ABSTRACT
Field experiments were conducted in 2013 and 2014 to evaluate the predation efficacy of four commercially available predatory mites (Phytoseiidae): Phytoseiulus persimilis, Neoseiulus californicus, N. fallacis, and Amblyseius andersoni, for control of twospotted spider mite (Tetranychus urticae Koch) and Lewis spider mite (Eotetranychus lewisi) in strawberries. In the 2013 experiment, a grower standard treatment (P. persimilis and N. californicus) was compared to single treatments of each phytoseiid species. The 2014 experiment excluded N. fallacis as a treatment, but all other treatments are the same as in 2013. For both seasons, all treatments decreased both two-spotted and Lewis spider mite populations immediately after releases equivalently. However, none of the treatments tested were able to keep twospotted and Lewis spider mite populations below the economic threshold level. These findings indicate that several releases will be needed throughout the growing season in order to maintain spider mite levels below the economic thresholds.
Introduction
In California, strawberries (Fragaria × ananassa Duchesne) are a top ten commodity with a total acreage of approximately 61,310 acres and a total value of approximately $2.8 billion (NASS-USDA, Citation2015). They are also the top crop grown in Ventura County, California, with an annual value of $690 million and approximately 40,000 acres. Spider mites (Tetranychidae) are the main arthropod pest of strawberry (Oatman and McMurtry, Citation1966). Stress caused by high populations of spider mite feeding during plant establishment can stunt plants and decrease production and yield (Oatman et al., Citation1981, Citation1982; Sances et al., Citation1982; Walsh et al., Citation1998). Numerous spider mite species can be found on strawberry, but the twospotted spider mite (TSSM), Tetranychus urticae Koch, is the dominant spider mite found across all growing regions (Oatman, Citation1971; Strand, Citation2008). However, in Ventura County, heavy infestations of another spider mite, Lewis spider mite (Eotetranychus lewisi), have been reported (Howell and Daugovish, Citation2013). Lewis spider mite has typically been reported as an occasional pest of citrus, stone fruit, and greenhouse poinsettias (Doucette, Citation1962; Jeppson et al., Citation1975; McMurtry, Citation1985; Pérez-Santiago et al., Citation2007). It’s occurrence as a pest on strawberry in coastal California is relatively new, and knowledge about its biology and management is limited.
In field production, the twospotted spider mite is managed with a combination of miticides and repeated releases of predatory mites (Phytoseiidae). Predatory mites differ in their diet selection, behavior, and environmental needs (McMurtry and Croft, Citation1997); therefore, there is a continuous need to evaluate their predation efficacy on various species of spider mites, crops, and environments. For example, Phytoseiulus persimilis is a specialist, feeding only on spider mites in the genus Tetranychus (McMurtry and Croft, Citation1997). In laboratory bioassays, Howell and Daugovish (Citation2013) have shown P. persimilis to not feed on Lewis spider mite, conversely Amblyseius andersoni, Neoseiulus californicus, and N. fallacis will feed on both spider mite species and are possible candidates to manage both populations of Lewis and twospotted spider mites.
Given the importance of releasing predatory mites to assist in spider mite control, we evaluated the efficacy of A. andersoni, N. californicus, and N. fallacis in managing Lewis and twospotted spider mites in a field setting. Treatments were compared to the grower standard treatment consisting of a combination of P. persimilis and N. californicus. Second-year field studies examined A. andersoni, N. californicus, and the grower standard only.
Materials and methods
Predatory mites
Predatory mites used in the experiments were obtained from the following insectaries: P. persimilis and A. andersoni (Bioline Agrosciences, Inc., Oxnard, CA, USA), N. californicus (Associates Insectary, Santa Paula, CA, USA), and N. fallacis (Rincon-Vitova, Ventura, CA, USA), and used within 24 h. Before releasing, a representative sample of each bottle was observed under the dissecting scope to ensure the bottles contained abundant active predatory mites (1000–2000 predators per bottle).
