ABSTRACT
Finding effective non-fumigant solutions to soil disinfestation is an international priority for sustainable strawberry production. In these studies at Ventura, California, combinations of steam, solarization, and mustard meal were evaluated to manage troublesome soil-borne pathogens: Macrophomia phaseolina and Fusarium oxysporum. Treatments containing steam and solarization reduced levels of these pathogens in soil 70–96% compared to untreated soil and had lower rates of late season pathogen-related plant mortality. All treatments improved plant canopy growth and fruit production of ‘San Andreas’ strawberry 58% to 83% compared to untreated control. Even though these treatments did not eradicate pathogen spores and sclerotia in soil, they enhanced early and whole season fruit production and when feasible can be applied in non-fumigated and organic strawberry fields to help sustain production.
Introduction
California produces nearly 90% of the strawberries in the United States (CSC, Citation2011). While the current production system relies primarily on chemical fumigation of soil, disinfestation with non-fumigant methods has been a priority research area of the strawberry industry (Fennimore et al., Citation2013; Muramoto et al., Citation2014). There is a current need for alternative production methods to sustain yields after the phase out of methyl bromide and because of increasing regulatory restrictions on alternative chemical fumigants (USDS, Citation2014). Among non-fumigant treatments, the application of steam to soil prior to strawberry planting has been evaluated (Samtani et al., Citation2012). Steam increases soil temperatures to levels that are lethal to soil-borne pathogens and weeds (Baker and Roistacher, Citation1957). The time and temperature of steam-induced heating required to kill soil-borne pests varies among soil types and conditions, but the general target for mortality of most weed species and pathogenic fungi has been reported to be 65 °C for 30 min (Baker and Roistacher, Citation1957). The efficacy of steam application may be enhanced when combined with other technologies that in themselves do not provide adequate soil disinfestation as stand-alone treatments in coastal California strawberry production regions, such as solarization and incorporation of mustard seed meal (Daugovish et al., Citation2011b). Solarization of wetted beds covered with transparent polyethylene (PE) film in coastal production areas can raise the soil temperature near the bed surface to 50 °C but has little effect at depths of 0.3 m or greater (Daugovish et al., Citation2011b; Stapleton et al., Citation1997). Seed meal and biomass of mustards and other Brassicaceae spp. incorporated into wet soil have been shown to produce biocidal isothiocyanates (ITCs) that suppressed several nematode species, weed propagates, and some fungal pathogens (Daugovish et al., Citation2004; James et al., Citation2004). However, the effects of Brassica-derived ITCs are inconsistent and variable, and combinations of mustard-derived ‘biofumigation’ with other methods of soil disinfection have been suggested (Dominguez et al., Citation2014; Fennimore et al., Citation2014).
In recent years, pathogenic fungi, Macrophomina phaseolina and Fusarium oxysporum, have become increasingly problematic in California, causing plant decline and collapse in all strawberry production regions in both fumigated and non-fumigated soil (Koike et al., Citation2012). Alternative chemical soil fumigants can provide some protection from pathogens in the early to mid-season production period; however, the decline accelerates late in the season with increased soil temperatures, fruit loads, and other stress factors (Koike et al., Citation2012). Alternative fumigants commonly applied to strawberry beds via drip lines (e.g., 1,3-D + chloropicrin combination) have not been as effective as the methyl bromide + chloropicrin combination, of which methyl bromide has been phased out (Zveibil et al., Citation2012).
Non-fumigated fields and buffer zones in fumigated fields that contain these pathogens are especially vulnerable and may experience severe or complete plant loss due to infection as early as 6 weeks after planting (Daugovish et al., Citation2011a). Research on the efficacy of non-fumigant soil treatments on management of M. phaseolina and F. oxysporum has been limited. Rosskopf et al. (Citation2014) reported significant reduction in introduced pathogen survivorship in soil after anaerobic disinfestation of soil in Florida. Trials at Huelva, Spain showed that combinations of solarization + chicken manure (or sugar beet vinasse) reduced incidence of charcoal rot caused by M. phaseolina compared with the untreated control, and provided fruit yields similar to 1,3-D + chloropicrin fumigated soil (Chamorro et al., Citation2014). Dominguez et al. (Citation2014) also reported that biosolarization (solarization combined with Brassica pellets, chicken manure, or dried olive pomace) reduced plant mortality due to charcoal rot by 50–90% over untreated controls and provided significant yield improvements. In the Central Coast of California, steam treatments alone or in combination with mustard seed meal were as effective as 1,3-D + chloropicrin in controlling weeds and provided comparable strawberry yields (Fennimore et al., Citation2014); however, no effects on pathogens were documented in that study.
