ABSTRACT
The objective of this work was to quantify changes in the germination capacity of Juglans nigra seeds following storage and then artificial stratification. Seeds were stored at 5 and –20 °C under 5% and 14% internal moisture content (IMC) for 0, 8, 10, and 12 months under conditions with oxygen or under vacuum. Germination percentage (GP) was affected by storage temperature, IMC, and storage period. Seeds stored with 5% IMC succeeded in germinating regardless of the temperature. Seeds stored with 14% IMC did not germinate (–20 °C) or germinated during storage (5 °C). The GP was reduced with the time of storage in seeds at –20 °C and 5% IMC and this effect was avoided in storage under vacuum. In conclusion, the germination capacity of black walnut seeds did not change for up to 12 months at low above-zero temperature and low IMC (5 °C, 5% IMC), being the most cost-effective alternative for long-term storage of black walnut seeds in commercial nurseries. Vacuum storage avoided the decrease of around 50% in average germination capacity of seeds stored for a year at –20 °C.
Introduction
Black walnut (Juglans nigra L.), also called eastern black walnut and American walnut, is one of the most coveted hardwood tree species. Native to the deciduous forests of the eastern United States, its best known use is for its lumber. The wood is used for furniture of all kinds, cabinetwork, musical instruments, and gunstocks (Voulgaridis and Vassiliou, Citation2005; Williams, Citation1990). In Argentina it is mainly used as a rootstock for European walnut (Juglans regia), since it induces precocity to the grafted variety (Lemus, Citation1997) and confers tolerance to Phytophthora cinnamonii, root nematodes, Armillaria, and crown gall (Iannamico et al., Citation2006).
Black walnut seed production can be unsteady, with large seed crops occurring at irregular intervals followed by years of poor yield. Thus, storage of the seeds is of paramount importance to cover the annual needs of nurseries, since it ensures availability of seeds in years with insufficient seed production (MINAGRI, Citation2014).
Storage may be defined as the preservation of viable seeds from the time of collection until they are required for sowing (Holmes and Buszewicz, Citation1958). Inadequate storage causes seed deterioration, which results in lower seed quality, viability, and vigor (Kapoor et al., Citation2010). Changes during storage involve important seed physiological modifications in the cell tissues, such as the loss of food reserves, build-up of toxic metabolic by-products, aging of cell membranes, and nuclear DNA alterations (Copeland and McDonald, Citation2001; Palma et al., Citation2000; Rivera et al., Citation2007; Zhou et al., Citation2009). Most oil-bearing seeds like walnut and cashew are very susceptible to microbial attack as a result of improper long-term storage. Microbial attack leads to hydrolysis of the oil in the seed and hence to the formation of free fatty acids, which are responsible for the rancid flavor/odor and seed deterioration (Onilude et al., Citation2010). These authors identified eight fungal species and three bacteria with lipase activity, which was enhanced when cashew nuts were stored in an atmosphere with >30% relative humidity.
According to their behavior during storage, seeds are classified into orthodox and recalcitrant (Roberts, Citation1973). Orthodox seeds are those that can be dried down to a low internal moisture content (IMC) of around 5% (wet basis) and successfully stored at low or sub-freezing temperatures for long periods without seed injury (FAO, Citation1991). Recalcitrant seeds, on the other hand, are those that cannot survive drying below relatively high moisture content (often in the range of 15–50%, wet basis). Intolerance to desiccation is an important agronomic disadvantage because it makes long-term seed conservation difficult (FAO, Citation1991; Farrant et al., Citation1993; Gentil, Citation2001).
The last stage in the ripening of orthodox seeds involves an extensive cell desiccation, which starts with the disruption of the vascular connection between the mother plant and the seed (Bewley and Black, Citation1994; Kermode and Finch-Savage, Citation2002). During this period seeds acquire the desiccation tolerance, which improves their viability and storage potential (Hoekstra et al., Citation1994; Nkang, Citation2002). The decrease in the seed’s IMC reduces the respiration rate and triggers several other biochemical processes, which protect the organelles and the cell walls (Walters et al., Citation2005), delaying seed aging and extending viability (Pérez Camacho et al., Citation2012). Recalcitrant seeds, on the other hand, do not undergo dehydration while on the mother plant, and must be sown immediately after harvest, or artificially stratified over winter before sowing in the spring, to maintain their viability (Farrant et al., Citation1993).