2013 field experiment
An organic field (28.3 ha) containing mixed populations of Lewis and twopsotted spider mite, the latter being the dominant species, was located in Somis, California. Strawberry plants, cv. Ventana, were planted in early Oct. 2012 on raised beds covered by black plastic mulch. On average, beds were ~91.5 m × 1.2 m (l × w), with four rows of strawberry planted across, and two drip lines. Two weeks prior to experimental set-up and baseline counts (11 Feb.), Chromobacterium subtsugae (Grandevo, Marrone Bio Innovations) was sprayed to decrease spider mite populations. Throughout the experimental period, Chromobacterium subtsugae was sprayed on surrounding beds, not on experimental beds. Treatments were set up in areas where abundant populations of both spider mites were present. A full bed was used for one treatment, with three subplots: front and end (~3 m from each end of the bed), and middle (~45 m into the bed). Each subplot contained 12 plants. Four treatments were evaluated: (1) Amblyseius andersoni, (2) N. californicus, (3) N. fallacis, and (4) Grower Standard (P. persimilis + N. californicus). Each species of predatory mites were released by hand at a rate of ~61,000 predators per hectare per release. The ‘Grower Standard’ rate was 61,000 predators of each species/ha; N. californicus released once and P. persimilis released three times total. Predatory mites were released 3 days (14 Feb.), 4 weeks (15 Mar.), and 7 weeks (28 Mar.) after baseline counts. Plants were sampled on 21 Feb., 27 Feb., 7 Mar., 21 Mar., 4 Apr., and 18 Apr.
2014 field experiment
An organic field (Oxnard, CA) with 3.6 ha of strawberry (cv. Ventana) was used for the 2014 experiment, following the same procedures as 2013. The experiment was set up as a complete randomized block design with four replicates. Three treatments evaluated were: (1) A. andersoni, (2) N. californicus, and (3) Grower Standard (P. persimilis + N. californicus). N. fallacis was not used due to a shortage of mites that year. Predatory mites were released following the same procedure and predator release rates as the 2013 field season. The Grower Standard release and rates were also similar (N. californicus released once, P. persimilis released three times). Baseline samples were taken on 27 Jan. Predatory mites were released 1 (6 Feb.), 4 (25 Feb.), and 6 (5 Mar.) weeks after baseline counts. Plants were sampled on 10 Feb., 18 Feb., 5 Mar., 11 Mar., and 26 Mar.
Sampling protocol
Sampling began 11 Feb. 2013 and 27 Jan. 2014, with a baseline sample taken before experimental releases (week 1). On sampling days, 6 mid-tier trifoliates were randomly collected from each subplot (72 trifoliates per treatment) and taken back to the laboratory. The number of mobile (adults + nymphs + larvae) Lewis and twospotted spider mites were counted using a stereo microscope. After predatory mites were released, the number of predators, species, and predator eggs were counted (data not shown).
Statistical analysis
Data from the three subplots were pooled for each bed and analyzed using repeated measure ANOVA with time as a repeated measure (v. 22.0, SPSS Inc., Chicago, IL, USA) to compare values between treatments over the sampling period. To satisfy the assumptions of ANOVA, data was transformed (√(y + 0.5 or log (x + 1)). The Greenhouse-Geisser correction was used to adjust degrees of freedom in any case where data violated the assumption of sphericity or homogeneity of covariance (Greenhouse and Geisser, Citation1959). Means were separated using Tukey HSD (p < 0.05) if any differences were found. Due to the low number of predator numbers and predator eggs, data were not analyzed and are not shown.
Results and discussion
2013 experiment
Lewis spider mite mobiles varied throughout the experiment (p < 0.0001, F6,72 = 4.149). There was no significant difference in Lewis mite mobiles between treatments throughout the sampling period (p = 0.291, F18,72 = 1.300; ). Baseline counts showed that all treatments began with Lewis spider mite populations below the economic threshold of five per trifoliate. After the first predatory mite release, all treatments decreased spider mite populations and kept them low for the first 3 weeks. As the season progressed, plants became stressed with production, temperatures increased, and Lewis spider mites increased at a faster rate than the predatory mites, becoming unmanageable for the predatory mites. Two more releases were made, and populations of Lewis spider mites decreased dramatically after each release (). Although no statistical differences were found between treatments, A. andersoni and N. californicus kept Lewis spider mite populations lower than the Grower Standard and N. fallacis treatments (). The grower standard had a higher population of Lewis spider mite than the other treatments. This may be due to P. persimilis being released the past two times for that treatment. This may have made P. persimilis the dominant predatory mite, but does not predate on Lewis spider mite.
Figure 1. 2013 experiment, mean (± SE) number of (a) Lewis spider mite and (b) twospotted spider mite mobiles (adult + nymphs + larvae) per trifoliate in each treatment. Week 1 is the baseline sample taken before predators were released. Arrows indicate predatory mite release. Grower Standard = P. persimilis + N. californicus. Data are presented as means of four replicates.