The objective of this project was to evaluate the efficacy of combinations of non-fumigant treatments in fields infested with weeds and M. phaseolina and F. oxysporum on pathogen survival and strawberry crop performance.
Materials and methods
Field experiments
The study was conducted in a commercial production field with continuous annual strawberry production at Ventura, CA during the 2010–11 and 2011–12 seasons. The soil was a clay loam, with a pH of 7.2, and 0.9% organic matter. The experiment was located in a non-fumigated buffer zone (due to proximity to residential areas) with at least a 3-year history of consistent M. phaseolina and F. oxysporum recovery from soil and declining strawberry plants. Fields, beds, and treatments were prepared similarly for both seasons. Sites were pre-irrigated in August; beds formed were 30 cm high with 170 cm distance between bed centers. Treatments were applied to 20-m-long sections on beds in September, and arranged in a randomized complete block design with four replications. Treatments were: (1) steam + mustard, (2) steam + solarization, (3) solarization + mustard, and (4) untreated control. Mustard pellets, 2200 kg/ha, were placed on bed tops and incorporated with an implement that rototills the soil to a depth of 35 cm and reshapes the bed in the same pass. Two irrigation drip lines (Toro, Aqua-Traxx delivering about 4 L/min/100 m via 20-cm-spaced emitters; Toro Micro-Irrigation, El Cajon, CA, USA), were placed at 45 cm from bed edges at 6 cm depth, and beds were covered with either black (no solarization) or transparent (with solarization) low density polyethylene mulch (0.15 mm thick, Guardian AgroPlastics, Tampa, FL, USA). HOBO sensors placed at 15 cm depth measured soil temperature and moisture for solarization treatments for 8 weeks after treatment application until planting. During solarization periods, there was no precipitation and average day-night air temperatures were 16.1 °C and 16.3 °C, while solar radiation averaged 259 W/m2 and 220 W/m2 in 2010 and 2011, respectively. A custom built propane-fueled stationary steam generator was used to produce steam, applied via four hoses per bed with 15-cm-long spikes placed in soil 15 cm apart. Felt blankets were placed over the beds with hoses during steam application to provide insulation and raise the temperature at 15 cm to 70 °C for 20 min.
Planting holes were mechanically cut on 12 Oct. to permit placement of bare root ‘San Andreas’ strawberry transplants. Transplants were irrigated with overhead sprinklers for 6 weeks during establishment and then irrigation was switched to drip for the rest of the season. Plants were grown following standard production and pest management practices (Strand, Citation2008).
For each experimental plot, a subplot of 20 plants was selected and used all for measurements throughout the experimental period. The plant canopy was measured on 29 Nov. 2010 and 20 Nov. 2011, and canopy areas calculated using the formula for an ellipse (plant length/2 × plant width/2 × π). Marketable and unmarketable fruit weights were recorded weekly throughout the fruiting season. In beds with transparent mulch, weed densities were measured on 16 Nov. 2010 and 28 Nov. 2011 in whole plots by removing weeds through planting holes from underneath the mulch and weighing dry biomass. Plant mortality was assessed monthly during both seasons by counting and removing dead or severely declined, non-productive plants from whole plots. These plants were analyzed in the laboratory for presence of pathogens to confirm the causes of mortality.
Pathogen sampling and analyses
Soil samples (composite of 50 cores along the diagonal of each plot) were taken on 12 Dec. 2010 and 17 Dec. 2011 at 0–15 and 15–30 cm depths. Samples were processed and analyzed for the presence of Fusarium oxysporum f. sp. fragariae and Macrophomina phaseolina. Inoculum density of F. oxysporum f. sp. fragariae was quantified by soil dilution plating. Each composite soil sample was thoroughly mixed by hand from which 3.1 grams of soil was removed and added to 200 mls of 1% sodium hexametaphosphate in de-ionized water. This suspension was stirred vigorously on a magnetic stir plate for a minimum of 5 min. While the suspension was stirring, 7.5 ml was withdrawn and added to 42.5 ml of sterile 0.1% water agar. This suspension was mixed for 5 min as described above. While the suspension was stirring, a subsample of 250 µl was withdrawn and spread over the surface of Komada’s selective medium in 10-cm-diameter Petri plates (Komada, Citation1975). Twenty plates were inoculated for each sample and incubated under fluorescent lights as described by Scott et al. (Citation2010). After 7–10 days, colonies of F. oxysporum f. sp. fragariae were identified.
