower than 3 (2.9) (). However, disease continued to progress and by the 8-week period all inoculated cultivars were severely diseased and no significant differences were seen (). All plants inoculated with M. phaseolina-infested toothpicks exhibited crown discoloration and the pathogen was recovered from all plants when surface-sterilized (1% NaOCl for 3 min) crown tissue was excised and placed into petri plates containing LA-CMA. All control plants that received sterilized toothpicks showed no disease symptoms (); these plants were not included in the statistical analysis.
Table 1. Susceptibility of strawberry cultivars to toothpick crown inoculation with a California isolate of Macrophomina phaseolina.
Pathogenicity of diverse Macrophomina phaseolina isolates on strawberry
At the 6-week period, strawberry isolates 11-9, 11-15, and 11-18 caused severe disease levels (3.2, 3.0, and 3.3, respectively) that were statistically higher (P = 0.05) than severity caused by strawberry isolates 11-12 and 11-17 (1.2 and 1.3, respectively) (). Plants inoculated with these five isolates exhibited crown discoloration and M. phaseolina was recovered from crown tissue that was surface sterilized and plated onto LA-CMA as described above. However, strawberry isolate 11-16 did not cause any foliar symptoms or crown discoloration; the disease severity rating was statistically lower (P = 0.05) than those of strawberry isolates 11-9, 11-15, and 11-18 but was not significantly different from strawberry isolates 11-12 and 11-17 (). All non-strawberry (watermelon, thyme, apple) isolates caused no foliar symptoms or crown discoloration and disease severity ratings were significantly lower (P = 0.05) than those for strawberry isolates 11-9, 11-15, and 11-18 (). Symptomless strawberry plants were not tested for M. phaseolina. All control plants that received sterilized toothpicks showed no disease symptoms; these plants were not included in the statistical analysis.
Table 2. Pathogenicity of strawberry and non-strawberry Macrophomina phaseolina isolates to strawberry (cv. San Andreas).
Susceptibility of cover crops to Macrophomina phaseolina isolated from strawberry
After 6 weeks, the majority of the Macrophomina-inoculated strawberry plants exhibited foliar dieback and wilting (8 symptomatic plants of 10 total for isolate 08-12; 6 symptomatic plants of 10 total for isolate 08-17) (). None of the 10 strawberry plants inoculated with sterile sand-cornmeal showed symptoms. None of the Macrophomina-inoculated or sterile sand-cornmeal inoculated cover crop species showed any foliar symptoms of disease (). When strawberry plants were unpotted and examined, 8 of 10 plants inoculated with isolate 08-12 had internally discolored, diseased roots while 6 of 10 plants inoculated with isolate 08-17 had such roots (). Control strawberry plants inoculated with sterile sand-cornmeal showed no root necrosis or disease symptoms. Roots of mustard, oat, rye, and vetch showed no discolored, necrotic, or symptomatic roots (). For fava bean, however, eight of nine plant sets inoculated with isolate 08-12 exhibited externally dark, discolored roots; for isolate 08-17, five out of nine plant sets had such roots (). Fava bean plants inoculated with sterile sand-cornmeal had white, asymptomatic roots.
Table 3. Susceptibility of cover crops and strawberry to root inoculation with two isolates of Macrophomina phaseolina recovered from strawberry.
After disease assessments were completed, roots were collected and tested for M. phaseolina. For strawberry, M. phaseolina was recovered from 5 out of 20 (isolate 08-12) and 4 out of 20 (isolate 08-17) discolored root pieces (). For all strawberry isolations, root pieces were heavily colonized by a Trichoderma species. For mustard, oat, rye, and vetch all roots appeared symptomless, thus white, healthy root pieces were randomly selected and tested; all such root pieces were negative for M. phaseolina with the exception of one root piece from mustard that was inoculated with isolate 08-12 (). For the darkened roots of fava bean, all 30 pieces were negative for M. phaseolina.