Regardless of the cause of deterioration, viability of orthodox seeds can be prolonged by reducing their respiration rate during storage (Pérez Camacho et al., Citation2012), either by lowering the temperature, the IMC, or storing in sealed containers (FAO, Citation2013). In the latter case, respiration decreases oxygen concentration around the seed. However, a minimum threshold of respiratory rate is necessary to support the multiple cell changes that occur during stratification and that are correlated with the breaking of seed dormancy, and consequently with seed germination and seedling growth (Hopper et al., Citation1985). These changes involved a reduction in stored protein and lipids and an increased number of mitochondria and endoplasmic reticulum in the hypocotyl cells of black walnut embryos (Somers and Van Sambeek, Citation2003). If these changes do not occur during seed storage, e.g., by the absence of oxygen, seed can remain viable for a long time but will require additional stratification (Williams, Citation1971).
The objective of this work was to quantify the changes in the germination capacity of black walnut seeds under different pre-stratification storage conditions in order to improve the time of conservation while maintaining their post-stratification germination capacity.
Materials and methods
Fruits were obtained from a 50-year-old J. nigra stand at the J.F. Villarino Experimental Field of the Facultad de Ciencias Agrarias, Universidad Nacional de Rosario, located in Zavalla (33° 01′ S, 60° 53′ W), Santa Fe province, Argentina. Fruits were collected following the FAO recommendations (FAO, Citation1991) from at least 15 trees after natural fruit drop, and then mixed to form a single sample. Fruits were husked and washed over sieves, then immediately soaked in water to remove floaters, usually non-viable seeds (Flores et al., Citation2013).
The first experiment was performed following a randomized complete block design with a factorial arrangement and four 50-seed replications per treatment. The three factors assessed were storage temperature (with two levels, 5 and –20 °C); internal moisture content (IMC) (two levels, 5% and 14%); and storage period (three levels, 8, 10, and 12 months). To lower the IMC of freshly collected seeds (~20%) down to the IMC of each treatment, seeds were exposed to the air and frequently shaken to promote uniform drying (Turnbull, Citation1975). IMC was determined by change in weight. Seeds that were to be stored at 5 °C were placed in 3-L glass jars filled to the brim and sealed (FAO, Citation1991). The jars were placed on shelves in the storage chamber at 5 ± 1 °C. Seeds for storage at –20 °C were placed in paper bags, then in coextruded heat shrinkable polyethylene bags (50 × 25 cm) to avoid moisture loss.
The second experiment was performed following a completely randomized block design with a factorial arrangement, with four 50-seed replications for each treatment. Seed were stored at –20 °C and 5% IMC and the two factors assessed were the effect of the storage atmosphere (two levels, vacuum and no vacuum), and storage period (three levels, 8, 10, and 12 months). For vacuum treatments, bags were sealed with an ST 120 sealer equipped with a Busch vacuum pump, whereas in treatments with no vacuum, polyethylene bags were sealed with adhesive tape. In both experiments, seeds were stratified in moist sand at a constant temperature (5 °C) for 4 months after the storage treatments to overcome physiological dormancy (Baskin and Baskin, Citation1998). Control seeds were stratified immediately after collection. After stratification, seeds were tested with the standard germination test for 28 days (ISTA, Citation2003). The results were expressed as percentage of normal seedlings, and those with stubby primary root, shortened epicotyl, primary root missing, and damaged epicotyl were considered as abnormal. The variables analyzed in both experiments were germination percentage (GP), germination velocity index (GVI), mean time to maximum germination (MTMG), percentage of normal seedlings (%NS), and mean dry weight of normal seedlings (DWNS). GVI represents the percentage of the hypothetical maximum germination velocity that would be reached if all seeds germinated within the first days of sowing (Silva and Nakagawa, Citation1995). MTMG expresses the average time needed to reach maximum germination (Edmond and Drapala, Citation1958). To determine mean dry weight, seedlings with their cotyledons removed were oven dried at 80 °C for 2 h, and weighed on a precision scale (AOSA, Citation2002; Nakagawa, Citation1994).
Statistical analysis was performed with Infostat software. Data were analyzed with analysis of variance (ANOVA) and means were compared using the Tukey test at the 5% probability level. Normality and homoscedasticity were tested graphically (Q-Q plot, and residuals versus predictors plot, respectively).