Inoculum density of Macrophomina phaseolina in soil samples was determined following a slightly modified method described by Mihail (Citation1992). Soil samples were dried in a greenhouse and each sample pulverized separately. A 5-g aliquot of soil from each sample was mixed with approximately 250 ml of 10% bleach solution:water in a blender for 30 s and the contents were allowed to settle for 3 min. Each sample was then washed with distilled water through a 74-µm US standard sieve. The residue was back washed into 125-ml sterilized flask with sterilized water. Further, 100 ml of cooled potato dextrose agar (PDA) amended with rifampicin (Sigma-Aldrich, St. Louis, MO, USA) (0.05 g/L) and Tergitol (Sigma-Aldrich) (1 ml/L) was added to each sample and mixed well. The mixture was poured into 5 to 6 Petri plates and incubated at 31 °C to 32 °C in the dark for 3 to 4 days. The number of Macrophomina colonies forming fluffy white mycelium surrounded by a central area with black sclerotia was enumerated under a stereomicroscope.
Results and discussion
Strawberry plant performance
In 2010, plants were 45–68% larger in all non-fumigant treatments compared to untreated control, indicating beneficial treatment effects on early plant growth (). In 2011, again the plants in solarization + mustard and steam + solarization treatments were, on average, 60% larger than in the untreated control, while in the steam + mustard treatment, plants were similar to other non-fumigant combinations and the untreated control (). Soil temperature at 15 cm under transparent mulch was 31 °C on average in August and 27 °C in September with night-day range of 23 °C to 44 °C during the solarization period in both years. Soil temperature at 15 cm depth under black mulch was on average 23 °C in August and 21 °C in September with night-day range of 22 °C to 25 °C during the same period in both years. These soil temperature differences suggest a mild solarization effect under transparent mulch.
Table 1. Performance of strawberry cv. San Andreas after non-fumigant soil treatments at Ventura, CA in 2010–11 and 2011–12.
The increase in plant canopy area resulted in 63–83% early fruit yield increase in all treatments compared with the untreated control in 2011 and 58–72% yield increase in 2012 in solarization + mustard and steam + solarization plots. The steam + mustard treatment provided early yields similar to other treatments, including untreated control consistent with previous plant canopy area measurements (). In general, treatments that included solarization (transparent mulch) tended to have greater early vigor and fruit production than treatments with black mulch (untreated and steam + mustard). This is consistent with previous findings of earlier plant development and productivity in beds with transparent mulch as opposed to opaque mulch due to warmer soil in the root zone (Daugovish et al., Citation2011b; Johnson and Fennimore, Citation2005). These yield improvements with non-fumigant treatments in winter months are especially valuable since the fresh market fruit prices are typically highest during that part of the season (USDA, Citation2014).
Total marketable yields were 50–84% greater than in the untreated control in 2011 for all treatments except solarization + mustard, and 37–45% greater in 2012, except for the steam + mustard treatment (). Even though the yields were statistically similar among treatments, application of steam combined with solarization tended to improve early and total yields in both seasons were compared to other treatments indicating potential benefit of combining these techniques. In studies by Samtani et al. (Citation2012), at Salinas, California the combination of solarization with steam also provided a 27% yield increase over the solarization application alone and a 50% improvement over the untreated control.
Pathogen survivorship, strawberry mortality, and weed control
Fusarium oxysporum survivorship was reduced at all sampling depths, 70–76% in the steam + solarization treatment compared to untreated control in both seasons (). The number of CFUs/g of soil in other treatments was not statistically different from the untreated control. Treatments that included steam effectively reduced viability of M. phaseolina inoculum (76–96% in 2010–11 and 80–92% in 2011–12) compared to untreated control, but only at 15–30 cm soil depths. At 0–15 cm depth in treatments containing steam application, and at all depths in other treatments, pathogen levels were not significantly different from the untreated control (). The reduction of pathogen levels in steam + solarization treated plots at 15–30 cm depth likely contributed to increased plant growth and fruit production, as previously discussed ().
Table 2. Treatment effects on pathogen populations in soil and resident weed densities at Ventura, CA in 2010–11 and 2011–12.
No significant plant mortality was observed in any treatments until April in both seasons (). As expected, with the onset of warmer temperatures in late spring and increased plant stress with heavy fruit loads, the mortality accelerated in all plots. Both M. phaseolina and F. oxysporum were consistently isolated from tissues of declining plants, confirming that levels of pathogen survivorship in soil were sufficient to cause the diseases. Plants in the solarization + mustard treatment had the highest levels of late-season mortality, reaching 62% on 31 May 2011 and 34% on 8 June 2012 (). This is likely due to lack of efficacy of this treatment against soil-borne pathogens () and accelerated development of disease in warmer soil with transparent mulch compared to cooler soils under opaque mulch. This would be consistent with previous reports showing that warmer temperatures favor development of disease caused by both M. phaseolina (Zveibil et al., Citation2012) and F. oxysporum (Koike and Gordon, Citation2015). High levels of late-season mortality in the solarization + mustard plots had a negative effect on total fruit yield in 2011, even though early yield was significantly greater than in the untreated control ().