Preliminary molecular characterization of Macrophomina phaseolina isolated from strawberry
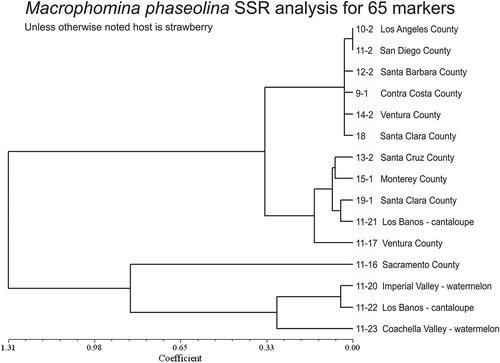
Of the 182 SSR loci evaluated, 65 were found to generate amplicons in all isolates evaluated and exhibit polymorphisms compared with results for isolates recovered from other hosts that were reported in Arias et al. (Citation2011). With the California isolates, those from strawberry were grouped separately from watermelon and cantaloupe with two exceptions (): an isolate recovered from cantaloupe in Los Baños, CA (11-21) grouped with the strawberry isolates and a strawberry isolate from Sacramento, CA (11-16) grouped with cucurbit isolates. With the exception of isolates 11-16 and 11-21, additional isolates that have been examined all exhibit an absolute grouping of strawberry isolates within the same main clade (data not shown).
Discussion
Macrophomina phaseolina as a pathogen of strawberry is not a new finding, having been reported at least as early as 1981 in Egypt (Madkour and Aly, Citation1981). However, almost all of the significant outbreaks have been recent developments reported since 2005. This pathogen now is causing economic damage on strawberry in Argentina (Baino et al., Citation2011), Australia (Fang et al., Citation2011a, Citation2011b), Chile (Sanchez et al., Citation2013), France (Baudry and Morzieres, Citation1993), Iran (Sharifi and Mahdavi, Citation2012), Israel (Zveibil and Freeman, Citation2005), Spain (Aviles et al., Citation2008), and the United States—California (Koike, Citation2008) and Florida (Mertely et al., Citation2005). Macrophomina has also been recovered from strawberry in Turkey, though pathogenicity was not confirmed (Benlioglu et al., Citation2004). In California the disease has developed throughout the major coastal strawberry producing counties and is associated with the use of alternative soil treatments in place of the methyl bromide + chloropicrin flat fume, pre-plant treatment. Inoculation of various strawberry cultivars using the very severe toothpick crown inoculation method demonstrated differences in susceptibility but only for a short period of time. These results showed that the cultivars may have subtle differences in susceptibility but that none of them are resistant; this finding is consistent with previous studies that used inoculated strawberry roots with sand-cornmeal inoculum (Koike et al., Citation2013). Differences in strawberry cultivar susceptibility to M. phaseolina have been found by other researchers as well (Aviles et al., Citation2009).
Preliminary host range experiments indicated that possible differences in virulence might exist between M. phaseolina isolates obtained from strawberry. Strawberry isolates 11-9 and 11-18 caused moderately severe symptoms (3.2 and 3.3, respectively), while two other strawberry isolates (11-12 and 11-16) caused few or no symptoms (1.2 and 1.0, respectively). For other crops, M. phaseolina isolates also show variation in virulence or aggressiveness (Dhingra and Sinclair, Citation1973; Kaur et al., Citation2012; Purkayastha et al., Citation2006).
In addition, our studies provide evidence that strawberry isolates of M. phaseolina may have a host preference for strawberry. In this experiment only M. phaseolina isolates from strawberry caused disease on toothpick inoculated strawberry, although isolate 11-16 from strawberry did not. As noted below, the genotype of this isolate was different from other strawberry isolates and grouped with cantaloupe/watermelon isolates (). Isolates from watermelon, thyme, and apple did not cause foliar or crown symptoms when inoculated with toothpicks into strawberry crowns. These results are consistent with other studies that indicated some M. phaseolina isolates manifested host preferences or restricted host ranges (Cloud and Rupe, Citation1991; Manici et al., Citation1995; Mayek-Perez et al., Citation2001; Mihail and Taylor, Citation1995; Reyes-Franco et al., Citation2006; Su et al., Citation2001). However, diversity of isolates from throughout the world, various crop rotations implemented in fields, and different inoculation and incubation conditions likely account for some reports in which M. phaseolina isolates show no such host preferences (Zazzeini and Tosi, Citation1989; Zveibil et al., Citation2012).
Using sand-cornmeal inoculum to inoculate roots of various cover crops, we demonstrated that M. phaseolina isolates from strawberry failed to infect and cause disease on fava bean, mustard, oat, rye, and vetch. Others have reported M. phaseolina as causing disease on fava bean, mustard, oat, and various Vicia species but not V. sativa (vetch) (Farr and Rossman, Citation2015). Interestingly, roots of inoculated fava bean developed a dark, external, stain-like discoloration while control fava bean roots remained white. Macrophomina was not recovered from any of the stained fava bean roots, clearly demonstrating lack of disease development. The reason for this reaction of fava bean roots to inoculation is beyond the scope of this study and is presently open to speculation. These cover crops, therefore, do not appear to be additional hosts of M. phaseolina that would increase soil inoculum levels if planted in rotation with strawberry in California.