Results
In the first experiment, GP was affected by storage temperature (p < 0.0001), IMC (p < 0.0001), and storage period (p = 0.001). Interactions between temperature and storage period (p = 0.0002), temperature and IMC (p < 0.0001), and storage time and IMC (p = 0.001) were also observed.
Control seeds, which were stratified immediately after collection, had an average 87% GP. IMC was the variable with the greatest influence on the GP during storage. Seeds stored with low IMC reached 74% GP on average. On the other hand, seeds kept at 5 °C and high IMC started to germinate inside the jars after 3 months, and were deteriorated and covered with fungi at the end of the 8–12 months storage periods evaluated in this experiment. Thus, the GP of seeds at 5 °C and high IMC could not be determined (seeds germinated during storage) ().
Figure 1. Influence of pre-stratification storage at different internal moisture content (IMC) (a), storage temperature (b), and storage time (c), on the post-stratification germination capacity of black walnut seeds. Seeds were stratified for 4 months after each storage treatment. Control seeds reached 87.8% of germination. Data of high IMC treatments was calculated only from seeds stored at –20 °C since values from those stored at 5 °C could not be determined because germination occurred during storage. Columns with different letters indicate that the mean value significantly differs according to the Tukey’s test (p ≤ 0.05).
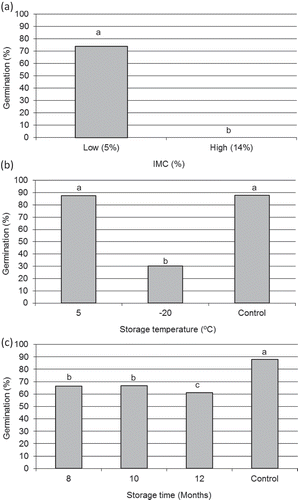
Storage temperature also affected GP, which dropped by 65% when storage temperature was lowered from 5 °C (87%) to –20 °C (30%) on average (). In relation with the variable storage period, GP decreased by 8% on average when the storage time was increased from 10 to 12 months ().
Storage conditions at 5 °C and low IMC, followed by 4 months of stratification, proved best, reaching a germination percentage near 90% for all of the storage periods evaluated (8 to 12 months) (). This value did not show significant differences in comparison with control seeds. On the other hand, seeds stored at –20 °C and low IMC reached 60.5% of GP on average, which was 30.8% lower in comparison with the GP of seeds stored at 5 °C and low IMC. Also, at –20 °C storage temperature, germination decreased sharply (–31.7%) when the storage period was increased from 10 to 12 months. This effect was not observed in seeds kept at 5 °C and low IMC, which explains the interaction between temperature and storage period ().
Table 1. Effects of the time of storage on the germination (%) of black walnut seeds kept under different temperature, internal moisture content (IMC), and vacuum during storage.z
Similarly, the lower GP observed in seeds kept at –20 °C and low IMC as compared to seeds stored at 5 °C and low IMC explains the interaction between temperature and IMC (). Seeds kept at –20 °C and high IMC failed to germinate, regardless of storage period. However, seeds in these treatments, unlike those kept at 5 °C and high IMC, were not deteriorated at the end of the storage time, although they were covered by an ice layer. Therefore, only seeds stored with a low IMC succeeded in germinating after the storage treatments (8, 10, and 12 months), regardless of the storage temperature ().
The other variables analyzed, namely GVI, MTMG, %NS, and DWNS, were also affected by storage temperature, storage period, and IMC. The effects and interactions were similar to those found for the GP (). The best result for these variables was obtained in seeds kept at 5 °C and low IMC. Under these storage conditions, GVI was as high as 21% and MTMG was 5 days on average. Seeds stored at –20 °C and low IMC decreased their germination velocity by 48%, reaching a GVI of 11% and a MTMG of 10 days on average. These variables were not assessed in treatments with high IMC, since seeds either germinated in the jars during storage (5 °C) or failed to germinate (–20 °C).