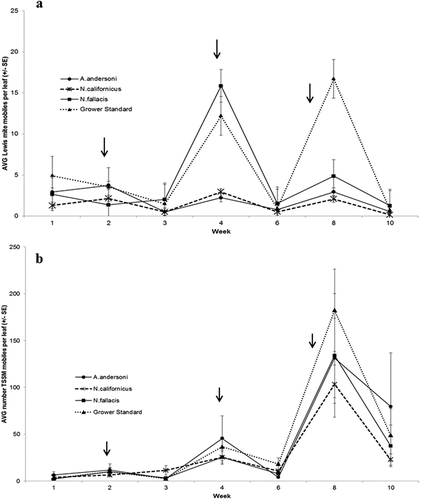
Twospotted spider mite mobiles varied throughout the sampling period (p < 0.0001, F6,72 = 17.77), with no significant difference between treatments throughout the experiment (p = 0.824, F18,72 = 0.447; ). Populations of twospotted spider mite also increased similarly to Lewis spider mite during weeks 4 and 8 ().
Immediately after predatory mite releases, the number of both Lewis and twospotted spider mite mobiles decreased in all treatments. However, 1 to 2 weeks after releases, spider mite populations began to increase again.
2014 experiment
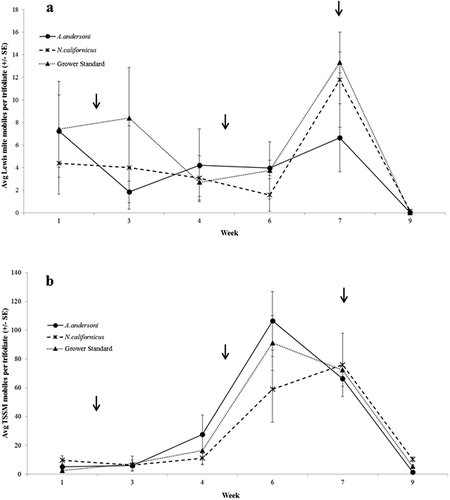
The results for the 2014 field experiment followed a similar pattern to those in 2013. Lewis spider mite mobiles varied throughout the experiment (p < 0.0001, F5,45 = 8.91), but the number of mobiles did not differ between treatments throughout the sampling period (p = 0.819, F10,45 = 0.583; ). A. andersoni kept Lewis spider mite mobiles under 10 mites per trifoliate throughout the experimental sampling period. The grower standard and N. californicus treatments increased to 13 and 12 (respectively) Lewis spider mites per trifoliate during week 7. Twospotted spider mite mobiles also varied throughout the experimental period (p < 0.0001, F5,45 = 50.02), however, there was no difference between treatments throughout the sampling period (p = 0.856, F2,9 = 0.158; ). As the season progresses and temperatures increase, as well as an increase in plant stress, spider mite populations also increase rapidly. A spike in spider mite mobiles can be seen for both species week 6 through 7 (). Similar to the patterns we saw in 2013, after predatory mite releases spider mite populations would decrease, but began to increase a week or two after predator releases. However, by the end of the sampling period (week 9), spider mite populations decreased close to zero.
Figure 2. 2014 experiment, mean (± SE) number of (a) Lewis spider mite and (b) twospotted spider mite mobiles (adult + nymphs + larvae) per trifoliate in each treatment. Week 1 is the baseline sample taken before predators were released. Arrows indicate predatory mite release. Grower Standard = P. persimilis + N. californicus. Data are presented as means of four replicates.
Predatory mites (phytoseiids) can be released as biocontrol of Lewis and twospotted spider mites in coastal California strawberry production, however, the timing and rate of release needs further examination. All four treatments, including the grower standard (P. persimilis + N. californicus), were able to decrease spider mite populations immediately after releases, but were not able to keep spider mite populations low 1 to 2 weeks after releases. This may be due to several factors. First, predatory mites will stay in areas with abundant prey. Once prey levels begin to decrease, predatory mites may decide to disperse (walking or aerially) to more desirable plants. Predatory mites that specialize on one prey type, e.g., P. persimilis, may disperse more often than other predatory mites that can feed on alternative food sources (pollen, smaller arthropods), such as A. andersoni, N. californicus, and N. fallacis. Another reason may be due to predatory mites having lower reproductive rates than Lewis and twospotted spider mite. At 25 °C, the lifecycle of the twospotted spider mite can be as brief as 3 to 5 days, and females can lay up to 30 eggs per day for 1 week (Hoy, Citation2011). At 25 °C, most predatory mites have a life cycle of 1 week and can lay two to four eggs per day for ~10 days (Hoy, Citation2011). This allows twospotted spider mites to out-produce the predatory mites. Microclimates within the plant zone can also play a role in the effectiveness of predatory mites. If conditions become too hot or cold, too humid or dry, predatory mites may choose to disperse or may die.