Further, 65 SSR markers were found to amplify DNA fragments among the subgroup of isolates that were evaluated. Interestingly, with few exceptions the strawberry isolates were grouped separately from isolates recovered from other hosts. This finding was also observed by Baird et al. (Citation2010) with their strawberry isolates (primarily from Florida) and a pole bean isolate from Alabama grouping separately from 104 isolates recovered from other hosts. Host association by genotype of M. phaseolina has also been observed in other crops (Arias et al., Citation2011). The one strawberry isolate (11-16) that was outside of this clade in also did not cause disease on this host when inoculated with a toothpick. In conjunction with the pathogenicity test data this suggests there may be a host preference for the genotypes recovered from strawberry, although additional pathogenicity and virulence tests need to be conducted to further clarify this possible relationship (cantaloupe isolate 11-21 has the same genotype as the strawberry isolates and needs to be included in these trials). Results from the analysis with 65 loci will be used to identify a manageable number of markers to use in a more extensive analysis of pathogen genotypes that is currently in progress. This analysis will have a larger isolate sample size that includes isolates recovered from all strawberry production areas in California. Representative isolates from each genotype will be evaluated for pathogenicity on the same hosts used in this report.
Acknowledgments
We thank the following persons for their assistance: Patty Ayala, Scott Cosseboom, Kat Kammeijer, and Dan Legard.
Funding
We thank the following organizations for their financial support: California Strawberry Commission and the USDA-ARS National Plant Disease Recovery System.
Additional information
Funding
Literature cited
- Arias, R.S., J.D. Ray, A. Mengistu, and B.E. Scheffler. 2011. Discriminating microsatellites from Macrophomina phaseolina and their potential association to biological functions. Plant Pathol. 60:709–718.
- Aviles, M., S. Castillo, J. Bascon, T. Zea-Bonilla, P.M. Martin-Sanchez, and R.M. Perez-Jimenez. 2008. First report of Macrophomina phaseolina causing crown and root rot of strawberry in Spain. Plant Pathol. 57:382.
- Aviles, M., S. Castillo, C. Borrero, M.L. Castillo, T. Zea-Bonilla, and R.M. Perez-Jimenez. 2009. Response of strawberry cultivars: ‘Camarosa’, ‘Candonga’, and ‘Ventana’ to inoculation with isolates of Macrophomina phaseolina. Acta. Hort. 842:291–294.
- Baino, O.M., S.M. Salazar, A.C. Ramallo, and D.S. Kirschbaum. 2011. First report of Macrophomina phaseolina causing strawberry crown and root rot in northwestern Argentina. Plant Dis. 95:1477.
- Baird, R.E., P.A. Wadl, T. Allen, D. McNeill, X. Wang, J.K. Moulton, T.A. Rinehart, H.K. Abbas, T. Shier, and R.N. Trigiano. 2010. Variability of United States isolates of Macrophomina phaseolina based on simple sequence repeats and cross genus transferability to related genera within Botryosphaeriaceae. Mycopathologia 170:169–180.
- Baudry, A., and J.P. Morzieres. 1993. First report of charcoal rot of strawberry in France. Acta Hort. 348:485–488.
- Benlioglu, S., A. Yildiz, and T. Doken. 2004. Studies to determine the causal agents of soilborne fungal diseases of strawberry in Aydin and to control them by soil disinfestation. J. Phytopathol. 152:509–513.
- Cloud, G.L., and J.C. Rupe. 1991. Morphological instability on a chlorate medium of isolates of Macrophomina phaseolina from soybean and sorghum. Phytopathology 81:892–895.
- Dhingra, O.D., and J.B. Sinclair. 1973. Location of Macrophomina phaseoli on soybean plants related to culture characteristics and virulence. Phytopathology 63:934–936.
- Fang, X., D. Phillips, H. Li, K. Sivasithamparam, and M.J. Barbetti. 2011a. Severity of crown and root diseases of strawberry and associated fungal and oomycete pathogens in Western Australia. Australasian Plant Pathol. 40:109–119.
- Fang, X., D. Phillips, H. Li, K. Sivasithamparam, and M.J. Barbetti. 2011b. Comparisons of virulence of pathogens associated with crown and root diseases of strawberry in western Australia with special reference to the effect of temperature. Sci. Hort. 131:39–48.