Table 2. Influence of storage temperature, internal moisture content (IMC), and vacuum for different periods of storage (months) on germination velocity index (GVI), mean time of maximum germination (MTMG), percentage of normal seedlings (NS), and mean dry weight of normal seedlings (DWNS) of black walnut seeds.z
The %NS of seeds stored at 5 °C and low IMC was near 75% regardless of storage period. This value decreased by 30% in seeds stored at –20 °C and low IMC (52% on average). In seeds kept at –20 °C, a marked decrease (34%) in %NS occurred when the storage period was increased from 10 to 12 months, showing an interaction between temperature and storage time ().
DWNS from seeds stored at 5 °C and low IMC was 603 mg on average, a value that did not differ from that of seedlings obtained from control seeds (599 mg). This value decreased by approximately 20% when the storage temperature was lowered from 5 °C to –20 °C, and a further 17% when the storage period at –20 °C was increased from 8 to 12 months ().
In the second experiment, GP was affected by storage conditions and by storage period. Also, there was an interaction between both variables (p < 0.0001) (). The GP of seeds stored at –20 °C under vacuum conditions was 32% higher on average than the GP of seeds stored without vacuum. Thus, vacuum treatments allowed seeds stored at –20 °C to reach germination percentages (>87.5%) similar to those of non-stored (control) seeds (87.8%), preventing their deterioration even under the longest storage period evaluated (12 months). Vacuum also prevented the decline in the GP observed in seed kept without vacuum, mainly when the storage time increased from 10 to 12 months. This explains the interaction observed between the storage atmosphere and storage time variables ().
GVI, MTMG, %NS, and DWNS, were also affected by vacuum conditions. The best results for these variables were obtained with –20 °C and vacuum, regardless of storage time. The GVI increased 89% by vacuum, whereas the TMG decreased by 50%. Vacuum also increased the %NS (+44%) and the DWNS (+24%) ().
Discussion
Loss of seed viability during storage is largely governed by their respiration rate. Thus, any method that reduces the rate of respiration without otherwise damaging the seed generally results in longer seed viability (Pérez Camacho et al., Citation2012). The usual methods for decreasing the seeds’ respiration rate include lowering the internal moisture content as well as the temperature and oxygen concentration in the storage atmosphere (Copeland and McDonald, Citation2001; FAO, Citation1991; Melgoza et al., Citation2003; Ruiz et al., Citation2008). However, the most widely used technique is the reduction of the internal moisture content because it is the most cost effective (Pérez Camacho et al., Citation2012; Walters et al., Citation2005). Williams (Citation1971) was able to store black walnut seed in outdoor stratification pits (90–120 cm deep) for as long as 4 years (40% germination) compared with 2 years in cold chamber both under dry and moist conditions (15% germination). In the mentioned experiment there was no subsequent seed stratification, so the germination capacity of those treatments that cause a sharp decrease in the seed metabolic activity during storage could be underestimated. The progressive morphological, anatomical, physiological, and hormonal changes that occurred during stratification and that were necessary for normal seedling germination could be limited in treatments with a minimal metabolic activity during storage (Somers and Van Sambeek, Citation2003). Instead, in our work all treatments included pre-stratification with the objective to retain seed viability followed by stratification to determine their germination capacity.
Our results confirm that IMC was the most important storage factor in extending seed viability. Seeds stored with low IMC (5%) presented high germination percentage, faster germination, and greater proportion of normal seedlings regardless of the storage temperature. However, it is important to point out that no high-temperature storage treatments were included in this study since the two temperatures evaluated (5 and –20 °C) are able to restrict or virtually stop seed metabolism (Harrington, Citation1972).
Since seeds kept at high IMC (14%) and low temperatures overcame dormancy and germinated inside the jars, storage under these conditions is suitable only for short storage periods and for tree and shrub species with dormant seeds (FAO, Citation1991; Hong and Ellis, Citation1992). Under these conditions, storage time should be similar to the period required by seeds to overcome their physiological dormancy (Baskin and Baskin, Citation1998), which in black walnut should not exceed 4 months (Flores, Citation2013; Van Sambeek et al., Citation1989).
Another result that emerged from our data was that black walnut seeds were able to overcome seed dormancy without the requirement of placing them on a moist substrate, and that their own internal moisture content was sufficient when its value was 14%. Consequently, storage under low temperature (5 °C) and high IMC (14%) conditions replaced the requirement of seed stratification because the storage conditions were similar in both cases (Hong and Ellis, Citation1992).