Figure 1. Mortality of strawberry plants of cv. San Andreas due to Macrophomina phaseolina and Fusarium oxysporum at Ventura, CA during 2010–11 (A) and 2011–12 (B) seasons. Letters ‘a’ near treatment means indicate their significant difference from other treatment means at the same date according to Fisher’s LSD test (P > 0.05).
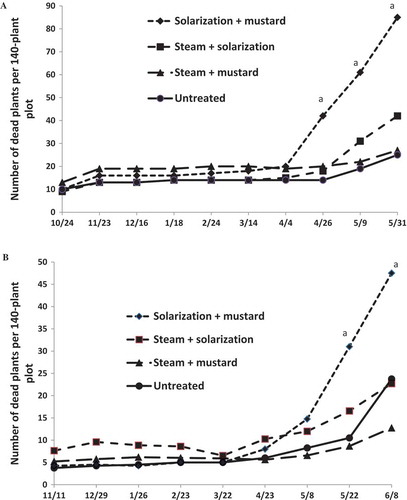
Lower levels of plant mortality (15–20%) in treatments with black mulch (steam + mustard and untreated control), compared to those with transparent mulch, may be attributed to slower rates of both root growth and disease progression in cooler soil, also reported by Daugovish et al. (Citation2013).
In treatments with transparent mulch, the predominant weed species were Malva parviflora, Chenopodium album, Euphorbia maculata, and Sonchus oleraceus. The steam + solarization treatment reduced total weed densities 60% and 96% in 2010 and 2011, respectively, compared to solarization + mustard treatment (). Similar beneficial effects of steam and solarization on weed control were also reported by Fennimore et al. (Citation2013), resulting in reduced costs of hand weeding.
Overall, this study showed that soil treatment with steam in combination with solarization can reduce the levels of M. phaseolina and F. oxysporum pathogens and weed propagates in soils, but were unable to eradicate them in infested strawberry fields. All non-fumigant treatments, regardless of the effect on pathogens, provided significant early yield improvements compared to untreated control and fruit production comparable to that of 1,3 D + chloropicrin fumigated soil (Dominguez et al., Citation2014; Fennimore et al., Citation2013). Thus, in the absence of fumigation in restricted buffer zones and organic fields these non-fumigant combinations, when feasible, can play an important role in sustaining strawberry production.
Literature cited
- Baker, K. and C. Roistacher. 1957. Principles of heat treatment of soil, p. 138–161. In: K. Baker (ed.). The UC system for producing healthy container grown plants. UC Agriculture Experiment Station Extension Service, Manual 23, Berkeley, CA, USA.
- California Strawberry Commission (CSC). 2011. Annual California Strawberry Acreage Survey. 29 Sept. 2014. http://www.californiastrawberries.com/files/Static%20Page%20Files/2012_Acreage_Survey_sm.pdf.
- Chamorro, M., L. Miranda, P. Domínguez, J. Medina, C. Soria, F. Romero, J.M. Lopez Aranda, and B. de los Santos. 2014. Evaluation of biosolarization for the control of charcoal rot disease (Macrophomina phaseolina) in strawberry. Crop Protection 67:279–286.
- Daugovish, O., J. Downer, O. Becker, G. Browne, and J. Dunniway. 2004. Mustard-derived biofumigation for vegetable crops and strawberries. J. Ital. Inst. Ind. Crops 3:335–338.
- Daugovish, O., S. Fennimore, T. Gordon, S. Koike, J. Muramoto, C. Shannon, and K. Subbarao. 2013. Non-fumigant treatments for management of Fusarium oxysporum and Macrophomina phaseolina on strawberry cultivar San Andreas. Proc. Annu. Intl. Conf. Methyl Bromide Altern. Emissions Reductions, San Diego, CA, 4–6 November. http://mbao.org/.
- Daugovish, O., S. Koike, T. Gordon, and H. Ajwa. 2011a. Fumigant and strawberry variety evaluations in Macrophomina phaseolina and Fusarium oxysporum infested fields. Proc. Annu. Intl. Conf. Methyl Bromide Altern. Emissions Reductions, San Diego, CA, 31 October–2 November. http://mbao.org/.