- Farr, D.F., and A.Y. Rossman. 2015. Fungal Databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA. 28 March 2015. http://nt.ars-grin.gov/fungaldatabases/.
- Kaur, S., G.S., Dhillon, S.K. Brar, G.E. Vallad, R. Chand, and V.B. Chauhan. 2012. Emerging phytopathogen Macrophomina phaseolina: Biology, economic importance and current diagnostic trends. Crit. Rev. Microbiol. 38:136–151.
- Koike, S.T. 2008. Crown rot of strawberry, caused by Macrophomina phaseolina, in California. Plant Dis. 92:1253.
- Koike, S.T., T.R. Gordon, O. Daugovish, H. Ajwa, M. Bolda, and K. Subbarao. 2013. Recent developments on strawberry plant collapse problems in California caused by Fusarium and Macrophomina. Intl. J. Fruit Sci. 13:76–83.
- Madkour, M.A., and M.H. Aly. 1981. Cell wall degrading enzymes produced during pathogenesis of Macrophomina phaseolina on strawberry plants. Phytopathol. Z. 100:36–43.
- Manici, L.M., F. Caputo, and C. Cerato. 1995. Temperature responses of isolates of Macrophomina phaseolina from different climatic regions of sunflower production in Italy. Plant Dis. 79:834–838.
- Mayek-Perez, N., C. Lopez-Castaneda, M. Gonzalez-Chavira, R. Garcia-Espinosa, J. Acosta-Gallegos, O. Martinez de la Vega, and J. Simpson. 2001. Variability of Mexican isolates of Macrophomina phaseolina based on pathogenesis and AFLP genotype. Physiol. Mol. Plant Pathol. 59:257–264.
- Mertely, J., T. Seijo, and N. Peres. 2005. First report of Macrophomina phaseolina causing a crown rot of strawberry in Florida. Plant Dis. 89:434.
- Mihail, J.D. 1992. Macrophomina. In: L.L. Singleton, J.D. Mihail, and C.M. Rush (eds.). Methods for research on soilborne phytopathogenic fungi. American Phytopathological Society Press, St. Paul, MN.
- Mihail, J.D., and S.J. Taylor. 1995. Interpreting variability among isolates of Macrophomina phaseolina in pathogenicity, pycnidium production, and chlorate utilization. Can. J. Bot. 73: 1596–1603.
- Purkayastha, S., B. Kaur, N. Dilbaghi, and A. Chaudhury. 2006. Characterization of Macrophomina phaseolina, the charcoal rot pathogen of cluster bean, using conventional techniques and PCR-based molecular markers. Plant Pathol. 55:106–116.
- Reyes-Franco, M.C., S. Hernandez-Delgado, R. Beas-Fernandez, M. Medina-Fernandez, J. Simpson, and N. Mayek-Perez. 2006. Pathogenic and genetic variability within Macrophomina phaseolina from Mexico and other countries. J. Phytopathol. 154:447–453.
- Sanchez, S., M. Gambardella, J.L. Henriquez, and I. Diaz. 2013. First report of crown rot of strawberry caused by Macrophomina phaseolina in Chile. Plant Dis. 97:996.
- Sharifi, K., and M. Mahdavi. 2012. First report of strawberry crown and root rot caused by Macrophomina phaseolina in Iran. Iranian J. Plant Pathol. 47:161.
- Su, G., S.-O. Suh, R.W. Schneider, and J.S. Russin. 2001. Host specialization in the charcoal rot fungus, Macrophomina phaseolina. Phytopathology 91:120–126.
- USDA. 2014. National Agricultural Statistics Service. US Vegetable Commodity Rankings. http://www.nass.usda.gov/Statistics_by_Subject/index.php?sector=CROPS. Accessed 25 Feb. 2015.
- Zazzeini, A., and L. Tosi. 1989. Chlorate sensitivity of Sclerotium bataticola isolates from different hosts. J. Phytopathol. 126:219–224.
- Zveibil, A., N. Mor, N. Gnayem, and S. Freeman. 2012. Survival, host-pathogen interaction, and management of Macrophomina phaseolina on strawberry in Israel. Plant Dis. 96:265–272.
- Zveibil, A., and S. Freeman. 2005. First report of crown and root rot in strawberry caused by Macrophomina phaseolina in Israel. Plant Dis. 89:1014.