Seeds stored at freezing temperatures (–20 °C) and high IMC were also non-viable at the end of the 8–12 month periods of storage, but in this case the more likely cause of seed death was the formation of ice crystals inside the cells, which causes a direct damage on membrane and nucleic acid structures (Levitt, Citation1980). In contrast, ice formation does not occur at low IMC conditions because dehydration is a strategy of seeds to avoid ice formation and chilling injury (DeVries et al., Citation2007; Woltz et al., Citation2005). This explains the large differences in chilling tolerance found in seeds with different IMC levels when they were stored at –20 °C.
In the second experiment, it was demonstrated that the exclusion of oxygen, when seeds were stored at low IMC and freezing temperatures, allowed obtaining a GP similar to that of non-stored seeds (control), avoiding a declination of around 50% in the main seed’s quality attributes after a year of storage. Seed deterioration involves the oxidation of lipids, cell and mitochondrial membranes, DNA, and RNA (Bailly, Citation2004; Osborne, Citation1994; Rajjou and Debeaujon, Citation2008; Rajjou et al., Citation2008), which points to the key role of oxygen in seed deterioration during storage (Groot et al., Citation2012). In addition, it has been reported that the increase in oxygen levels during storage triggers chromosome aberrations during cell division (Abdalla and Roberts, Citation1968). Even though the detrimental effect of the presence of oxygen during storage is well established, its role has hardly been considered in the practice of commercial or gene-bank seed storage (Groot et al., Citation2012), and only low moisture content and low temperatures during storage have long been considered as the key factors in prolonging seed viability (Cromarty et al., Citation1982). Despite the adequate behavior of black walnut seeds at –20 °C, desiccated orthodox seeds should be stored in the range of –5 °C and +5 °C in order to save a considerable amount of energy (Pérez-García et al., Citation2007).
In conclusion, our research showed that the germination capacity, GP, GVI, MTMG, %NS, and the DWNS, of black walnut seeds did not change for up to 12 months of storage at low above-zero temperature (5 °C) and low IMC (5%). Furthermore, vacuum avoided the decrease of around 50% in average of the germination capacity of seeds stored for a year at –20 °C and low IMC.
Acknowledgments
The authors thank Gabriela Venturi for her assistance in writing the manuscript in English, and Jerry Van Sambeek for his assistance in the discussion of results.
Funding
This work was principally supported by the Universidad Nacional de Rosario and the Universidad Nacional del Litoral (CAI+D 50120110100002 LI).
Additional information
Funding
Literature cited
- Abdalla, F.H., and E.H. Roberts. 1968. Effects of temperature, moisture, and oxygen on the induction of chromosome damage in seeds of barley, broad beans, and peas during storage. Ann. Bot. 32:119–136.
- Association Official Seed Analysts (AOSA). 2002. Seed vigor testing handbook, AOSA Contribution No. 32. AOSA, Washington, DC, USA.
- Bailly, C. 2004. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 14:93–107.
- Baskin, C.C., and J.M. Baskin. 1998. Seeds: Ecology, biogeography, and evolution of dormancy and germination. Academic Press, San Diego, CA.
- Bewley, J.D., and M. Black. 1994. Seeds: Physiology of development and germination. Plenum Press, New York, NY.
- Copeland, O.L., and M.B. McDonald. 2001. Principles of seed science and technology, 4th ed. Kluwer Press, New York, NY.
- Cromarty, A., R.H. Ellis, and E.H. Roberts. 1982. The design of seed storage facilities for genetic conservation. International Board for Plant Genetic Resources, Rome, Italy.
- DeVries, M., A.S. Goggi, and K.J. Moore. 2007. Determining seed performance of frost-damaged maize seed lots. Crop Sci. 47:2089–2097.
- Edmond, J.B., and W.J. Drapala. 1958. The effect of temperature, sand and soil, and acetone on germination of okra seed. Proc. Am. Soc. Hort. Sci. 71:728–734.
- FAO. 1991. Seed storage, p. 195–237. In: R.L. Willan (ed.). A guide to forest seed handling. FAO, Rome, Italy.
- FAO. 2013. Normas para bancos de germoplasma de recursos fitogenéticos para la alimentación y la agricultura. FAO, Roma, Italy. 22 May 2014. http://www.fao.org/3/a-i3704s.pdf.