- Daugovish, O., J. Muramoto, C. Shannon, M. Bolda, and S. Koike. 2011b. Anaerobic soil disinfestation for southern California strawberries. Proc. Annu. Intl. Conf. Methyl Bromide Altern. Emissions Reductions, San Diego, CA, 31 October–2 November. http://mbao.org/.
- Domínguez, P., L. Miranda, C. Soria, B. de los Santos, M. Chamorro, F. Romero, O. Daugovish, J.M. López-Aranda, and J. Medina. 2014. Soil biosolarization for sustainable strawberry production. Agron. Sustainable Dev. 34:821–829.
- Fennimore, S., F.N. Martin, T.C. Miller, J.C. Broome, N. Dorn, and I. Greene. 2014. Evaluation of a mobile steam applicator for soil disinfestation in California strawberry. HortScience 49:1–8.
- Fennimore, S., R. Serohijos, J. Samtani, H. Ajwa, K.V. Subbarao, F. Martin, O. Daugovish, D. Legard, G. Browne, J. Muramoto, C. Shennan, and K. Klonsky. 2013. TIF film, substrates and non-fumigant disinfestation maintain yields. Calif. Agr. 67:139–146.
- James, R., G. Knudsen, and M. Mora. 2004. Pre-plant soil treatment effects on production of Douglas fir seedlings at USDA Forest Service nursery. Forest Health Prot. Report 04-10. USDA Forest Service, Missoula, MT, USA.
- Johnson, M. and S. Fennimore. 2005. Weed and crop response to colored plastic mulches in strawberry production. HortScience 40:1371–1375.
- Koike, S.T. and T.R. Gordon. 2015. Management of Fusarium wilt of strawberry. Crop Protection 73:67–72.
- Koike, S.T., T.R. Gordon, O. Daugovish, H. Ajwa, M. Bolda, and K. Subbarao. 2012. Developments on strawberry plant collapse problems in California caused by Fusarium and Macrophomina. Intl. J. Fruit Sci. 13:76–83.
- Komada, H. 1975. Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soils. Rev. Plant Prot. Res. 8:114–125.
- Mihail, J.D. 1992. Macrophomina, p. 134–136. In: L.L. Singleton, J.D. Mihail, and C.M. Rush (eds.). Methods for research on soilborne phytopathogenic fungi. APS Press, St. Paul, MN.
- Muramoto, J., C. Shennan, G. Baird, M. Zavatta, S. Koike, M. Bolda, O. Daugovish, S. Dara, K. Klonsky, and M, Mazzola. 2014. Optimizing anaerobic soil disinfestation for California strawberries. Acta Hort. 1044:215–220. http://www.actahort.orgbooks1044/1044_25.htm.
- Rosskopf, E.N., N. Kokalis-Burelle, J. Hong, D.M. Butler, J. Noling, Z. He, B. Booker, and F. Sances. 2014. Comparison of anaerobic soil disinfestation and drip-applied organic acids for raised-bed specialty crop production in Florida. Acta Hort. 1044:221–228.
- Samtani, J., C. Gilbert, J. Weber, K. Subbarao, R. Goodhue, and S. Fennimore. 2012. Effect of steam and solarization treatments on pest control, strawberry yield, and economic returns relative to methyl bromide fumigation. HortScience 47:64–70.
- Scott, J.C., T.R. Gordon, D.V. Shaw, and S.T. Koike. 2010. Effect of temperature on severity of Fusarium wilt of lettuce caused by Fusarium oxysporum f. sp. lactucae. Plant Dis. 94:13–17.
- Stapleton, E., C.E. Bell, and J.E. DeVay. 1997. Soil solarization. University of California ANR publication #21377, Davis, CA, USA.
- Strand, L. 2008. Integrated pest management for strawberries, 2nd ed. Univ. of Calif. Statewide Intl. Pest Mgt. Project, Agr. Nat. Res. Publ. 3351.
- USDA. 2014. United States Department of Agriculture, Agricultural Marketing Service: Fruits and Vegetables. 28 Mar. 2015. http://marketnews.usda.gov/mnp/fv-nav-byCom?navClass=FRUITS&navType=byComm.
- USDS (United States Department of State). 2014. Methyl bromide critical use nomination for preplant soil use for strawberries grown for fruit in open field. 19 Sept. 2014. http://www.epa.gov/ozone/mbr/CUN2014/2014CUNStrawberryFruit.pdf.
- Zveibil, A., N. Mor, N. Gnayem, and S. Freeman. 2012. Survival, host-pathogen interaction, and management of Macrophomina phaseolina on strawberry in Israel. Plant Dis. 96:265–272.