Predatory mites and their eggs were monitored throughout the experiment, but were found in low numbers until the end of the experiment. During the beginning of experiment, the main predators found in treatment beds were those that were released. Towards the last 2 weeks of sampling, a few N. californicus would be found in other treatments. N. californicus occurs naturally in California and has been observed in fields near the end of the winter berry season. This was observed in both 2013 and 2014, but their numbers were still low. Naturally occurring N. californicus have been observed by growers and Pest Control Advisors, but their late arrival in the season does not have much of an impact since spider mite populations naturally decrease towards the end of the season.
These results show that predatory mites can successfully decrease both twospotted and Lewis spider mites, but may need to be released earlier in the season when spider mite populations are low, and rates and number of releases may need to be increased for successful control. Research on the proper timing, rates, and possible combinations, e.g., releasing A. andersoni, N. californicus, or N. fallacis at the beginning of the season, and releasing P. persimilis before twospotted spider mite populations begin to build up, needs further research.
Acknowledgments
We wish to thank Cameron Chandler and William Rutan for field and laboratory support, Glen Hasegawa and Success Valley Farms for field sites, and Bioline Agrosciences, Inc. and Associates Insectary for predatory mites.
Funding
We gratefully acknowledged the California Strawberry Commission for their financial support.
Additional information
Funding
Literature cited
- Doucette, C.F. 1962. The lewis mite, Eotetranychus lewisi, on greenhouse Poinsettia. J. Econ. Entomol. 55:139–140.
- Greenhouse, S.W. and S. Geisser. 1959. On methods in the analysis of profile data. Psychometrika. 24:95–112.
- Howell, A.D. and O. Daugovish. 2013. Biological control of Eotetranychus lewisi and Tetranychus urticae (Acari: Tetranychidae) on strawberry by four phytoseiids (Acari: Phytoseiidae). J. Econ. Entomol. 106(1):80–85.
- Hoy, M.A. 2011. Agricultural acarology: Introduction to integrated mite management, p. 86, 162. CRC Press, Boca Raton, FL.
- Jeppson, L.R., H.H. Keifer, and E.W. Baker. 1975. Mites injurious to economic plants, p. 127–252. University of California Press, Ltd, London, England.
- McMurtry, J.A. 1985. Citrus, p. 339–346. In: W. Helle and M.W. Sabelis (eds.). Spider mites: Their biology, natural enemies, and control, vol. 1B. Elsevier, Amsterdam, Netherlands.
- McMurtry, J.A. and B.A. Croft. 1997. Life-styles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol. 42:291–321.
- NASS-USDA. 2015. Strawberries—Acres planted & production in $ 2014. NASS-USDA, Washington D.C., USA.
- Oatman, E.R. 1971. Mite species on strawberry in southern California. J. Econ. Entomol. 64:1313–1314.
- Oatman, E.R. and J.A. McMurtry. 1966. Biological control of the twospotted spider mite on strawberries in southern California. J. Econ. Entomol. 59:433–439.
- Oatman, E.R., F.V. Sances, L.F. LaPré, N.C. Toscano, and V. Voth. 1982. Effects of different infestation levels of the twospotted spider mite on strawberry yield in winter plantings in southern California. J. Econ. Entomol. 75:94–96.
- Oatman, E.R., J.A. Wyman, H.W. Browning, and V. Voth. 1981. Effects of releases and varying infestation levels of the twospotted spider mite on strawberry yield in southern California. J. Econ. Entomol. 74:112–115.
- Pérez-Santiago, G., G. Otero-Colina, V.A. González Hernández, M.E. Ramírez Guzmán, H. González Hernández, and A. López Jiménez. 2007. The population level of Eotetranychus lewisi and the concentration of carbohydrates in peach trees. Exp. Appl. Acarol. 43:255–263.
- Sances, F.V., N.C. Toscano, E.R. Oatman, L.F. Lapre, M.W. Johnson, and V. Voth. 1982. Reductions in plant processes by Tetranychus urticae (Acarina: Tetranychidae) feeding on strawberry. Environ. Entomol. 11:733–737.
- Strand, L. 2008. Integrated pest management for strawberries, 2nd ed. University of California Statewide Integrated Pest Management Program, Agriculture and Natural Resources Publication 3351.
- Walsh, D.B., F.G. Zalom, and D.V. Shaw. 1998. Interaction of the two spotted spider mite (Acari: Tetranychidae) with yield of day-neutral strawberries in California. J. Econ. Entomol. 91(3):678–685.