- Farrant, J.M., N.W. Pammenter, and P. Berjak. 1993. Seed development in relation to desiccation tolerance: A comparison between desiccation sensitive (recalcitrant) seeds of Avicennia marina and desiccation tolerant types. Seed Sci. Res. 3:1–13.
- Flores, P.C. 2013. Factores que afectan la calidad fisiológica y la capacidad de almacenamiento de las semillas de nogal negro (Juglans nigra L.). Universidad Nacional de Rosario, Rosario, Argentina, Tesis doctoral.
- Flores, P.C., D. Poggi, M.S. García, M. Catraro, and N.F. Gariglio. 2013. The pulp of Juglans nigra fruit affects seed germination and root growth. Seed Technol. 35:41–53.
- Gentil, D.F. 2001. Conservação de sementes do cafeeiro: Resultados discordantes ou complementares?. Bragantia 60:149–154.
- Groot, S.P.C., A.A. Surki, R.C.H. de Vos, and J. Kodde. 2012. Seed storage at elevated partial pressure of oxygen: A fast method for analysing seed ageing under dry conditions. Ann. Bot. 110:1149–1159.
- Harrington, J.F. 1972. Seed storage and longevity, p. 134–245. In: T.T. Kozlowsky (ed.). Seed biology, vol. 3. Academic Press, New York, NY.
- Hoekstra, F., A. Haigh, F. Tettero, and T. van Roekel. 1994. Changes in soluble sugars in relation to desiccation tolerance in cauliflower seeds. Seed Sci. Res. 4:143–147.
- Holmes, G.D., and G. Buszewicz. 1958. The storage of seed of temperate forest tree species. For. Abstr. 19:313–322.
- Hong, T.D., and R.H. Ellis. 1992. The survival of germination orthodox seeds after desiccation and hermetic storage. J. Exp. Bot. 43:239–247.
- Hopper, G.M., D.J. Parrish, and D.W. Smith. 1985. Adenylate energy charge during stratification and germination of Quercus rubra. Can. J. For. Res. 15(5):829–832.
- Iannamico, L., P. Calvo, and H.R. Castro. 2006. The behavior of ten late sprouting walnut cultivars in the Alto Valle of Rio Negro, Patagonia (Argentina). Proc. V Intl. Walnut Symp., Sorrento, Italy, 9–13 Nov. 2004. (Acta Hort. 705:493–498).
- International Seed Testing Association (ISTA). 2003. International rules for seed testing. ISTA, Zurich, Switzerland.
- Kapoor, R., A. Arya, M.A. Siddiqui, A. Amir, and H. Kumar. 2010. Seed deterioration in Chickpea (Cicer arietinum L.) under accelerated ageing. Asian J. Plant Sci. 9:158–162.
- Kermode, A.R., and W.E. Finch-Savage. 2002. Desiccation sensitivity in orthodox and recalcitrant seeds in relation to development, p. 149–184. In: M. Black and H.W. Pritchard (eds.). Desiccation and survival in plants: Drying without dying. CABI Publishing, New York, NY.
- Lemus, G. 1997. Propagación y variedades de nuez. Seminario: Avances tecnológicos en el cultivo del nogal. INIA, Santiago.
- Levitt, J. 1980. Responses of plants to environmental stresses. Academic Press, New York, NY.
- Melgoza, C.A., M.M.H. Royo, N.C.R. Morales, and T.J.S. Sierra. 2003. Germination of locoweed seed (Astragalus mollissimus Torr) at different temperatures ranges and water stress levels. Rev. Mex. Cienc. Pecu. 41:85–89.
- Ministerio de Agricultura, Ganadería y Pesca de la República Argentina (MINAGRI). 2014. Consideraciones para la cosecha, almacenamiento y germinación de semillas.12 November 2015. http://www.minagri.gob.ar/new/0-0/forestacion/viveros/consideraciones.htm.
- Nakagawa, J. 1994. Testes de vigor baseados na avaliação das plántulas, p. 49–85. In: R.D. Vieira and N.M. Carvalho. Testes de vigor em sementes. FUNEP Jaboticabal, Sao Paulo, Brazil.
- Nkang, A. 2002. Carbohydrate composition during seed development and germination in two sub-tropical rainforest tree species (Erythrina caffra and Guilfoylia monostylis). J. Plant Physiol. 159:473–483.
- Onilude, A.A., R.O. Igbinadolor, and S.M. Wakil. 2010. Effect of varying relative humidity on the rancidity of cashew (Anacardium occidentale L.) kernel oil by lipolytic organisms. Afr. J. Biotechnol. 9(31):4890–4896.
- Osborne, D.J. 1994. DNA and desiccation tolerance. Seed Sci. Res. 4:175–185.
- Palma, R.M.P., H.A. López, and M.J.C. Molina. 2000. Condiciones de almacenamiento y germinación de semillas de Cenchrus ciliaris L y Andropogon gayanus Kunth. Agrociencia 34:41–48.
- Pérez Camacho, I., V. González Hernández, O. Ayala Garay, J. Carrillo Salazar, G. García de los Santos, A. Peña Lomelí, and E. Cruz Crespo. 2012. Calidad fisiológica de semillas de Physalis ixocarpa en función de madurez a cosecha y condiciones de almacenamiento. Rev. Mex. Cienc. Agr. 3:67–78.
- Pérez-García, F., M.E. González-Benito, and C. Gómez-Campo. 2007. High viability recorded in ultra-dry seeds of 37 species of Brassicaceae after almost 40 years of storage. Seed Sci. Technol. 35:143–153.
- Rajjou, L., and I. Debeaujon. 2008. Seed longevity: Survival and maintenance of high germination ability of dry seeds. C.R. Biol. 331:796–805.
- Rajjou, L., Y. Lovigny, S.P.C. Groot, M. Belghazi, C. Job, and D. Job. 2008. Proteome-wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 148:620–641.
- Rivera, R.M.F., L.R. Sosa, E.A. Fernández, M.I. Reale, and V. Villarreal. 2007. Efecto del estrés hídrico a distintas temperaturas sobre la germinación de semillas de Bulnesia retama (Gill. ex. Hook.) Griseb.–Zigofiláceas–en San Luis, Argentina. Int. J. Exp. Bot. 76:5–17.
- Roberts, E.H. 1973. Predicting the storage life of seeds. Seed Sci. Technol. 1:499–514.
- Ruiz, E.F.H., L.P. Marrero, P.O. Cruz, A.B. Murillo, and H.J.L. García. 2008. Agroclimatic factor influences in the basil productivity (Ocimum basilicum L.) in an arid area of Baja California Sur, Mexico. Revista Ciencias Técnicas Agropecuarias 7:44–47.
- Silva, J.B., and J. Nakagawa. 1995. Estudo de fórmulas para cálculo da velocidade de germinaçăo. Informativo Abrates 5:62–73.
- Somers, P.W., and J.W. Van Sambeek. 2003. Physiological and ultrastructural changes in black walnut embryos during stratification and germination. Ann. Rep. Northern Nut Growers Assoc. 94:107–119.
- Turnbull, J.W. 1975. Seed extraction and cleaning. In: Report on FAO/DANIDA training course on forest seed collection and handling, vol. 2. FAO, Rome, Italy.
- Van Sambeek, J.W., J.E. Preece, T.L. Lindsay, and G.R. Gaffney. 1989. In vitro studies on black walnut embryo dormancy. Annu. Rep. Northern Nut Growers Assoc. 80:55–59.
- Voulgaridis, V., and V.G. Vassiliou. 2005. Walnut wood and its utilisation to high value products. Acta Hort. 705:69–81.
- Walters, C., L.M. Hill, and L.J. Wheeler. 2005. Dying while dry: Kinetics and mechanisms of deterioration in desiccated organisms. Integr. Comput. Biol. 45:751–758.
- Williams, R.D. 1971. Stratified walnut seeds still viable after four years in storage. Tree Planters’ Notes 22(4):1–2.
- Williams, R.D. 1990. Black walnut, p. 391–399. In: R.M. Burns and B.H. Honkala (eds.). Silvics of North America. Hardwoods Agriculture Handbook. USDA Forest Service, Washington D.C.
- Woltz, J.M., D.B. Eglin, and D.M. TeKrony. 2005. Freezing point temperatures of corn seed structures during seed development. Agron. J. 97:1564–1569.
- Zhou, L., E. Jiang, and B. Li. 2009. Effect of wood vinegar on seed germination and water implantation of corn. J. Northeast Agr. Univ. 16:6–11.
